About this course:
This course explores the epidemiology, pathophysiology, and risk factors associated with acute respiratory distress syndrome (ARDS). It also reviews the clinical manifestations, evaluation, diagnostic criteria, and supportive management strategies for ARDS.
Course preview
Acute Respiratory Distress Syndrome
This course explores the epidemiology, pathophysiology, and risk factors associated with acute respiratory distress syndrome (ARDS). It also reviews the clinical manifestations, evaluation, diagnostic criteria, and supportive management strategies for ARDS.
Upon completion of this module, learners should be able to:
- review the epidemiology of and risk factors for ARDS
- describe the pathophysiology and clinical manifestations associated with ARDS
- describe the evidence-based guidelines for the evaluation and diagnosis of ARDS
- discuss the various supportive management strategies for ARDS
- discuss the pathophysiology of mechanical ventilation (MV) and the recommended ventilator strategies for managing patients with ARDS
Healthcare providers (HCPs) are responsible for providing high-quality, evidence-based care to optimize patient outcomes. As new treatments emerge, people are living longer, healthier lives. As the US population ages, more people live with chronic health conditions. With more people managing complex chronic conditions, the number of patients being admitted to intensive care units (ICUs) is increasing. Traditionally, HCPs working with critically ill patients have focused on stabilizing immediate, life-threatening cardiopulmonary symptoms. As survival from critical illness has improved, the medical focus has shifted to preventing the sequelae of critical illness, including neuromuscular weakness, cognitive impairment, psychological disorders, and chronic respiratory disorders. Critically ill patients are often admitted to ICUs so that HCPs can effectively manage physiological responses to illness. ARDS (is a distinct type of respiratory failure characterized by poor oxygenation and stiff (non-compliant) lungs associated with capillary endothelial injury and diffuse alveolar damage. ARDS typically occurs in patients who are already critically ill due to a major injury or another disease. MV is an important aspect of managing patients with ARDS, but mortality remains high despite significant advances in treatment. HCPs caring for critically ill patients must be familiar with the pathophysiology, risk factors, and clinical manifestations of ARDS. In addition, HCPs should understand the criteria for ARDS diagnosis and treatment strategies, including the use of MV (Dirkes & Kozlowski, 2019; Hyzy & McSparron, 2025; Siegel, 2025).
Background
The Centers for Disease Control and Prevention (CDC) defines chronic diseases as conditions that last more than one year and require ongoing medical attention and/or limit activities of daily living (ADLs). Chronic disease is the leading cause of death and disability in the United States. An estimated 6 out of 10 American adults have at least one chronic disease, and 4 out of 10 have two or more chronic diseases. Chronic conditions such as heart disease, cancer, chronic lung disease, diabetes mellitus (DM), Alzheimer’s disease, and chronic kidney disease (CKD) significantly contribute to the $4.9 trillion spent on US health care costs annually. The current life expectancy for adults in the United States is 78.4 years (CDC, 2024a, 2024b, 2024c).
As the population lives longer with complex chronic conditions, more advanced medical treatments will be necessary. More than 5 million patients are admitted to ICUs in the United States annually for intensive or invasive monitoring, including airway, breathing, or circulation support; stabilization of acute or life-threatening medical problems; and comprehensive management of an injury or illness. Although the ICU patient population is heterogeneous, the most common indications for admission include cardiac, respiratory, and neurologic conditions. Respiratory failure with ventilator support is among the top five reasons for ICU admissions for adults. In addition, the most common technologic support required in an ICU is MV, accounting for 20% to 40% of admissions in the United States. Although current data is sparse, annual critical care costs increased by 92% between 2000 ($56 billion) and 2010 ($108 billion), and daily ICU costs increased by 61% during that same span ($4,300 in 2010). Health care expenditures are expected to rise 5.4% annually from 2022 to 2031 (Society of Critical Care Medicine [SCCM], 2024).
Acute Respiratory Distress Syndrome
A distinctive type of acute hypoxemic respiratory failure was first recognized in the 1960s. In 1967, Ashbaugh and colleagues presented a case series of 12 patients who developed respiratory failure after various clinical insults. These patients presented with hypoxemia and decreased lung compliance but did not respond to traditional respiratory treatment. Instead, these patients often required MV with positive end-expiratory pressure (PEEP) to combat atelectasis and hypoxemia. ARDS was initially named adult respiratory distress syndrome until it was discovered that it could also occur in children. ARDS also replaced other names, including shock lung, wet lung, acute lung injury (ALI), noncardiac pulmonary edema, and stiff lung. In 1994, during an American-European Consensus Conference, it was established that ALI and ARDS would be distinguished based on the severity of the patient’s hypoxemia, with ARDS representing a higher level of hypoxemia. Hypoxemia would be evaluated using the ratio of partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2). This definition was essential in establishing the diagnosis, guiding treatment options, prevention, and continued research. Referred to as the Berlin definition, it was most recently updated in 2024 and categorizes patients based on severity. Mild, moderate, and severe ARDS are defined by specific parameters utilized in the diagnostic process. The Berlin definition of ARDS is the most widely accepted; it was initially formulated by the European Society of Intensive Care Medicine (ESICM) and endorsed by the American Thoracic Society (ATS) and the SCCM (Banavasi et al., 2021; Ignatavicius et al., 2023; Siegel, 2025).
The estimated incidence of ARDS ranges from 62.4 to 78.9 cases per 100,000 person-years in the United States. It is estimated that ARDS affects nearly 3 million people globally each year, with some variation based on geographical location. Within the United States, ARDS affects approximately 200,000 people annually and results in 74,500 deaths. Approximately 25% of ARDS cases are initially classified as mild, while 75% are moderate or severe. However, as many as 33% of mild cases will progress to moderate or severe. In a multicenter, international study of 30,000 ICUs, the researchers found that nearly 10% of ICU admissions and 23% of patients requiring MV met the criteria for ARDS....
...purchase below to continue the course
In 2019, an outbreak of COVID-19 caused significant morbidity and mortality related to severe respiratory distress, similar to ARDS. Many patients diagnosed with COVID-19 require ICU care due to the rapid progression of respiratory symptoms; older adults and those with comorbidities are at higher risk of death. Although significant research has taken place over the last few years, there are still many unknowns about COVID-19. More specifically, researchers are finding that although COVID-19–related ARDS has similar features to typical ARDS, there are differences in the factors that cause the condition and the treatment strategies for COVID-19 ARDS. Studies conducted during the pandemic found that ARDS develops in 42% of patients presenting with COVID-19 pneumonia, and 61% to 81% of patients are admitted to an ICU. Although the development of severe disease from COVID-19 has decreased, COVID-19 ARDS appears to have poorer outcomes than typical ARDS. For typical ARDS, there is a 40% mortality rate for patients admitted to the ICU compared to 65.7% to 94% for COVID-19 ARDS patients admitted to the ICU (Beloncle, 2023; Gibson et al., 2020; Li & Ma, 2020).
HCPs caring for patients with ARDS and those requiring MV must understand the following concepts (Hickey et al., 2024; Hinkle et al., 2021; Patel, 2025b; Respiratory Therapy Zone, 2025):
- Ventilation is the movement and exchange of gases (oxygen [O2] and carbon dioxide [CO2]) between the lungs and the atmosphere. A ventilator forces O2 into the lungs while CO2 is removed from the body during exhalation. During MV, the CO2 level in the blood can be modified by changing the tidal volume (TV) or respiratory rate (RR).
- Oxygenation of mechanically ventilated patients, which boosts the O2 supply to the lungs, can be achieved by increasing the FiO2 or the PEEP.
- TV is the volume of air moved in and out of the lungs with each respiratory cycle.
- PEEP refers to the positive pressure applied by the ventilator at the end of each respiratory cycle. In mechanically ventilated patients, the pressure will remain greater than the atmospheric pressure to prevent the alveoli from collapsing.
- FiO2 is the percentage of O2 in the air mixture that the ventilator delivers to a patient.
- Flow refers to the speed at which the ventilator delivers breaths, measured in liters per minute (LPM).
- Compliance is the elasticity and expandability of the lungs and chest wall, determined by the change in volume divided by the change in pressure.
- Hypoxia refers to the lack of O2 as evidenced by a decrease in PaO2.
- Hypercarbia refers to an elevation in CO2 as evidenced by an increase in the partial pressure of carbon dioxide (PaCO2).
Pathophysiology
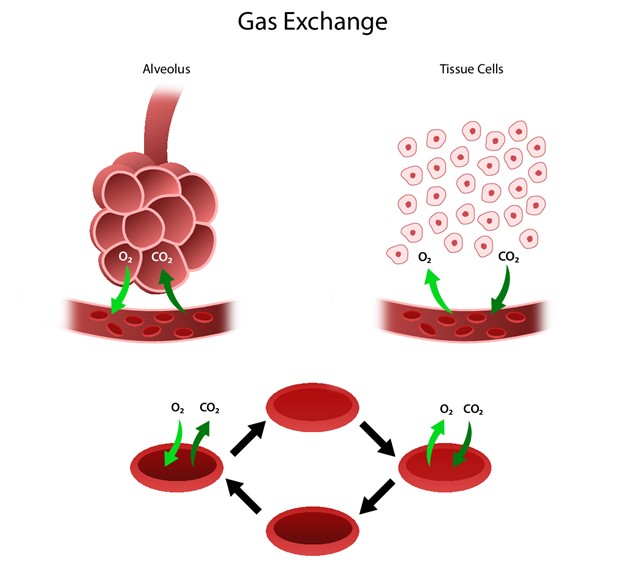
The respiratory system facilitates life-sustaining processes, including O2 transport, respiration, ventilation, and gas exchange. Human cells rely on the oxidation of carbohydrates, fats, and proteins to produce energy. Without a continuous supply of O2, cells in the brain, heart, and other essential organs cannot survive. O2 is transported to body tissues, and CO2 is removed from those tissues, by the circulating blood through the thin walls of the capillaries. O2 diffuses through capillary walls to the interstitial fluid and eventually to the cells, while CO2 diffuses in the opposite direction (from the cells to the blood). After these tissue capillary exchanges, blood enters the systemic venous circulation and travels to the pulmonary circulation. The O2 concentration in the alveoli is higher than the concentration in the blood; therefore, O2 diffuses from the alveoli into the blood. Similarly, CO2 has a higher concentration in the blood than in the alveoli, so it diffuses from the blood into the alveoli (refer to Figure 1; Hinkle et al., 2021).
Figure 1
Human Gas Exchange
Ventilation
Ventilation requires movement of the walls of the thoracic cage and the diaphragm—the movements of the thoracic cage and diaphragm alternate by increasing and decreasing the chest’s capacity. When the chest capacity increases, air enters the trachea, passes through the bronchi and bronchioles, and inflates the alveoli in the lungs (inspiration). As the thoracic cavity expands, the pressure inside the thorax is lower than the atmospheric pressure; air flows from a region of higher pressure to one of lower pressure. During expiration, the diaphragm relaxes and the lungs recoil, decreasing the size of the thoracic cavity and increasing the pressure in the alveoli, causing air to flow from the lungs to the atmosphere. The inspiratory phase of respiration requires active energy, while the expiratory phase is passive (Brinkman & Sharma, 2023; Hinkle et al., 2021; Powers & Dhamoon, 2023).
In addition to air pressure variances, airway resistance, lung compliance, and lung volumes also impact ventilation. The size or diameter of the airway, lung volumes, and airflow velocity determine airway resistance. Any process that changes the diameter of the airway will affect airway resistance and airflow rate. With increased airway resistance, a more significant respiratory effort will be required to achieve normal ventilation. Compliance allows the thoracic cavity to expand (change in volume) based on air pressure variances. Alveolar surface tension and the amount of connective tissue and water in the lungs determine lung compliance. Standard compliance allows the lungs and thorax to stretch easily when pressure is applied. Increased compliance occurs when the lungs have lost their elastic recoil and become permanently overextended (e.g., COPD). Decreased compliance occurs when the lungs become stiff, requiring increased energy expenditure by the patient to achieve normal ventilation levels. ARDS, pulmonary edema, pleural effusion, body mass index (BMI) above 40 kg/m2, pneumothorax, and pulmonary fibrosis can result in decreased lung compliance (Brinkman & Sharma, 2023; Hinkle et al., 2021; Powers & Dhamoon, 2023).
Perfusion
Gas exchange depends on pulmonary diffusion and perfusion. Pulmonary diffusion is how O2 and CO2 are exchanged in the body due to the differences in gas concentrations in the alveoli and capillaries. The alveolar–capillary membrane is ideal for diffusion due to its large, thin surface area, allowing for gas exchange from areas of high concentration to those of low concentration. Pulmonary perfusion refers to the blood flow through the pulmonary vasculature and is determined by pulmonary artery pressure, gravity, and alveolar pressure. Blood is pumped into the lungs by the right ventricle through the pulmonary artery. The pulmonary artery supplies both lungs by dividing into the right and left branches. The normal range for systolic blood pressure in the pulmonary artery is about 20 to 30 mm Hg, and the diastolic pressure is 5 to 15 mm Hg, making pulmonary circulation a low-pressure system. Due to these lower pressures, the pulmonary vasculature can accommodate varying amounts of blood flow. Because pulmonary capillaries lie between adjacent alveoli, alveolar pressure also influences perfusion. If the alveolar pressure increases, the capillaries are squeezed, impeding perfusion (Hinkle et al., 2021; Powers & Dhamoon, 2023).
Adequate gas exchange depends on effective ventilation and perfusion, resulting in an adequate ventilation–perfusion (V/Q) ratio. Four V/Q states can occur in the lungs: normal V/Q ratio, low V/Q ratio, high V/Q ratio, and absence of ventilation and perfusion. In a healthy lung, the V/Q ratio is 1:1, indicating equal amounts of blood and gas moving through the alveoli. Low V/Q ratios (shunt) happen when perfusion exceeds ventilation. When blood bypasses the alveoli without gas exchange, shunting results in hypoxia. Low V/Q ratios can occur with obstruction of the distal airways (e.g., tumor, atelectasis, pneumonia, or mucus plug). High V/Q ratios (dead space) develop when ventilation exceeds perfusion, resulting in an inadequate blood supply for gas exchange in the alveoli (e.g., pulmonary emboli [PE], pulmonary infarction, cardiogenic shock). A silent unit occurs in the absence of ventilation and perfusion caused by blockages (e.g., pneumothorax, ARDS; Hinkle et al., 2021; Powers & Dhamoon, 2023).
Neurologic Control of Ventilation
Ventilation control depends on the neuronal network in the brainstem, which controls the activities of the motor neurons that innervate the respiratory muscles. The inspiratory and expiratory centers in the medulla oblongata and pons control the rate and depth of ventilation. The pneumotaxic center in the upper pons controls the respiration pattern, while the apneustic center in the lower pons stimulates the inspiratory medullary center to promote deep inspiration. In addition, various groups of receptor sites in the brain assist with respiratory control. Central chemoreceptors within the medulla respond to chemical changes in cerebrospinal fluid (CSF). An increase or decrease in pH triggers these receptors, resulting in a change in the rate and depth of respiration. Peripheral chemoreceptors from the aorta to the arch and in the carotid arteries respond to changes in PaO2, followed by changes in PaCO2 and pH. Within the lungs, various receptors—including stretch, irritant, and juxtacapillary mechanoreceptors—respond to changes in resistance by altering breathing patterns. Proprioceptors in the muscles and chest wall react to body movements such as range of motion (ROM) exercises, stimulating an increase in ventilation. Finally, baroreceptors in the aorta and carotid bodies respond to changes in arterial blood pressure, causing reflex hypoventilation or hyperventilation (Hinkle et al., 2021).
Pathophysiology of Acute Respiratory Distress Syndrome
Healthy lung function requires dry, patent alveoli that are closely situated by well-perfused capillaries. The normal pulmonary capillary endothelium is selectively permeable, allowing a small amount of fluid into the interstitium. Hydrostatic and oncotic forces control fluid movement while preventing alveolar edema. ARDS involves the activation and dysregulation of multiple overlapping and interacting injury response pathways, coagulation, and inflammation in the lungs and systemically. These pathways are critical to the body’s normal response to injury or infection, but excessive or diffuse activation is harmful. The degree to which the specific pathways are involved and the degree of lung versus systemic involvement can vary, making ARDS complex and heterogeneous (Diamond et al., 2024; Patel, 2025a; Siegel, 2025).
ARDS progresses through different phases. The exudative phase is characterized by alveolar-capillary damage. During this early phase of ARDS, pulmonary or systemic inflammation causes the release of cytokines and other proinflammatory molecules. The cytokines activate alveolar macrophages and recruit neutrophils to the lungs. This process releases oxidants, leukotrienes, platelet-activating factors, and proteases, damaging the capillary and alveolar endothelium and disrupting the barrier between the capillaries and the airspaces. The barrier disruption allows fluid, protein, and cellular debris to flood the airspace and interstitium, leading to disruption of surfactant, airspace collapse, V/Q mismatch, pulmonary hypertension, and shunting. When the airspaces are flooded or collapsed, no inspired gas can enter, leaving blood with a mixed venous O2 content to perfuse the alveoli regardless of how high the FiO2 is. These changes lead to impaired gas exchange, decreased lung compliance, and pulmonary hypertension. For patients with COVID-19, research has shown that injury to the alveolar epithelial cells predominates and that endothelial cells are less damaged, causing less exudate. The second phase of ARDS (the proliferative phase) is characterized by improved lung function and healing. Finally, the fibrotic phase indicates the end of the acute disease process (Diamond et al., 2024; Li & Ma, 2020; Patel, 2025a; Siegel, 2025).
Risk Factors for ARDS
Many factors may increase the risk of ARDS, including infectious and noninfectious triggers. These triggers can cause injury to the lung directly due to local inflammation or indirectly due to systemic inflammation and injury mediators. More than 60 possible causes of ARDS have been identified, and other causes will likely emerge over time. Sepsis (i.e., pulmonary and nonpulmonary) is the most common cause of ARDS, with pulmonary sepsis (i.e., pneumonia) causing about 60% of ARDS cases. Among the noninfectious triggers, the more common causes of ARDS include aspiration of gastric contents, pancreatitis, and severe traumatic injuries that result in hypovolemic shock or multiple blood transfusions. However, with advancements in MV, transfusion strategies, and the use of crystalloids for fluid resuscitation, the number of ARDS cases resulting from a traumatic injury has decreased. Alcohol use, cigarette smoking, and exposure to air pollutants are common indirect factors that increase the risk of ARDS. Some genetic components have also been shown to minimally increase the risk of ARDS, specifically the haptoglobin variant Hp-2 gene, which is more common in European ancestry. More recently, there has been an increase in ALIs related to e-cigarettes, vaping, and drug-induced ARDS (i.e., chemotherapeutic agents and immunotherapies; Bos & Ware, 2022; National Heart, Lung, and Blood Institute [NHLBI], 2022; Patel, 2025a; Siegel, 2025). Less common causes of direct and indirect lung injuries can be found in Table 1.
Table 1
Less Common Direct and Indirect Causes of ALIs
Direct causes | Indirect causes |
|
|
(Bos & Ware, 2022; NHLBI, 2022; Patel, 2025a; Siegel, 2025)
The lung injury prediction score (LIPS) can identify patients at low risk for developing ARDS, but may not be as accurate in identifying high-risk patients. The LIPS is a sum of points assigned for various predisposing conditions for ARDS, with a score less than four indicating low risk and a score of greater than four indicating a high risk. In a prospective cohort study of 5,584 patients, the LIPS had a negative predictive value (i.e., the percent of patients with a LIPS score of less than four that developed ARDS) of 97%. However, for scores greater than four, the LIPS had a sensitivity of 68% and a specificity of 78%. More recent studies have found that a high LIPS score, combined with a high C-reactive protein (CRP) level, may increase the accuracy of predicting patients at high risk for ARDS (Ahmed et al., 2020; Siegel, 2025). Clinicians can use the LIPS to determine the risk for ARDS. The LIPS criteria can be found in Table 2.
Table 2
Lung Injury Prediction Score Criteria
Criteria | Points |
Shock | 2 points |
Aspiration | 2 points |
Sepsis | 1 point |
Pneumonia | 1.5 points |
Orthopedic spine surgery | 1.5 points |
Acute abdominal surgery | 2 points |
Cardiac surgery | 2.5 points |
Aortic vascular surgery | 3.5 points |
Traumatic brain injury | 2 points |
Smoke inhalation | 2 points |
Near drowning | 2 points |
Lung contusion | 1.5 points |
Multiple fractures | 1.5 points |
Alcohol abuse | 1 point |
BMI greater than 30 kg/m2 | 1 point |
Hypoalbuminemia | 1 point |
Chemotherapy | 1 point |
FiO2 greater than 0.35 or greater than 4 LPM | 2 points |
Tachypnea (greater than 30 breaths/min) | 1.5 points |
Oxyhemoglobin saturation less than 95% | 1 point |
Acidosis (pH less than 7.35) | 1.5 points |
Diabetes mellitus | 1 point |
(Gajic, n.d.; Siegel, 2025)
Clinical Manifestations
Patients with ARDS can present with clinical manifestations of ARDS in addition to features associated with the inciting event. However, based on the numerous potential inciting events, manifestations of ARDS can often be nonspecific, making diagnosis challenging. HCPs should be aware of various clinical findings associated with ARDS since prompt recognition and treatment are critical to reducing morbidity and mortality. The characteristic features of ARDS include dyspnea and hypoxemia that progressively worsen within 6 to 72 hours of the inciting event. Symptoms can quickly progress from mild shortness of breath to severe respiratory distress within 12 to 24 hours, often requiring MV to prevent hypoxia. Patients may also report restlessness, anxiety, increased respiratory or heart rate, coughing, and fatigue. Physical exam findings may include acute confusion, cyanosis, diaphoresis, tachypnea, tachycardia, and diffuse crackles. Patients often maintain low O2 saturation levels despite 100% O2 administration (Diamond et al., 2024; Patel, 2025a; Siegel, 2024).
In many cases, the underlying cause will be easily identified as pneumonia or sepsis. Clinical features associated with pneumonia or sepsis may include fever, hypotension, leukocytosis, lobar consolidation, and lactic acidosis. However, in other cases, the causative factor may be more challenging to determine. HCPs should ask about recent trauma and potential recent exposures, such as smoke or other toxic inhalation, sick contacts, and environmental exposures. In addition, HCPs should also ask about a history of asthma, cancer, cardiac dysfunction, pulmonary fibrosis, stem cell transplant, recent surgeries, smoking history, substance use, or recent changes in prescription medications (Diamond et al., 2024; Patel, 2025a; Siegel, 2024).
Evaluation and Diagnosis
ARDS can be challenging to diagnose and often masquerades as another disorder or disease initially, making prompt treatment difficult. HCPs should start with a medical history, focusing on previous respiratory conditions, heart disease, recent infections, recent travel, and other relevant information. The HCP should complete a thorough physical assessment, looking carefully for any signs of change in fluid status or inflammatory response. The abdomen should include tenderness, distention, or absent bowel sounds. A thorough skin assessment should also be completed, checking for rashes, wounds, burns, or track marks (Diamond et al., 2024; Siegel, 2024).
Laboratory and Diagnostic Testing
Laboratory testing for patients with suspected ARDS should include a complete blood count (CBC) with a differential, comprehensive metabolic profile (CMP), serum magnesium, calcium, and phosphorus levels, liver function tests, coagulation studies, and arterial blood gas (ABG) analysis. Other laboratory tests that may be indicated to evaluate for common etiologies include troponin, CRP, D-dimer, lactate levels, brain natriuretic peptide (BNP; for cardiogenic pulmonary edema), and lipase (for pancreatitis; Diamond et al., 2024; Siegel, 2024).
In addition to laboratory testing, all patients with suspected ARDS should have a chest radiograph. Abnormal imaging is critical for diagnosing ARDS and evaluating various etiologies (e.g., pneumonia). Although not necessary to diagnose ARDS, a computed tomography (CT) of the chest may help identify pneumothorax, pleural effusion, barotrauma, or mediastinal lymphadenopathy. An electrocardiogram (ECG) should be performed to evaluate for cardiac dysfunction, such as arrhythmias or ischemia. A sputum or endotracheal (ET) aspirate should be collected and sent for gram stain and culture (Diamond et al., 2024; Siegel, 2024).
Additional testing may be required when acute cardiogenic pulmonary edema is suspected, since it can mimic ARDS. Most HCPs will use the combination of clinical assessment and BNP or N-terminal proBNP (NT-proBNP), with or without transthoracic echocardiography (TEE), to confirm or exclude acute cardiogenic pulmonary edema. Since acute cardiogenic pulmonary edema is usually due to left ventricular systolic or diastolic dysfunction, clinical features can include crackles, murmurs, S3/S4 gallops, elevated jugular venous pressure, and lower extremity edema. A BNP or NT-proBNP can be obtained, but this test alone is not a reliable indicator for distinguishing ARDS from acute cardiogenic pulmonary edema. A BNP level of less than 100 pg/mL may favor ARDS, but a level greater than 100 pg/mL neither confirms acute cardiogenic pulmonary edema nor excludes ARDS. TEE may be performed when a diagnosis of acute cardiogenic pulmonary edema cannot be excluded by clinical evaluation, laboratory findings, or imaging but is not routinely performed in patients with low suspicion of acute cardiogenic pulmonary edema. In rare cases, when the diagnosis and etiology of ARDS remain unclear, additional testing may be performed, including an echocardiogram, heart catheterization, or bronchoscopy (Diamond et al., 2024; Siegel, 2024).
Diagnosis
For most patients, ARDS is a clinical diagnosis, as histopathology (i.e., diffuse alveolar damage) obtained through a lung biopsy is rarely performed. The Berlin Definition of ARDS is the gold standard for diagnosis. It should be used once acute cardiogenic pulmonary edema and other causes of hypoxemic respiratory failure and bilateral lung infiltrates are excluded. In 2024, a global definition of ARDS was proposed that expanded on the previous Berlin Definition (Diamond et al., 2024; Gorman et al., 2022; Matthay et al., 2024; Siegel, 2024). The Berlin Definition requires that all the following criteria be present for a diagnosis of ARDS (Matthay et al., 2024; Siegel, 2024):
- acute onset: respiratory symptoms must have begun within one week of a known clinical insult or new or worsening symptoms in the past week; in the new global definition, the clinical onset may be slower and present before intubation in some cases
- bilateral infiltrates on chest radiography or CT scan of noncardiac origin; the new global definition also includes diagnosis by ultrasonography performed by a trained operator; opacities must not be fully explained by lobar collapse, pleural effusions, pulmonary nodules, or lung collapse
- respiratory failure must not be fully explained by fluid overload or cardiac failure: an echocardiogram to exclude hydrostatic pulmonary edema is required if there are no risk factors for ARDS
- a moderate to severe impairment of oxygenation must be present: defined by the ratio of PaO2 to FiO2; the new global definition also includes the ratio of peripheral oxyhemoglobin saturation (SpO2) to FiO2as an alternate assessment of oxygenation; the severity of hypoxemia is used to define the severity of ARDS, and the new global definition includes diagnosis in nonintubated patients:
- Intubated patients:
- mild ARDS: PaO2/FiO2 is between 200 and 300 mm Hg on ventilator settings that include PEEP or continuous positive airway pressure (CPAP) 5 cm H2O or greater; alternatively, the SpO2/FiO2 is between 235 and 315 mm Hg (if the SpO2 is below 98%)
- moderate ARDS: PaO2/FiO2 is between 100 and 200 mm Hg on ventilator setting with PEEP 5 cm H2O or greater; alternatively, the SpO2/FiO2 is between 148 and 235 mm Hg (if the SpO2 is below 98%)
- severe ARDS: PaO2/FiO2 is 100 mm Hg or less on ventilator settings with PEEP 5 cm H2O or greater; alternatively, SpO2/FiO2 148 mm Hg or less (Diamond et al., 2024; Gorman et al., 2022; Matthay et al., 2024; Siegel, 2024)
- Nonintubated patients:
- ARDS is defined as PaO2/FiO2 of 300 mm Hg (or SpO2/FiO2 of 315 mm Hg if the SpO2 is below 98%) or less while on humidified high-flow O2 delivered via nasal cannula (HFNC) at 30 L/minute or greater or noninvasive ventilation (i.e., CPAP at 5 cm H2O or greater end-expiratory pressure)
- Resource-limited settings:
- the new global definition provides an exception for resource-limited settings; PEEP 5 cm H2O or minimum flow rate for HFNC is not required for diagnosis
- Intubated patients:
An ABG analysis is necessary to calculate the PaO2/FiO2 for a patient with ARDS. The PaO2 is measured in mm Hg, while the FiO2 is expressed as a decimal between 0.21 and 1. For a patient with a PaO2 of 60 mm Hg while receiving 80% O2 (FiO2 0.8), the PaO2/FiO2 ratio would be 75 mm Hg (60/0.8). The ratio of oxyhemoglobin saturation measured by pulse oximetry (SpO2) to FiO2 may be an appropriate alternative if an ABG cannot be obtained. The Berlin definition does not specify criteria relating to the underlying etiology. Therefore, uncertainty remains regarding which conditions should or should not be included in the ARDS diagnosis. In general, disorders that cause diffuse alveolar damage and have the potential to resolve are included (e.g., inhalation injuries or diffuse viral or bacterial pneumonia; Gorman et al., 2022; Matthay et al., 2024; Siegel, 2024).
Histopathologic Diagnosis and Staging
Patients with ARDS typically progress through three pathologic stages: early exudative, fibroproliferative, and fibrotic. Although histopathological samples are not routinely collected for the diagnosis of ARDS, they are obtained in certain circumstances and, more commonly, post-mortem. The early exudative stage occurs in the first 7 to 10 days. It is characterized by diffuse alveolar damage as evidenced by interstitial edema, type II cell hyperplasia, acute and chronic inflammation, and hyaline membrane formation. The fibroproliferative stage occurs after 7 to 10 days. It is characterized by the resolution of pulmonary edema and the proliferation of type II alveolar cells, squamous metaplasia, interstitial infiltration by myofibroblasts, and early collagen deposition. The fibroproliferative phase may vary in length, but it is estimated to be between 2 and 3 weeks. For patients who survive, the final stage is the fibrotic stage, characterized by the obliteration of normal lung architecture, fibrosis, and cyst formation. The severity of the disease determines the degree of fibrosis (Gorman et al., 2022; Siegel, 2024).
Clinical Course of ARDS
For patients with ARDS, the first few days are characterized by hypoxemia that requires moderate to high concentrations of inspired O2 and PEEP. The patient will have persistent bilateral alveolar infiltrates and diffuse crackles. For most patients, these first few days are usually the most tenuous. Mortality in patients with ARDS is not usually due to respiratory failure but rather sepsis or multiorgan failure. For patients who survive, the subsequent days are characterized by diminishing infiltrates and improved oxygenation, allowing for weaning to begin. Some patients will have persistent hypoxemia and remain ventilator-dependent, which signifies the fibroproliferative phase. These patients will have low lung compliance, high dead space, pulmonary hypertension, and persistent hypoxemia. Finally, the fibrous stage will begin for patients who survive the fibroproliferative phase. This stage is characterized by a gradual improvement in hypoxemia and pulmonary infiltrates over weeks to months. Cardiopulmonary function generally returns to baseline by 6 to 12 months (Gorman et al., 2022; Siegel, 2024).
Treatment Strategies for ARDS
ARDS can be difficult to manage, with high mortality rates that are dependent on numerous factors, including the severity of the disease, age, and comorbidities. MV and other supportive therapies remain the recommended strategies for managing ARDS. Treatment goals include increasing O2 delivery, decreasing O2 consumption, and avoiding further injury. Most patients will require invasive MV (i.e., via an ET tube or tracheostomy), particularly those with moderate or severe ARDS. Noninvasive ventilation (NIV; via a mask or nasal cannula) may be appropriate for some patients with mild or early ARDS (Diamond et al., 2024; Patel, 2025a; Siegel & Siemieniuk, 2025).
Noninvasive Ventilation
NIV should be used only for patients who are hemodynamically stable and easily oxygenated. These patients require frequent evaluation by an HCP to identify the early signs that intubation is needed. Mild ARDS can be treated with 70% to 100% O2 delivered noninvasively using a non-rebreather face mask. However, since the COVID-19 pandemic, HFNC and noninvasive positive pressure ventilation (NIPPV) use has increased. These strategies may prevent ET intubation and the complications associated with MV. However, if excessive spontaneous breathing effort is needed, there is a risk of self-inflicted lung injury. In a clinical trial that compared HFNC, face mask NIPPV, and standard O2, researchers found that HFNC was more effective at preventing intubation in patients with a PaO2/FiO2 of 200 or less on 5 cm H2O or greater PEEP. In addition, there was a higher 90-day mortality rate with standard O2 and face mask NIPPV compared to HFNC. Researchers hypothesized that the face mask NIPPV has a higher mortality rate due to excessive TV, which can worsen lung injury. NIV should be used cautiously because delayed intubation can increase mortality in moderate to severe ARDS. MV should be considered if O2 saturation falls below 90% with NIV. Since high FiO2 supplementation can increase the risk of O2 toxicity, HCPs should decrease FiO2 to 50% to 60% as soon as possible. The target PaO2 of 55 to 80 mm Hg is recommended for patients with ARDS (Patel, 2025a; Siegel & Hyzy, 2025).
Mechanical Ventilation
Most patients with ARDS will require MV to improve oxygenation and reduce O2 demand by relaxing the respiratory muscles. Normal respiratory function works as a negative-pressure system. During inspiration, the diaphragm moves downward, creating negative pressure in the pleural cavity. This negative intrathoracic pressure decreases right atrial (RA) pressure, which creates a pulling effect on the inferior vena cava (IVC), resulting in increased venous return. Conversely, MV alters normal respiratory function by pushing air into the upper airways and alveoli, creating positive pressure in the thoracic cavity. MV can significantly reduce a patient’s work of breathing (WOB). This reduction in WOB allows blood flow to be redistributed to more critical organs, limits CO2 and lactate generation, and improves acidosis. However, the positive intrathoracic pressure related to MV increases RA pressure and decreases venous return, reducing preload (i.e., the force stretching the cardiac muscle before constriction). With less blood reaching the right and left ventricles, cardiac output decreases. With decreased cardiac output and preload, mean arterial pressure (MAP) will drop if there is no compensatory increase in systemic vascular resistance (SVR; Hickey et al., 2024; King Han & Mirza, 2024; Patel, 2025b).
HCPs who manage MV patients must understand lung pressures and lung compliance. Healthy lung compliance in adults is approximately 100 mL/cm H2O. Therefore, when positive-pressure ventilation delivers 500 mL of air to a healthy lung, a 5 cm H2O increase in alveolar pressure will occur (refer to Figure 2). Various respiratory disorders can significantly impact lung compliance. For example, any respiratory disorder that destroys the lung parenchyma (e.g., COPD) can increase lung compliance. Conversely, ARDS, pneumonia, pulmonary fibrosis, and pulmonary edema can cause stiffening of the lungs, resulting in decreased lung compliance. Stiffening can increase the risk of barotrauma during MV, where small increases in volume can lead to significant increases in pressure. HCPs should understand two pressure types when managing a patient receiving MV: peak pressure and plateau pressure. Peak pressure measures airway resistance and is achieved when air is pushed into the lungs during inspiration. Plateau pressure is a static pressure achieved at the end of full inspiration, measuring alveolar pressure and lung compliance. Normal plateau pressure is below 30 cm H2O, and higher pressures increase the likelihood of barotrauma (Hickey et al., 2024; King Han & Mirza, 2024; Patel, 2025b).
Figure 2
Biology of Ventilation
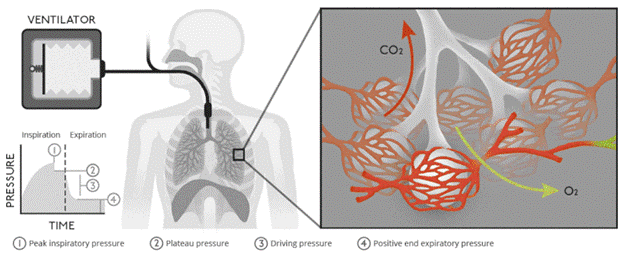
(Lutz, 2020)
The ATS, the ESICM, and the SCCM published a clinical practice guideline on using MV in adults with ARDS (Fan et al., 2017). Ventilator settings are particularly important for patients with ARDS, as evidence suggests that some strategies can exacerbate alveolar damage and perpetuate lung injury. Therefore, a low TV or lung-protective approach is recommended using a volume-limited assist control mode (AC). AC ventilation is a volume-controlled mode that sets a fixed TV that the ventilator will deliver at set intervals or when the patient initiates a breath (Diamond et al., 2024; Fan et al., 2017; Patel, 2025b; Siegel & Hyzy, 2025). The NHLBI ARDS Clinical Network Mechanical Ventilation Protocol (ARDSnet) is a low TV approach, which includes the following recommendations:
- TV from 4 to 8 mL/kg of ideal body weight
- respiratory rate up to 35 breaths per minute (bpm)
- SpO2 between 88% and 95%
- plateau pressure less than 30 cm H2O
- pH between 7.30 and 7.45
- inspiratory-to-expiratory time ratio less than 1
- PEEP should be high enough to maintain open alveoli and minimize FiO2 until a plateau pressure of 28 to 30 cm H2O is reached; PEEP is typically set at 5 cm H2O, and FiO2 to 1 and weaned soon after to target O2 saturation (Diamond et al., 2024; Fan et al., 2017; Patel, 2025b; Siegel & Hyzy, 2025)
Conventional MV strategies for ARDS focused on normalizing ABG values. However, more recent research has shown that low TV reduces mortality. Low TVs require an increased respiratory rate (up to 35 bpm) to ensure adequate alveolar ventilation and CO2 removal. These settings can increase the risk of respiratory acidosis, which is generally well tolerated when the pH remains 7.15 or above (ideally between 7.30 and 7.45). If the pH drops below 7.15, a bicarbonate infusion may be beneficial. O2 saturation levels between 88% and 95% are recommended to limit oxygen toxicity. Low TV and hypercarbia can cause dyspnea, causing ventilator dyssynchrony. Ventilator dyssynchrony can lead to increased WOB, discomfort, and poor gas exchange. HCPs should administer analgesics (e.g., fentanyl [Sublimaze] or morphine [Duramorph]) and a sedative (e.g., propofol [Diprivan]). Initial dosing for propofol (Diprivan) is 5 mcg/kg/min, titrating up to 50 mcg/kg/min. PEEP improves oxygenation in patients with ARDS by increasing the volume of aerated lung through alveolar recruitment. This allows for a decrease in FiO2 to prevent O2 toxicity. HCPs should titrate the PEEP, using the least amount necessary to ensure adequate oxygenation on a nontoxic FiO2. For most patients with ARDS, this level of PEEP will be between 8 and 15 cm H2O; however, some patients with severe ARDS may require PEEP levels greater than 20 cm H2O. These patients should be monitored closely for O2 toxicity (Diamond et al., 2024; Fan et al., 2017; Patel, 2025b; Siegel & Hyzy, 2025).
Other Supportive Measures
After the initiation of low TV ventilation, HCPs should monitor the patient’s clinical response, including vital signs, lung sounds, ABG analysis, and plateau pressure. Adjustments to the ventilator settings should be made as needed. For patients who improve with low TV ventilation, HCPs should attempt weaning as tolerated, specifically reducing the PEEP and FiO2. If the patient tolerates the initial weaning process, switching to a partial-assist or spontaneous ventilator mode can be considered. The weaning process will vary but could take as little as 24 to 48 hours or days to weeks in other individuals. HCPs should initiate medical therapies to address the underlying etiology of ARDS (Siegel & Hyzy, 2025).
Refractory Hypoxemia
Many patients respond well to low TV ventilation, while others experience refractory hypoxemia (i.e., PaO2/FiO2 greater than 150 mm Hg). Signs of refractory hypoxemia can include ventilator dyssynchrony, high plateau pressures (i.e., 30 cm H2O or greater), or worsening hypoxemia. HCPs should be aware that abrupt changes in airway pressures could indicate a pneumothorax or an obstructed ET tube. When refractory hypoxemia occurs, HCPs should target the suspected reason for the intolerance to low TV ventilation. For some patients, additional supportive therapies may be indicated, such as conservative fluid management. The HCP should also consider an alternative diagnosis, evaluate for complications of ARDS or MV (e.g., PE or ventilator-associated pneumonia [VAP]), and treat the ventilator dyssynchrony. Other options include switching ventilator modes (i.e., pressure-limited) or increasing the inspiratory to expiratory ratio (Siegel & Hyzy, 2025; Siegel & Siemieniuk, 2025). Other supportive measures for refractory hypoxemia can include (Fan et al., 2017; Patel, 2025a; Siegel & Hyzy, 2025; Siegel & Siemieniuk, 2025):
- General measures that decrease O2 consumption should be implemented to improve arterial O2 saturation. Pain, anxiety, increased WOB, and fever can all increase O2 consumption. HCPs should consider antipyretics, analgesics, sedatives, and paralytics when appropriate.
- Conservative fluid management should be considered for patients with refractory hypoxemia. ARDS can increase vascular permeability, leading to pulmonary edema. HCPs should consider fluid restrictions and/or diuresis for patients with fluid overload. This entails a delicate balance between ensuring adequate circulating volume to preserve end-organ perfusion while lowering preload and fluid in the lungs.
- Treat ventilator dyssynchrony, which can increase WOB, discomfort, poor gas exchange, and lead to prolonged mechanical ventilation (PMV). Ventilator dyssynchrony can occur in 25% of patients receiving MV, leading to an increased need for sedation and/or a neuromuscular blockade. HCPs should review ventilator settings and consider minor trigger sensitivity and inspiratory flow adjustments.
- Prone positioning ventilation involves ventilating the patient with low TV ventilation while in the prone position. This strategy can improve oxygenation in patients with moderate to severe ARDS by allowing the recruitment of nonventilated lung regions and decreasing the risk of ventilator-induced lung injury (VILI). The clinical practice guidelines for MV in ARDS patients recommend prone positioning for more than 12 hours per day for severe ARDS. Prone positioning should be initiated early (within 7 days) to maximize the success of this strategy. If patients do not respond after a short trial of prone positioning (i.e., 6 to 8 hours or up to 20 hours), they are unlikely to improve with this strategy.
- High PEEP can be used for patients with refractory hypoxemia, which may help to open collapsed alveoli, resulting in decreased alveolar overdistention. If the alveoli remain open throughout the respiratory cycle, cyclic atelectasis is reduced, decreasing the risk of VILI. Fan and colleagues (2017) recommend that adults with moderate to severe ARDS receive high PEEP when low PEEP is insufficient.
- Recruitment maneuvers are a brief application of a high level of PEEP or CPAP, which recruits non-gas-exchanging parts of the lung to become involved in gas exchange. These maneuvers can be performed independently or as part of an open lung ventilation approach (i.e., a strategy that combines low TV ventilation with recruitment and subsequent titration of applied PEEP). Fan and colleagues (2017) recommend that adult patients with ARDS receive recruitment maneuvers; however, little guidance is given regarding the method, timing, and optimal patient population. Recruitment maneuvers are more often used in patients with moderate to severe ARDS. Typical PEEP levels are 35 to 40 cm H2O for 40 seconds.
- Pharmacological therapies may be beneficial in patients with severe ARDS. Neuromuscular blockades can improve oxygenation but remain controversial because of the risk of prolonged neuromuscular weakness. The impact of glucocorticoid administration on ARDS remains uncertain. Some studies have found that early administration of glucocorticoids in moderate to severe ARDS may reduce mortality. Glucocorticoids should be considered for patients with ARDS triggered by a steroid-responsive process (e.g., acute eosinophilic pneumonia, community-acquired pneumonia, or refractory sepsis) or patients with early (within 14 days of onset) refractory hypoxemia despite standard therapies. Commonly used regimens include
- Methylprednisone (Medrol) 2 mg/kg per day for 21 to 28 days, followed by a taper
- Dexamethasone (Decadron) 20 mg IV daily for 5 days, then 10 mg daily for 5 days
Inhaled pulmonary vasodilators (e.g., nitric oxide [INOmax], prostacyclin [Flolan], prostaglandin E1 [Prostasol]) may improve oxygenation in patients with refractory hypoxemia by selectively dilating vessels that perfuse well-ventilated lung regions, improving V/Q matching, and ameliorating pulmonary hypertension. These agents act locally and have short half-lives with very few systemic effects. Studies have found that neither of these agents is superior to the other; however, nitric oxide is associated with the risk of renal impairment.
- Extracorporeal membrane oxygenation (ECMO) has been more frequently used as a rescue therapy for improving oxygenation. ECMO is a system that drains blood from a large central vein, pumping it through a gas exchange device that oxygenates the blood and removes CO2. The blood is then reinfused into a large central vein or artery. Fan and colleagues (2017) recommend that additional evidence is needed to make a definitive clinical recommendation for ECMO in severe ARDS patients. ECMO should be reserved for patients with severe ARDS who have failed prone ventilation, high PEEP, and recruitment maneuvers. Early application (i.e., within 7 days) is critical to successful outcomes with ECMO.
Complications of ARDS
Patients with ARDS are at high risk for complications related to critical illness and MV. More specifically, patients requiring MV are at high risk for complications and poor outcomes, including death. VAP, barotrauma, PE, VILI, and sepsis are ventilator-associated events (VAEs) that can affect patients receiving MV. These complications can result in PMV, prolonged ICU stays, increased health care costs, and high mortality. VAE rates for ICUs nationwide are approximately 6.8 events per 1,000 ventilator days. These rates varied substantially by ICU type, with higher rates in trauma, surgery, and neurological units and lower rates in medical and cardiac units. Although patients are at risk for VAEs until extubation occurs, the highest risk is within the first 2 weeks of MV (specifically days 3 to 7). VAEs are divided into two subcategories to distinguish VAP from other ventilator-associated complications. The overall mortality rate for VAEs is 35%, and patients developing VAEs are twice as likely to die compared to patients who did not develop VAEs (Klompas, 2019; National Healthcare Safety Network, 2025).
Prolonged Mechanical Ventilation
PMV increases the risk of pneumonia, barotrauma, tracheal injuries, musculoskeletal deconditioning, and mortality. Strategies such as early mobilization (EM) of patients on MV can help prevent PMV and facilitate the weaning process (Fadila et al., 2022). Prolonged ventilatory dependence can stem from many factors, including respiratory insufficiency, reduced ventilatory capacity, and cardiovascular insufficiency. PMV can lead to diaphragmatic weakness and atrophy, especially when accompanied by passive modes (e.g., AC) of ventilation. Other factors contributing to respiratory muscle weakness include malnutrition, immobility, excessive steroids, sedative medications, and a systemic inflammatory response (SIR) associated with sepsis. Cardiovascular insufficiency (i.e., heart failure [HF]) can also increase the risk of PMV (Fadila et al., 2022). The following risk factors can increase the risk of PMV:
- history of obstructive pulmonary disease (e.g., COPD)
- history of restrictive pulmonary disease (e.g., pulmonary fibrosis, sarcoidosis)
- neuromuscular disorders
- ICU admission due to pneumonia, ARDS, head trauma, and postoperative intracerebral hemorrhage
- abnormal arterial CO2, serum blood urea nitrogen (BUN), serum creatinine, arterial pH, white blood count (WBC), or body temperature on the first ICU day
- age 85 years and older
- extended inpatient length of stay before ICU admission (Fadila et al., 2022; King Han & Mirza, 2024)
Barotrauma
Barotrauma is an example of a VILI caused by high lung inflation pressure, which leads to high transpulmonary pressure, regional lung overdistention, and air leakage. With ventilation, positive pressure is needed to overcome airway resistance, increase airflow, and overcome lung elasticity. Plateau pressure (recommended to be below 30 cm H2O) is commonly used as a representative indicator of transpulmonary pressure, measuring lung overdistention. Careful control of inspiratory pressures is a strategy to limit lung distention and prevent barotrauma. High inspiratory pressures can cause alveolar rupture, pneumothorax, pneumomediastinum, and subcutaneous emphysema due to barotrauma or air leakage (Haribhai & Mahboobi, 2022).
Other Complications
Patients with ARDS are often critically ill and require intensive, prolonged therapies that place them at risk for additional complications. Prolonged bed rest can lead to profound muscle weakness, skin breakdown, and deep vein thrombosis (DVT). Patients with ARDS and prolonged ventilation often experience gastrointestinal bleeding from stress ulcers. Acute delirium can also occur in patients who are critically ill. Since acute delirium is multifactorial, identifying and treating the underlying cause can be challenging. Additional complications can include nosocomial infections, central line-associated bloodstream infection (CLABSI), urinary tract infections, antibiotic resistance, renal failure, and nutritional deficiencies. Patients who experience a critical illness can suffer long-term consequences, including anxiety, depression, and posttraumatic stress disorder (PTSD; Diamond et al., 2024; Siegel, 2024).
References
Ahmed, M. E., Hamed, G., Fawzy, S., & Taema, K. M. (2020). Lung injury prediction scores: Clinical validation and C-reactive protein involvement in high risk patients. Medicina Intensiva, 44(5), 267–274. https://doi.org/10.1016/j.medin.2019.02.010
Banavasi, H., Nguyen, P., Osman, H., & Soubani, A. O. (2021). Management of ARDS - What works and what does not. The American Journal of the Medical Sciences, 362(1), 13–23. https://doi.org/10.1016/j.amjms.2020.12.019
Beloncle, F. M. (2023). Is COVID-19 different from other causes of acute respiratory distress syndrome? Journal of Intensive Medicine, 3(3), 212–219. https://doi.org/10.1016/j.jointm.2023.02.003
Bos, L. D. J., & Ware, L. B. (2022). Acute respiratory distress syndrome: Causes, pathophysiology, and phenotypes. The Lancet, 400(10358), 1145–1156. https://doi.org/10.1016/S0140-6736(22)01485-4
Brinkman, J. E., & Sharma, S. (2023). Physiology, pulmonary. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK482426
Centers for Disease Control and Prevention. (2024a). About chronic diseases. National Center for Chronic Disease Prevention and Health Promotion. https://www.cdc.gov/chronic-disease/about
Centers for Disease Control and Prevention. (2024b). Deaths and mortality. https://www.cdc.gov/nchs/fastats/deaths.htm
Centers for Disease Control and Prevention. (2024c). Living with a chronic condition. https://www.cdc.gov/chronic-disease/living-with/index.html
Diamond, M., Peniston, H. L., Sanghavi, D., & Mahapatra, S. (2024). Acute respiratory distress syndrome. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK436002
Dirkes, S. M., & Kozlowski, C. (2019). Early mobility in the intensive care unit: Evidence, barriers, and future directions. Critical Care Nurse, 39(3), 33–43. https://doi.org/10.4037/ccn2019654
Fadila, M., Rajasurya, V., & Regunath, H. (2022). Ventilator weaning. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK430712
Fan, E., Del Sorbo, L., Goligher, E. C., Hodgson, C. L., Munshi, L., Walkey, A. J., Adhikari, N. K. J., Amato, M. B. P., Branson, R., Brower, R. G., Ferguson, N. D., Gajic, O., Gattinoni, L., Hess, D., Mancebo J., Meade, M. O., McAuley, D. F., Pesenti, A., Ranieri, V. M., . . . Brochard, L. J. (2017). An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine, 195(9), 1253–1263. https://doi.org/10.1164/rccm.201703-0548ST
Gajic, O. (n.d.). Lung injury prediction score (LIPS). Retrieved September 7, 2025, from https://www.mdcalc.com/calc/10281/lung-injury-prediction-score-lips
Gibson, P. G., Qin, L., & Puah, S. H. (2020). COVID-19 acute respiratory distress syndrome (ARDS): Clinical features and differences from typical pre-COVID-19 ARDS. The Medical Journal of Australia, 213(2), 54–56.e1. https://doi.org/10.5694/mja2.50674
Gorman, E. A., O'Kane, C. M., & McAuley, D. F. (2022). Acute respiratory distress syndrome in adults: Diagnosis, outcomes, long-term sequelae, and management. The Lancet, 400(10358), 1157–1170. https://doi.org/10.1016/S0140-6736(22)01439-8
Haribhai, S., & Mahboobi, S. K. (2022). Ventilator complications. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK560535
Hickey, S. M., Sankari, A., & Giwa, A. O. (2024). Mechanical ventilation. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK539742
Hinkle, J. L., Cheever, K. H., & Overbaugh, K. J. (2021). Brunner & Suddarth's textbook of medical-surgical nursing (15th ed.). Wolters Kluwer.
Hyzy, R. C., & McSparron, J. I. (2025). Overview of initiating invasive mechanical ventilation in adults in the intensive care unit. UpToDate. Retrieved September 4, 2025, from https://www.uptodate.com/contents/overview-of-initiating-invasive-mechanical-ventilation-in-adults-in-the-intensive-care-unit
Ignatavicius, D., Rebar, C., & Heimgartner, N. M. (2023). Medical-surgical nursing: Concepts for clinical judgment and collaborative care (11th ed.). Elsevier.
King Han, M., & Mirza, S. H. (2024). Management and prognosis of patients requiring prolonged mechanical ventilation in long-term acute care hospitals (LTACH). UpToDate. Retrieved September 8, 2025, from https://www.uptodate.com/contents/management-and-prognosis-of-patients-requiring-prolonged-mechanical-ventilation-in-long-term-acute-care-hospitals-ltach
Klompas, M. (2019). Ventilator-associated events: What they are and what they are not. Respiratory Care, 64(8), 953–961. https://doi.org/10.4187/respcare.07059
Li, X., & Ma, X. (2020). Acute respiratory failure in COVID-19: Is it “typical” ARDS? Critical Care, 24, 198. https://doi.org/10.1186/s13054-020-02911-9
Lutz, E. (2020). Biology of ventilation [Image]. https://commons.wikimedia.org/wiki/File:Biology_of_ventilation.gif
Matthay, M. A., Arabi, Y., Arroliga, A. C., Bernard, G., Bersten, A. D., Brochard, L. J., Calfee, C. S., Combes, A., Daniel, B. M., Ferguson, N. D., Gong, M. N., Gotts, J. E., Herridge, M. S., Laffey, J. G., Liu, K. D., Machado, F. R., Martin, T. R., McAuley, D. F., Mercat, A., . . . Wick, K. D. (2024). A new global definition of acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine, 209(1), 37–47. https://doi.org/10.1164/rccm.202303-0558ws
National Healthcare Safety Network. (2025). Ventilator-associated event (VAE). Centers for Disease Control and Prevention. https://www.cdc.gov/nhsn/pdfs/pscmanual/10-vae_final.pdf
National Heart, Lung, and Blood Institute. (2022). Acute respiratory distress syndrome: Causes and risk factors. https://www.nhlbi.nih.gov/health/ards/causes
Patel, B. K. (2025a). Acute hypoxemic respiratory failure (AHRF, ARDS). Merck Manual Professional Version. https://www.merckmanuals.com/professional/critical-care-medicine/respiratory-failure-and-mechanical-ventilation/acute-hypoxemic-respiratory-failure-ahrf,-ards
Patel, B. K. (2025b). Overview of mechanical ventilation. https://www.merckmanuals.com/professional/critical-care-medicine/respiratory-failure-and-mechanical-ventilation/overview-of-mechanical-ventilation
Powers, K. A., & Dhamoon, A. S. (2023). Physiology, pulmonary ventilation and perfusion. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK539907
Respiratory Therapy Zone. (2025). Respiratory therapy normal values: Reference guide. https://www.respiratorytherapyzone.com/respiratory-therapy-normal-values
Siegel, M. D. (2024). Acute respiratory distress syndrome: Clinical features, diagnosis, and complications in adults. UpToDate. Retrieved September 7, 2025, from https://www.uptodate.com/contents/acute-respiratory-distress-syndrome-clinical-features-diagnosis-and-complications-in-adults
Siegel, M. D. (2025). Acute respiratory distress syndrome: Epidemiology, pathophysiology, pathology, and etiology in adults. UpToDate. Retrieved September 4, 2025, from https://www.uptodate.com/contents/acute-respiratory-distress-syndrome-epidemiology-pathophysiology-pathology-and-etiology-in-adults
Siegel, M. D., & Hyzy, R. C. (2025). Acute respiratory distress syndrome: Ventilator management strategies for adults. UpToDate. Retrieved September 8, 2025, from https://www.uptodate.com/contents/acute-respiratory-distress-syndrome-ventilator-management-strategies-for-adults
Siegel, M. D., & Siemieniuk, R. (2025). Acute respiratory distress syndrome: Fluid management, pharmacotherapy, and supportive care in adults. UpToDate. Retrieved September 8, 2025, from https://www.uptodate.com/contents/acute-respiratory-distress-syndrome-fluid-management-pharmacotherapy-and-supportive-care-in-adults
Society of Critical Care Medicine. (2024). Critical care statistics. https://www.sccm.org/Communications/Critical-Care-Statistics
Powered by Froala Editor