About this course:
This learning activity aims to increase the nurse's knowledge of the disease process of hyperlipidemia, risk factors, and management of the affected individual.
Course preview
Cholesterol Management, Coaching, and Patient Education
This learning activity aims to increase the nurse's knowledge of the disease process of hyperlipidemia, risk factors, and management of the affected individual.
This learning activity is designed to allow learners to:
- explain the functions of the liver as they relate to cholesterol production and processing
- identify diagnostic tests used in cholesterol assessment
- identify modifiable and non-modifiable risk factors in hyperlipidemia
- discuss the dietary implications concerning cholesterol management
- analyze the pharmacological treatment of hyperlipidemia
- interpret the American Heart Association (AHA) guidelines for the management of hyperlipidemia
Introduction
Different terms are used to identify an abnormal level of specific lipids or fats in the blood (Ibrahim et al., 2023; Rogers & Brashers, 2023):
- Hyperlipidemia is a condition characterized by high levels of lipids, or fats, in the blood. Hyperlipidemia indicates a high level of low-density lipoprotein cholesterol (LDL-C) and triglycerides. This high level of lipoproteins in the blood causes fat deposits in the heart, liver, and muscle.
- Hypercholesterolemia is a specific subtype of hyperlipidemia. With hypercholesterolemia, an individual has high LDL-C or low high-density lipoprotein cholesterol (HDL-C). Triglyceride levels are normal with hypercholesterolemia.
The prevalence of hyperlipidemia is a concern in the United States. Approximately 86 million people over the age of 20 have a total cholesterol level above 200 mg/dL, and about 25 million (10% of adults over the age of 20) have a total cholesterol level above 240 mg/dL. Of the patients who could benefit from cholesterol treatment, only 54.5% (47 million) of individuals are currently being treated. In the United States, between 2018 and 2020, 17% of individuals over the age of 20 had an HDL-C level below 40 mg/dL. One in three adults has an elevated LDL-C level. Increased cholesterol levels do not just affect adults; 7% of children and adolescents ages 6–19 years have high total cholesterol levels in the United States. Sex and ethnic or racial background affect the prevalence of high total cholesterol levels. Among patients assigned male at birth, non-Hispanic Asian adults have the highest prevalence of total cholesterol over 240 mg/dL at 13%, followed by non-Hispanic White adults at 9.6%, Hispanic adults at 9.3%, and non-Hispanic Black adults at 6.9%. Among those assigned female at birth, non-Hispanic White adults have the highest prevalence of total cholesterol over 240 mg/dL at 10.7%, followed by Hispanic adults at 10%, non-Hispanic Black adults at 9.3%, and non-Hispanic Asian adults at 8.7%. For most individuals, hyperlipidemia is a silent disease, meaning they experience no symptoms. Therefore, many people do not know there is a problem until their cholesterol level is checked by a healthcare professional (HCP). Hyperlipidemia raises an individual's risk for heart disease, the leading cause of death in the United States, and stroke, the fifth leading cause of death in the United States (Centers for Disease Control and Prevention [CDC], 2024b; U.S. Department of Health and Human Services [HHS], 2024a).
Pathophysiology
Cholesterol is a molecule that contributes to the formation of cell membranes and is a vital component in synthesizing vitamin D and steroid hormones, including estradiol, testosterone, aldosterone, and cortisol. Cholesterol is also a component of bile. Bile facilitates the body's absorption of fat and fat-soluble vitamins (A, D, E, and K). Cholesterol also plays a role in nerve impulse conduction. The body can synthesize cholesterol and utilize dietary cholesterol absorbed in the intestines. Endogenous cholesterol is made in the liver and comprises 75% of the total cholesterol needed by the body. Exogenous cholesterol is obtained through the diet and comprises 25% of the total cholesterol. Dietary cholesterol is present in all animal-based fats. Foods with significant cholesterol include fatty meats, eggs, and full-fat dairy products, including cheese, milk, cream, and yogurt. Once dietary cholesterol is absorbed, it is transported to the liver. The liver controls the blood concentration of cholesterol. Cholesterol is controlled by a negative feedback loop in the liver: the more cholesterol is returned to the liver (via lipoproteins), the more synthesis is inhibited. When the body has too much cholesterol, the excess is secreted into the bile and then excreted through the stool. A small amount is reabsorbed and transported to the liver (Cagen, 2023; Davidson & Pradeep, 2024b).
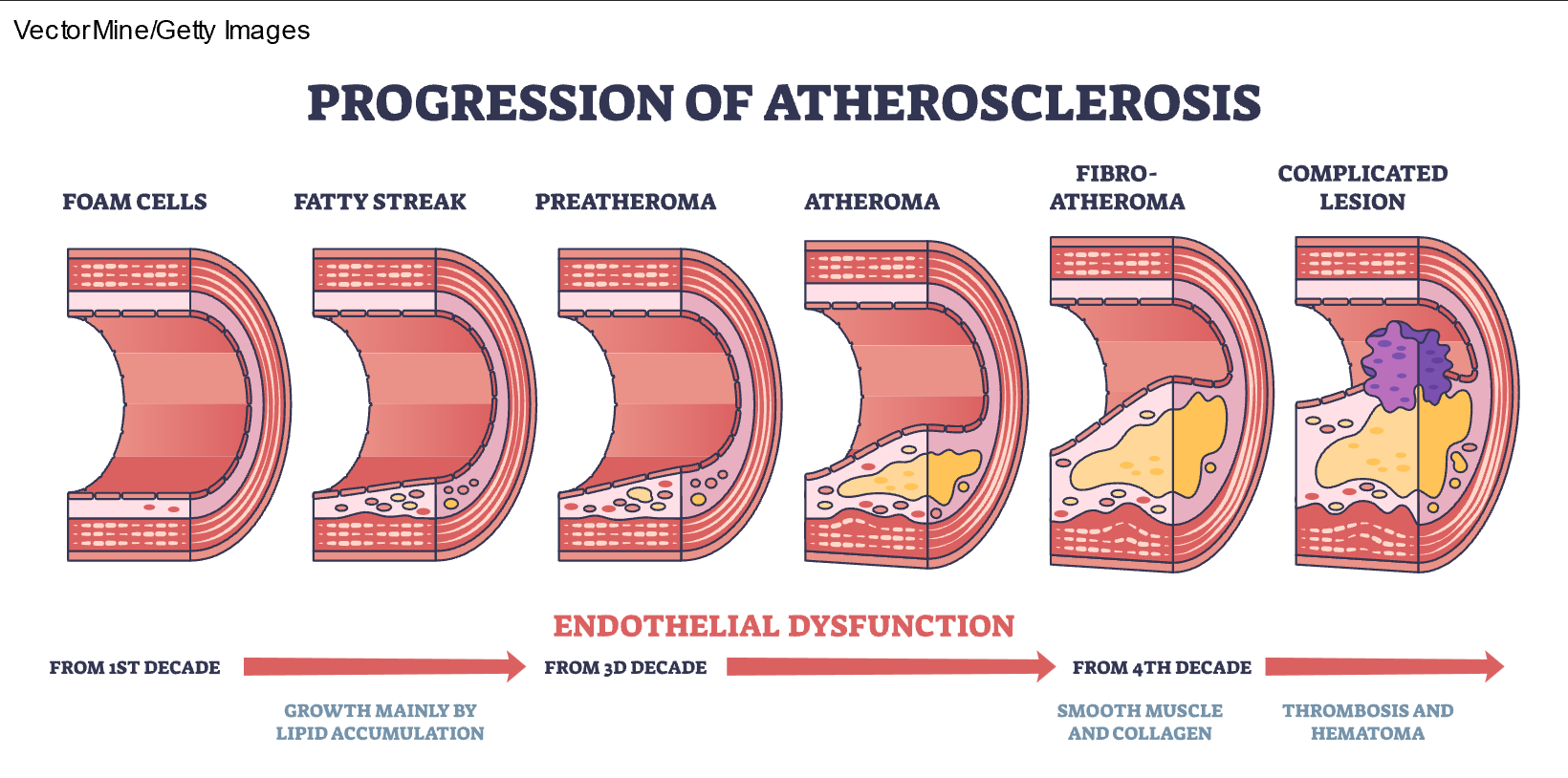
High consumption of dietary saturated fats increases LDL-C levels in the blood and can lead to hypercholesterolemia. Since blood is primarily composed of water, fat and cholesterol must bind with lipoproteins to travel through the bloodstream. LDL-C refers to lipoproteins high in cholesterol, and HDL-C contains a smaller amount of cholesterol. HDL-C is often referred to as "good" cholesterol since it helps remove other forms of cholesterol from the bloodstream and returns the excess to the liver, where it is broken down and excreted in the bile. LDL-C is known as "bad" cholesterol because it can build up along the walls of blood vessels, narrowing the vessel (refer to Figure 1). When there is more LDL-C in the body than needed, excess cholesterol accumulates in the arteries, resulting in atherosclerosis depicted in Figure 2). If a clot forms, it can become stuck in the narrow vessel, leading to myocardial infarction or stroke (Courtney, 2024; Rogers & Brashers, 2023).
Figure 1
Cholesterol
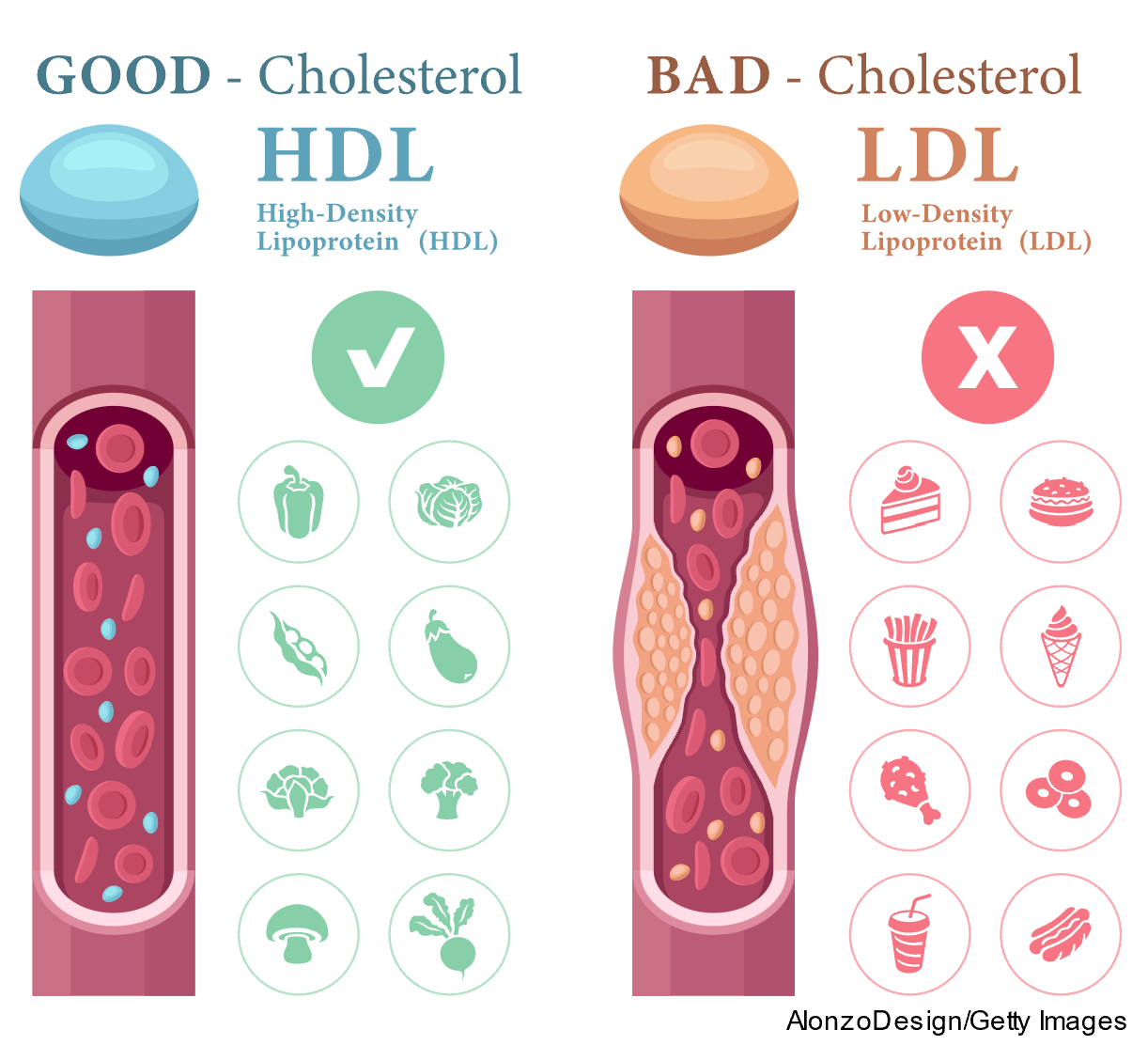
Figure 2
Progression of Atherosclerosis
...purchase below to continue the course
The 2018 Cholesterol Clinical Guidelines recommend that HCPs utilize a risk calculator to determine a patient's 10-year risk for developing heart disease. One such calculator is the Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator Plus, developed by the American College of Cardiology (ACC). This calculator estimates a patient's 10-year ASCVD risk, determines the impact of various interventions on overall risk, reassesses ASCVD risk over time during follow-up visits, and provides the HCP with resources to facilitate discussions with patients regarding ASCVD risk and risk-lowering interventions. Data required to assess risk accurately include the patient's age, sex, race, systolic and diastolic blood pressure, total cholesterol, HDL-C, and LDL-C. The risk estimator also considers whether the patient has a diagnosis of diabetes, their smoking status, and medication therapies, including hypertension treatment, use of statins, and acetylsalicylic acid (aspirin). While many modifiable and non-modifiable factors increase a patient's risk of developing hyperlipidemia and subsequently ASCVD, heart attack, or stroke, modifiable risk factors such as smoking, high blood cholesterol, diabetes, hypertension, and BMI over 30 have been found to contribute to up to 90% of coronary heart disease cases (ACC, n.d.; Wilson, 2024a, 2024b).
Smoking/Vaping
Smoking and vaping damage blood vessels and increase the risk of plaque buildup; they also reduce the amount of HDL-C in the blood, effectively lowering the amount of cholesterol carried back to the liver to be flushed from the body. High levels of HDL-C in the blood protect against heart disease. Smoking just one cigarette daily elevates the risk of coronary artery disease by 50%. The risk is even greater if the individual has other risk factors or has been diagnosed with hyperlipidemia, as smoking and vaping compound that risk (CDC, 2024c; Jackson, 2025; National Heart, Lung, and Blood Institute [NHLBI], 2024b; Majid et al., 2021). Refer to Table 1 for the benefits of smoking cessation, quickly or over time (HHS, 2024b).
Table 1
Health Benefits of Quitting Smoking Over Time
Length of time after quitting | Benefits |
Minutes | Heart rate drops. |
24 hours | Nicotine level in the blood drops to zero. |
Several days | Carbon monoxide level in the blood drops to level of someone who does not smoke. |
1 to 12 months | Coughing and shortness of breath decrease. |
1 to 2 years | Risk of heart attack drops sharply. |
3 to 6 years | Added risk of coronary heart disease drops by half. |
5 to 10 years | Added risk of cancers of the mouth, throat, and voice box drops by half. Risk of stroke decreases. |
15 years | Risk of coronary heart disease drops to close to that of someone who does not smoke. |
The adverse effects of smoking appear to be reversible upon quitting, emphasizing the role of HCPs to encourage and assist patients with cessation (Devonish et al., 2022).
Health Conditions
A patient with type 1 or type 2 diabetes mellitus (T1DM, T2DM) has an increased risk of developing hyperlipidemia. NIDDM lowers the amount of HDL-C and raises LDL-C levels in the blood. This combination elevates the risk of heart disease and stroke. Individuals with NIDDM are twice as likely to have heart disease or suffer a stroke than adults without NIDDM (CDC, 2024c; National Institute of Diabetes and Digestive and Kidney Diseases, 2021; Wilson, 2024b).
A BMI over 30 is linked to higher triglyceride and LDL-C levels and lower HDL-C levels, increasing the risk of heart disease. A patient losing 5%–10% of their total body weight can positively affect blood cholesterol levels and decrease their hyperlipidemia and heart disease risk. Even for a patient with a healthy overall weight, excessive abdominal adiposity raises the risk of heart disease. Excessive adiposity in the abdomen (central obesity) is defined as a waist measurement equal to or over 40 inches for patients assigned male at birth and equal to or over 35 inches for those assigned female at birth (Bays et al., 2024; CDC, 2024c; Wilson, 2024b).
Many other medical conditions can negatively affect cholesterol and increase a patient's risk of hyperlipidemia, heart disease, heart attack, and stroke, including:
- chronic kidney disease
- HIV/AIDS
- hypothyroidism
- polycystic ovarian syndrome
- sleep apnea
- lupus erythematosus (NHLBI, 2024a)
There are also medications used to treat unrelated conditions that can raise LDL-C or lower HDL-C, including:
- anti-arrhythmia drugs, such as amiodarone (Pacerone)
- beta-blockers, such as sotalol (Betapace)
- sodium-glucose transport protein 2 (SGLT2) inhibitors
- androgen deprivation therapy
- thiazide diuretics, such as hydrochlorothiazide (HydroDiuril)
- immunosuppressant drugs, such as cyclosporine (Neoral)
- retinoids, such as tretinoin (Retin-A)
- steroids, such as prednisone (Deltasone; Feingold, 2023; NHLBI, 2024a)
Family and Cultural History
Some patients may have a predisposition to hyperlipidemia due to family history. Familial hypercholesterolemia (FH) is the most prevalent monogenic genetic disorder with an autosomal dominant inheritance pattern. FH affects approximately 10 million people worldwide, occurring in 1 in 250 (approximately 1 million) people in the United States. Autosomal dominance means it only takes an abnormal gene from one parent for their child to be affected by the disease. The effects are much more severe when the affected gene is inherited from both parents, yielding homozygous familial hypercholesterolemia (HoFH). FH causes LDL-C and total cholesterol to be extremely high; however, triglyceride levels are unaffected. Patients with untreated HoFH can have a total cholesterol and LDL level greater than 600 mg/dL. Patients with untreated heterozygous FH have an LDL level greater than 250 mg/dL. In patients younger than 20, heterozygous FH is highly probable with an LDL level greater than 200 mg/dL. The condition is present from birth and can lead to early-onset heart disease, with many affected individuals experiencing heart attacks at an early age (CDC, 2024c; July, 2024; Rosenson & Durrington, 2025; Vaezi & Amini, 2022). Symptoms of HoFH and FH are similar but occur earlier in HoFH and include:
- tendonitis or arthralgias
- cutaneous, planar, tuberous, and tendon xanthomas
- xanthelasmas
- corneal arcus
- ischemic heart disease symptoms, aortic stenosis, peripheral vascular disease, or cerebrovascular disease (July, 2024)
Familial combined hyperlipidemia is an autosomal dominant disorder inherited by patients from their parents. Like FH, familial combined hyperlipidemia increases LDL-C and total cholesterol levels and decreases HDL-C; however, it also increases triglycerides. Familial combined hyperlipidemia affects 14% of patients with premature coronary artery disease. In the early years, patients may be asymptomatic; however, when symptoms appear, they mimic those seen with FH without xanthomas or xanthelasmas (Sweeney, 2025).
Familial hyperchylomicronemia syndrome (FCS) is an autosomal recessive disorder that affects metabolism and causes elevated triglycerides and chylomicrons. People with this rare disorder are at risk of acute pancreatitis. Experts suppose this disorder is underreported due to the vague symptomology such as xanthomas, bloating, abdominal pain, fatigue, indigestion, asthenia, joint pains, and difficulty concentrating. Hypertriglyceridemia symptoms, such as acanthosis nigricans, elevated BMI over 30, and Cushingoid features, can also be present. Diagnosis is often late and associated with an episode of pancreatitis. Mortality and morbidity are increased due to complications from cardiovascular disease and pancreatitis (Regmi & Rehman, 2023).
Often, a family history of hyperlipidemia is not related to genetics but to lifestyle or culture. Family members often share behaviors, lifestyle choices, and environments that can result in similar health concerns, including hyperlipidemia and heart disease. Culture and ethnicity also play a role in an individual's cholesterol levels. The Pacific Islander, Native Hawaiian and Asian American Cardiovascular Health Epidemiology study in 2025 found that of the 677,500 participants who self-reported as Chinese, Filipino, Native Hawaiian or other Pacific Islander, Japanese, Korean, Vietnamese, or other Southeast Asian, all had much higher rates of cholesterol than non-Hispanic White adults (AHA, 2025a, 2025b; CDC, 2024c; Tangney & Rosenson, 2024a).
Age and Gender
Hyperlipidemia can affect patients of all ages; however, it is more common in individuals over 45 due to an age-related decline in liver function. As the liver becomes less efficient, higher LDL-C levels are not effectively removed from the blood. This eventually leads to increased cholesterol levels, dyslipidemia, and an increased risk of heart disease, heart attack, and stroke. Until age 55 (or until menopause), those assigned female at birth tend to have lower LDL-C levels and higher HDL-C levels than those assigned male due to the cardiovascular-protective effects of estrogen (CDC, 2024c; Sweeney, 2025).
Physical Activity
A sedentary lifestyle leads to decreased HDL-C. When there is less HDL-C in the blood, LDL-C is less likely to be efficiently removed and returned to the liver. Not engaging in regular physical activity can also lead to weight gain and hyperlipidemia (Grundy et al., 2019). It is recommended that patients engage in the following amounts and types of physical activity to prevent hyperlipidemia (CDC, 2024a):
- Adults should get at least 150 minutes of moderate aerobic activity, 75 minutes of vigorous aerobic activity, or a combination of moderate and vigorous activity weekly. Moderate aerobic exercises include brisk walking, biking, swimming, and mowing. Vigorous aerobic exercise includes activities such as running, heavy yard work, and aerobic dancing.
- Adults are advised to complete strength training exercises for all major muscle groups at least twice weekly. Examples include lifting free weights, using weight machines or resistance bands, and rock climbing. The goal is to do a single set of exercises targeting each muscle group with enough weight or resistance to cause muscle fatigue after 12 to 15 repetitions.
- Exercise should occur throughout the week. If the goal is to lose weight, moderate aerobic activity must be increased to 300 minutes or more a week to offset the caloric intake. All exercise programs and changes to physical activity levels should occur under the direction of an HCP to minimize the risk of injury or adverse effects, such as a heart attack.
Diet
The top five most effective and well-studied diets to reduce cholesterol include the Mediterranean diet, the DASH diet, the Therapeutic Lifestyle Changes (TLC) diet, a vegetarian diet, and a vegan diet (NHLBI, n.d.; Tangney & Rosenson, 2024a). For more information, consult the NursingCE course Diets Decoded.
The TLC diet was created by the National Institute of Health's National Cholesterol Education Program to reduce cholesterol through dietary modifications. The recommendations of this diet include eating healthier fats, limiting foods high in cholesterol, increasing fiber intake, limiting salt and alcohol intake, and increasing the intake of fruits, vegetables, and fish high in omega-3 fatty acids. If the only goal of starting the TLC diet is lowering LDL-C, men are advised to limit their intake to 2,500 calories per day, and women are encouraged to limit their intake to 2,000 calories per day. If LDL-C levels have not decreased after following the diet for six weeks, it is recommended that 2 grams of plant stanols and sterols and 10–25 grams of soluble fiber daily are added to the diet (NHLBI, n.d.).
Some general dietary guidelines for a heart-healthy diet include the following (MedlinePlus, 2024; NHLBI, n.d.; Prokopidis et al., 2025; Tangney & Rosenson, 2024a; US Department of Agriculture, 2020):
- Reducing saturated fats can reduce blood levels of LDL-C. Saturated fats raise LDL-C levels more than any other dietary factor. Less than 20% to 35% of the total daily calories consumed should come from dietary fats, with less than 7% from saturated fat. Saturated fats appear in most meats, dairy products, chocolate, baked goods, and deep-fried and processed foods. Refer to Table 2 for the recommended maximum amount of fat per daily caloric intake.
- Trans fats or partially hydrogenated vegetable oil, as it is commonly referred to on food labels, should be eliminated entirely from the diet. Trans fats can raise LDL-C and lower HDL-C in the blood. Trans fats are commonly found in foods made with hydrogenated oils and fats, including margarine, crackers, store-bought cookies and cakes, and french fries. The US Food and Drug Administration (FDA; 2023) mandated that all food products be free of partially hydrogenated vegetable oils by 2021; however, extensions were granted, and the final ban went into effect on December 22, 2023.
- Cholesterol should be limited to under 200 mg per day. Foods high in cholesterol include organ meats, egg yolks, shrimp, and whole-milk dairy products.
- Eating foods rich in omega-3 fatty acids has heart-healthy benefits. Although omega-3 fatty acids do not directly affect LDL-C levels, they can increase HDL-C levels. They also decrease blood pressure and reduce the risk of blood clots, inflammation, and heart attack. Omega-3 fatty acids are found in salmon, mackerel, tuna, walnuts, and flaxseeds. According to several recommendations, fish should be incorporated into the diet at least twice weekly.
- Sodium intake should be limited to 2,300 mg daily. This includes all sodium, including what is present in foods and added during cooking or at the table. Research has shown that sodium increases the risk of heart disease. The top sodium sources in the American diet come from rolls and breads, pizza, cold cuts and cured meats, sandwiches, soups, chips and crackers, burritos and tacos, chicken, cheese, and eggs and omelets.
- Increasing soluble fiber can reduce cholesterol absorption through the digestive tract into the bloodstream. Soluble fiber is found in oatmeal, kidney beans, lentils, chickpeas, black-eyed peas, lima beans, brussels sprouts, apples, bananas, and oranges.
- Adding whey protein as a dietary supplement lowers LDL-C, total cholesterol, and blood pressure. Whey protein is found in dairy products and may account for the benefits of consuming dairy.
- Alcohol should be consumed in moderation. Alcohol intake increases daily caloric totals and may lead to weight gain. Excessive alcohol intake can also increase blood pressure and triglyceride levels, elevating the risk of developing heart disease. Moderate alcohol intake can increase HDL-C levels; however, the evidence is not strong enough to encourage anyone to incorporate alcohol into their diet that does not already do so. Patients assigned female at birth should limit alcohol intake to no more than a single alcoholic drink per day, and those assigned male should limit alcohol intake to no more than two alcoholic drinks per day. One drink is considered a 5 oz glass of wine, a 12 oz beer, or 1.5 oz of 40% distilled spirits.
- Increasing the intake of fruits and vegetables can increase cholesterol-lowering compounds known as plant stanols or sterols, which are discussed later. Sterols and stanols decrease cholesterol levels by affecting absorption in the digestive tract like soluble fiber. Three to five servings of vegetables and two to four servings of fruit are recommended daily.
Table 2
Maximum Amounts of Fats Per Day
Calories per Day | Total Fat | Saturated Fat |
1,500 | 33–58 grams | 10 grams |
2,000 | 44–78 grams | 13 grams |
2,500 | 56–97 grams | 17 grams |
(MedlinePlus, 2024)
Stress
Stress has physiological effects, causing veins to rupture and serum cholesterol levels to rise. Stress is related to the production of higher levels of LDL-C. Research has consistently demonstrated a negative effect of stress on cholesterol levels. A cross-sectional study in 2018–2019 of three hospitals, including 1,176 healthcare workers, revealed that occupational stress has a significant association with dyslipidemia. Prolonged chronic periods of anxiety and stress lead to cell damage and increased cholesterol production (AHA, 2023; Anni et al., 2021; NHLBI, n.d.; Zhang et al., 2021).
Sleep
Research has shown that both too much and too little sleep can negatively impact cholesterol levels. Sleep duration is closely associated with serum lipid and lipoprotein levels. A 2020 systematic review and meta-analysis of 13 studies, including 83,037 participants, showed that a long duration of sleep is connected with elevated total cholesterol levels. Other research studies have shown that snoring is associated with lower HDL-C. This could be due to other factors like elevated BMI and stress, as it is difficult to separate all possible confounders. A 2022 cross-sectional study of 41,061 participants suggested that shortened sleep is connected with a diet higher in sugar and fat and less inclination to exercise. Insomnia is linked to elevated BMI, metabolic syndrome, hypertension, stroke, and CAD in epidemiological studies. Some of these conditions lead to dyslipidemia. A 2019 cross-sectional study revealed that compared to people without insomnia, those with insomnia in the last month had elevated HDL-C and triglycerides. A lack of sleep also heightens stress and general anxiety levels, which also leads to increased LDL-C levels (Abdurahman et al., 2020; Jia et al., 2025; Zheng et al., 2024).
Diagnostics
When discussing cholesterol, HCPs should take a life-span approach. Due to the potentially dangerous effects of lifetime exposure to high cholesterol, particularly LDL, the current 2018 Guideline on the Management of Blood Cholesterol indicates that HCPs should consider selective screenings of children as young as two with a family history of early heart disease, heart attack, stroke, or high cholesterol. For children without known risk factors, it is recommended that cholesterol be tested between the ages of 9 and 11, and then again between ages 17 and 21. This early testing can help HCPs identify and correct high cholesterol levels early through lifestyle modifications, thereby decreasing the long-term risks associated with hyperlipidemia (Grundy et al., 2019).
The AHA recommends that all adults ages 20–45 have their cholesterol checked every four to six years if they are considered low risk. Recommending cholesterol monitoring for younger patients prompts HCPs to consider cholesterol screening and cardiovascular health more often for young adults. Young adults with risk factors for hyperlipidemia may often already show the first stages of atherosclerosis. After age 40, the HCP should use a risk assessment tool, described below, to calculate the 10-year risk of having a heart attack or stroke. Those assigned male at birth aged 45–65 and those assigned female at birth ages 55–65 should have their cholesterol checked every one to two years, depending on risk level. After age 65, all patients should have their cholesterol checked annually (Davidson & Pradeep, 2024a; Grundy et al., 2019; NHLBI, 2024b).
A lipid panel or lipid profile is a collection of laboratory tests used to measure the amount of cholesterol and triglycerides in a patient's blood. The patient should fast and not eat or drink anything but water for 9 to 12 hours before laboratory collection for this type of testing. A complete lipid profile measures the levels of different fats in the blood, including total cholesterol, LDL-C, HDL-C, and triglycerides. The cholesterol targets according to age for components of a lipid profile test are outlined in Table 3 (Vijan, 2024).
Table 3
Cholesterol Targets by Age
Age and Sex | Total Cholesterol | Non-HDL Cholesterol | LDL-C Cholesterol | HDL-C Cholesterol |
People 19 years and younger | less than 170 mg/dL | less than 120 mg/dL | less than 110 mg/dL | greater than 45 mg/dL |
Adults 20 years and older | less than 150 mg/dL | less than 130 mg/dL | less than 100 mg/dL | greater than or equal to 40 mg/dL |
(AHA, 2024; Wilson, 2024a)
The AHA and ACC recommend that HCPs focus on reducing LDL levels from the patient's baseline and then progressively decreasing total LDL into the targeted range. Table 4 outlines the risk categories for each type of cholesterol (ACC, n.d.; Grundy et al., 2019; Wilson, 2024a).
Table 4
Patient Risk Based on Lipid Panel Results
Cardiovascular Risk | Total Cholesterol | LDL-C | HDL-C |
Higher risk | greater than 240 mg/dL | greater than 160 mg/dL | Male: less than 40 mg/dL Female: less than 50 mg/dL |
At-risk | 200–239 mg/dL | 100–159 mg/dL | Male: 40–59 mg/dL Female: 50–59 mg/dL |
Lower risk | less than 200 mg/dL | less than 100 mg/dL | greater than or equal to 60 mg/dL |
(Davidson & Pradeep, 2024a)
Lipoprotein-a or Lp(a) can also gauge a patient's risk of developing cardiovascular disease. This test is not included in the standard lipid profile and must be ordered separately by the HCP. An individual's Lp(a) level is hereditary and determined by genetic history. Lp(a) is often completed for patients with a family history of early heart disease, heart attack, or stroke, or those who are unsure about their family history. Elevated levels of Lp(a) indicate that the patient is at an increased risk of developing cardiovascular disease despite cholesterol results that are within the normal ranges. If the results are elevated, the initiation of a statin is indicated to prevent cardiovascular disease (NHLBI, 2024b).
The AHA guidelines have included using coronary artery calcium (CAC) measurement when the risk of ASCVD is uncertain in patients ages 40–75. CAC involves using a CT scan to take cross-sectional images of the blood vessels surrounding the heart. These images show whether calcified plaque buildup is present. Measurements of plaque buildup can help HCPs determine which patients are most at risk for heart disease before symptoms appear. The test results yield a risk score called the Agatston score, which indicates the total area of calcium located within the vessels and measures the density of the calcium deposits. When calcium is present, the higher the Agatston score, the higher the patient's risk of developing heart disease. A score of zero indicates that no calcium is present in the heart vessels. A score of 1–99 suggests calcium is beginning to accumulate in the blood vessels. A score of 100–400 indicates moderate plaque deposits are present, and the patient is at a relatively high risk of heart attack or heart disease over the next three to five years. For these patients, medication intervention is indicated. A score greater than 400 indicates severe heart disease and a high risk of a heart attack. CAC testing should not be used on everyone but is recommended for patients with a risk score in the intermediate range (Grundy et al., 2019; Gupta et al., 2022).
AHA Management Guidelines
Cholesterol management is viewed as a shared endeavor between patients and their HCPs. HCPs should ensure that patients understand the implications of hyperlipidemia and the healthcare plan specific to them. The main goal of cholesterol-lowering treatment and lifestyle modifications is to lower the LDL level enough to reduce the risk of developing heart disease or having a heart attack. The following ten recommendations appear in the 2018 ACC/AHA Guideline on the Management of Blood Cholesterol and the 2022 update from the ACC regarding the role of non-statin therapies (Grundy et al., 2019; Lloyd-Jones et al., 2022):
Table 5
Top 10 Take-Home Messages to Reduce Risk of Atherosclerotic Cardiovascular Disease Through Cholesterol Management
1. | In all individuals, emphasize a heart-healthy lifestyle across the course of life. A healthy lifestyle reduces ASCVD risk at all ages. In younger individuals, healthy lifestyle can reduce development of risk factors and is the foundation of ASCVD risk reduction. In young adults 20 to 39 years of age, an assessment of lifetime risk facilitates the clinician–patient risk discussion (refer to No. 6) and emphasizes intensive lifestyle efforts. In all age groups, lifestyle therapy is the primary intervention for metabolic syndrome. |
2. | In patients with clinical ASCVD, reduce LDL-C with high-intensity statin therapy or maximally tolerated statin therapy. The more LDL-C is reduced on statin therapy, the greater will be subsequent risk reduction. Use a maximally tolerated statin to lower LDL-C levels by greater than 50%. |
3. | In very high-risk ASCVD, use an LDL-C threshold of 70 mg/dL (1.8 mmol/L) to consider addition of non-statins to statin therapy. Very high-risk includes a history of multiple major ASCVD events or one major ASCVD event and multiple high-risk conditions. In very high-risk ASCVD patients, it is reasonable to add ezetimibe (Zetia) to maximally tolerated statin therapy when the LDL-C level remains 70 mg/dL (1.8 mmol/L) or more. In patients at very high risk whose LDL-C level remains 70 mg/dL (1.8 mmol/L) or more on maximally tolerated statin and ezetimibe therapy, adding a PCSK9 inhibitor is reasonable, although the long-term safety (greater than three years) is uncertain and cost effectiveness is low. (Since publication of the 2018 AHA/ACC/multisociety cholesterol guideline, three additional non-statin therapies—bempedoic acid [Nexletol], evinacumab [Evkeeza], and inclisiran [Leqvio]—have received FDA approval for management of hypercholesterolemia.) |
4. | In patients with severe primary hypercholesterolemia (LDL-C level 190 mg/dL [4.9 mmol/L] or more), without calculating 10-year ASCVD risk, begin high-intensity statin therapy. If the LDL-C level remains 100 mg/dL (2.6 mmol/L) or higher, adding ezetimibe is reasonable. If the LDL-C level on statin plus ezetimibe remains 100 mg/dL (2.6 mmol/L) or more and the patient has multiple factors that increase subsequent risk of ASCVD events, a PCSK9 inhibitor may be considered—although the long-term safety (greater than three years) is uncertain and economic value is uncertain. |
5. | In patients 40 to 75 years of age with diabetes mellitus and LDL-C 70 mg/dL (1.8 mmol/L) or higher, start moderate-intensity statin therapy without calculating 10-year ASCVD risk. In patients with diabetes mellitus at higher risk, especially those with multiple risk factors or those 50 to 75 years of age, it is reasonable to use a high-intensity statin to reduce the LDL-C level by 50% or more. |
6. | In adults 40 to 75 years of age evaluated for primary ASCVD prevention, have a clinician–patient risk discussion before starting statin therapy. Risk discussion should include a review of major risk factors (e.g., cigarette smoking, elevated blood pressure, LDL-C, hemoglobin A1C [if indicated], and calculated 10-year risk of ASCVD); the presence of risk-enhancing factors (refer to No. 8); the potential benefits of lifestyle and statin therapies; the potential for adverse effects and drug–drug interactions; consideration of costs of statin therapy; and patient preferences and values in shared decision-making. |
7. | In adults 40 to 75 years of age without diabetes mellitus and with LDL-C levels ≥70 mg/dL (≥1.8 mmol/L), at a 10-year ASCVD risk of greater than or equal to 7.5%, start a moderate-intensity statin if a discussion of treatment options favors statin therapy. Risk-enhancing factors favor statin therapy (refer to No. 8). If risk status is uncertain, consider using CAC to improve specificity (refer to No. 9). If statins are indicated, reduce LDL-C levels by 30% or more, and if 10-year risk is greater than or equal to 20%, reduce LDL-C levels by 50% or more. |
8. | In adults 40 to 75 years of age without diabetes mellitus and 10-year risk of 7.5% to 19.9% (intermediate risk), risk-enhancing factors favor initiation of statin therapy (refer to No. 7). Risk-enhancing factors include family history of premature ASCVD; persistently elevated LDL-C levels greater than or equal to 160 mg/dL (4.1 mmol/L); metabolic syndrome; chronic kidney disease; history of preeclampsia or premature menopause (age 40 years or older); chronic inflammatory disorders (e.g., rheumatoid arthritis, psoriasis, or chronic HIV); high-risk ethnic groups (e.g., South Asian); persistent elevations of triglycerides greater than or equal to 175 mg/dL (1.97 mmol/L); and, if measured in selected individuals, apolipoprotein B is 130 mg/dL or more, high-sensitivity C-reactive protein is 2.0 mg/L or more, ankle-brachial index Is 0.9 or more, and lipoprotein (a) is 50 mg/dL or 125 nmol/L or more, especially at higher values of lipoprotein (a). Risk-enhancing factors may favor statin therapy in patients at 10-year risk of 5%—7.5% (borderline risk). |
9. | In adults 40 to 75 years of age without diabetes mellitus, with LDL-C levels greater than or equal to 70 mg/dL to 189 mg/dL (1.8 to 4.9 mmol/L), and at a 10-year ASCVD risk of 7.5% to 19.9%, if a decision about statin therapy is uncertain, consider measuring CAC. If CAC is zero, treatment with statin therapy may be withheld or delayed, except in cigarette smokers, those with diabetes mellitus, and those with a strong family history of premature ASCVD. A CAC score of 1 to 99 favors statin therapy, especially in those older than 55 years of age. For any patient, if the CAC score is more than 100 Agatston units or the 75th percentile, statin therapy is indicated unless otherwise deferred by the outcome of clinician–patient risk discussion. |
10. | Assess adherence and percentage response to LDL-C–lowering medications and lifestyle changes with repeat lipid measurement 4 to 12 weeks after statin initiation or dose adjustment, repeated every 3 to 12 months as needed. Define responses to lifestyle and statin therapy by percentage reductions in LDL-C levels compared with baseline. In ASCVD patients at very high-risk, triggers for adding non-statin drug therapy are defined by threshold LDL-C levels greater than 70 mg/dL (1.8 mmol/L) on maximal statin therapy (refer to No. 3). |
Pharmacologic Management
The current guidelines continue to emphasize the importance of a clinician-patient risk discussion. For those with a 10-year ASCVD risk more than 7.5%, it is recommended that a discussion occurs before a statin prescription is written. As suggested in the ACC/AHA cholesterol guidelines, this frank discussion should consider whether ASCVD risk factors have been addressed, evaluate whether an optimal lifestyle has been implemented, and review the potential for statin benefit versus the potential for adverse outcomes and drug-drug interactions. Collaborative decision-making between HCPs and patients is based on active participation by the patient in choosing treatments based on personal values, preferences, and related health conditions or comorbidities (Grundy et al., 2019).
LDL-Cholesterol Lowering Treatment
Statins
Statins inhibit cholesterol synthesis in the liver by blocking the protein HMG-CoA reductase from making cholesterol. As a result, liver cells try to compensate for low cholesterol by synthesizing more LDL receptors on the cell surface to increase LDL uptake from the blood. Statins are the most common pharmacologic treatment used to treat hyperlipidemia in people 10 years old or older. In very high-risk situations, a statin may be used for patients younger than 10 years old (Hill & Bordoni, 2023; NHLBI, 2024c; Rosenson, 2024b).
Examples of statins include atorvastatin (Lipitor), fluvastatin (Lescol and Lescol XL), lovastatin (Altoprev), pitavastatin (Livalo), pravastatin (Pravachol), rosuvastatin (Crestor), and simvastatin (Zocor). Statins may increase the risk of diabetes for patients who have already been diagnosed with prediabetes, have an elevated BMI, or have metabolic syndrome. Statins may also cause abnormal results on liver enzyme tests; however, actual liver damage is rare. Other adverse effects include myopathy (muscle damage) and hepatotoxicity. Statins are contraindicated in patients with alcohol use disorder, decompensated cirrhosis, acute liver failure, and those who are pregnant (Arcangelo et al., 2022; NHLBI, 2024c; Pignone & Cannon, 2024; Rosenson, 2024b).
The AHA divides statin therapy into three categories: high-intensity, moderate-intensity, and low-intensity. High-intensity statins lower LDL-C levels by more than 50%, moderate-intensity statins reduce LDL-C levels by 30%–49%, and low-intensity statins lower LDL-C levels by less than 30%. High-intensity statins include atorvastatin (Lipitor) and rosuvastatin (Crestor). Moderate-intensity statins include simvastatin (Zocor), pravastatin (Pravachol), lovastatin (Altoprev), fluvastatin XL (Lescol XL), and pitavastatin (Livalo). Low-intensity statins include low doses of lovastatin (Altoprev), simvastatin (Zocor), pravastatin (Pravachol), and fluvastatin (Lescol; Grundy et al., 2019; Pignone & Cannon, 2024).
Ezetimibe (Zetia)
Ezetimibe (Zetia) is a unique drug used to reduce cholesterol. Like statins, ezetimibe (Zetia) should not be used for patients under the age of 10. Ezetimibe (Zetia) acts on the small intestine cells to inhibit the absorption of dietary cholesterol. It does not inhibit cholesterol synthesis in the liver or increase bile acid excretion. Ezetimibe (Zetia) can be used alone or in combination with a statin. It is used to treat familial hypercholesterolemia when a statin is ineffective. Adverse effects include myopathy, rhabdomyolysis, hepatitis, pancreatitis, and thrombocytopenia (Arcangelo et al., 2022; Pignone & Cannon, 2024; UpToDate Lexidrug, n.d.-b).
Bile-Acid Sequestrants
Bile-acid sequestrants reduce LDL-C and were previously the first-line treatment for hyperlipidemia. Currently, they are used for patients who do not tolerate statins or whose cholesterol levels are not responding to statin therapy alone. Bile-acid sequestrants lower LDL-C by increasing LDL receptors on hepatocytes. Once administered, these agents bind with bile acids in the intestines and form an insoluble complex, preventing the bile acids' reabsorption. This accelerated excretion of bile acids creates a demand for increased synthesis of LDL-C by the liver, which prompts liver cells to increase their capacity for LDL uptake. Examples of bile-acid sequestrants include cholestyramine (Questran, Prevalite), colesevelam (Welchol), and colestipol (Colestid). Adverse effects are limited to the digestive system, since bile-acid sequestrants are not absorbed, and include constipation, bloating, indigestion, and nausea (Arcangelo et al., 2022; Rosenson, 2024a).
Bempedoic Acid (Nexletol)
Bempedoic acid (Nexletol) is a newer drug that works similarly to statins, except instead of inhibiting HMG CoA reductase, bempedoic acid (Nexletol) inhibits ATP-citrate lyase (ACL). It is less likely to cause myopathy since it is converted to its active form in the liver and not in the muscles. When combined with a statin, it can reduce LDL-C significantly. Adverse effects of bempedoic acid (Nexletol) include hyperuricemia, tendon rupture, respiratory tract infection, back pain, abdominal pain, anemia, and elevated liver enzymes (Bays et al., 2025; Rosenson, 2024a; UpToDate Lexidrug, n.d.-a).
PCSK9 Inhibitors
PCSK9 inhibitors lower LDL-C by decreasing the destruction of LDL receptors in the liver, which helps remove and clear LDL-C from the blood. They are administered subcutaneously every two to four weeks. PCSK9 inhibitors are often used in conjunction with a statin for patients at high risk of ASCVD or those with familial hypercholesterolemia. Examples include evolocumab (Repatha) and alirocumab (Praluent). Localized side effects include itching, pain, and inflammation at the injection site (NHLBI, 2024c; Rosenson, 2024a; Stroes & Stiekema, 2025).
Triglyceride-Lowering Treatment
Fibric Acid Derivatives (Fibrates)
Fibrates are the most effective drugs available for lowering triglyceride levels. In addition to lowering triglyceride levels, fibrates can also increase HDL-C levels. Fibrates have little to no effect on LDL-C. Fibrates work by reducing the very-low-density lipoproteins (VLDLs) produced by the liver. They also increase the removal of triglycerides from the blood. Examples of fibrates include gemfibrozil (Lopid), fenofibrate (Tricor), and fenofibric acid (Trilipix). Adverse effects include rash, nausea, abdominal pain, and diarrhea. Gallstones, myopathy, and liver injury have also been reported (Arcangelo et al., 2022; Rosenson, 2024a; Rosenson & Eckel, 2025).
Nicotinic Acid (Niacin)
Nicotinic acid (Niacin) decreases LDL-C and triglycerides, and raises HDL-C. The primary action of nicotinic acid (Niacin) is to reduce the production of VLDLs. LDL-C is a byproduct of VLDL degradation; therefore, reducing VLDL causes a decrease in LDL-C. The primary mechanism by which nicotinic acid (Niacin) reduces VLDLs is by inhibiting lipolysis in adipose tissue. Although nicotinic acid (Niacin) is effective, it causes various undesirable side effects, including intense flushing, nausea, vomiting, and diarrhea. Intense flushing of the face, neck, and ears affects most patients who take the medication and can be uncomfortable (Arcangelo et al., 2022; Rosenson, 2024a; Rosenson & Eckel, 2025).
Icosapent ethyl (Vascepa)
Icosapent ethyl (Vascepa) is used as an adjunct therapy with statins to reduce the risk of myocardial infarction, stroke, and unstable angina in patients with hyperlipidemia, including triglyceride levels at or over 150 mg/dL. Icosapent ethyl (Vascepa) is also indicated as an adjunct to dietary measures to reduce triglyceride levels in patients with severe hypertriglyceridemia (triglyceride level over 500 mg/dL). Adverse effects include peripheral edema, myalgia, constipation, gout, and atrial fibrillation (UpToDate Lexidrug, n.d.-c).
Familial Hypercholesterolemia Treatment
Lipoprotein Apheresis
Some patients with familial hypercholesterolemia may benefit from lipoprotein apheresis to lower their blood cholesterol levels. Lipoprotein apheresis is a dialysis-like process in which a filtering machine removes LDL-C from the blood, and the remainder of the blood is returned to the patient (NHLBI, 2024c).
Lomitapide (Juxtapid)
Lomitapide (Juxtapid) is a microsomal triglyceride transfer protein inhibitor used with dietary modifications to reduce the amount of LDL-C and total cholesterol in patients with HoFH. It works by inhibiting the microsomal triglyceride transfer protein (MTTP), which is necessary for VLDL assembly and excretion by the liver. Adverse effects include diarrhea, nausea, vomiting, constipation, bloating, and weight loss. Lomitapide (Juxtapid) may cause severe hepatotoxicity. Due to this, patients should be advised not to drink alcohol while taking lomitapide (Juxtapid). Due to the severity of hepatotoxicity, the FDA approved a risk evaluation and mitigation strategies (REMS) program. All prescriptions for lomitapide (Juxtapid) must be written by a registered HCP with the Juxtapid REMS program and filled at an approved pharmacy (UpToDate Lexidrug, n.d.-d).
Olezarsen (Tryngolza)
Olezarsen (Tryngolza) has been approved recently (February 2025) for use as an adjunct to a low-fat diet in patients with severe hypertriglyceridemia and risk for acute pancreatitis due to FCS. It works by targeting apolipoprotein C-III production, and research has demonstrated a decrease in both triglycerides and the incidence of pancreatitis. Adverse effects are injection site reactions, lowered platelet count, and arthralgias (Regmi & Rehman, 2023; Rosenson & Eckel, 2025).
Herbs, Supplements, and Specific Dietary Components
Garlic
Research is conflicting on garlic supplementation. A research review in 2023 showed that garlic supplementation lowers total cholesterol. An earlier meta-analysis and review in 2016 reported total and LDL-C reduction by 10% after two months or more of garlic supplementation. Other studies have not shown improvement in lipid levels with raw, powdered, or aged garlic. Although garlic is safe with minimal adverse effects, including breath and body odor, pyrosis (heartburn), and dyspepsia (upset stomach), it is not recommended as a lipid-lowering treatment (NCCIH, 2024).
Red Yeast Rice
Some red yeast rice contains a substantial amount of monacolin K, which is chemically identical to lovastatin (Altoprev). Consequently, these types of red yeast rice can have similar LDL-C-lowering effects; however, they also have the same adverse effects and drug interactions as lovastatin (Altoprev). The FDA has not approved red yeast rice supplements that contain over a trace amount of monacolin K. Due to variable potency and lack of standardization, red yeast rice is not recommended for clinical use. Red yeast rice may also be contaminated with citrinin, which can be nephrotoxic (NCCIH, 2024; Tangney & Rosenson, 2024).
Omega-3 Fatty Acids
Dietary intake of omega-3 fatty acids has been shown to reduce triglyceride levels but may elevate or lower cholesterol levels. A meta-analysis of seven trials consisting of 662 participants showed a decrease in LDL-C and triglycerides with an increase of HDL-C levels with krill oil supplementation. Dietary sources rich in omega-3 fatty acids include salmon, herring, trout, mackerel, nuts, flaxseed, chia seeds, and canola and soybean oils (MedlinePlus, 2024; Tangney & Rosenson, 2024).
Stanols and Sterols
Incorporating foods and dietary supplements containing added plant stanols or sterols is an adjunctive option in conventional treatment for high cholesterol levels. Although there is less evidence for the effectiveness of the supplements than for foods containing stanols or sterols, studies have shown that when taken with meals, stanol or sterol supplements can reduce cholesterol levels. The FDA has approved a health claim stating that the product may reduce the risk of heart disease when taken in amounts that are appropriate for some foods and dietary supplements that contain stanols or sterols. Examples of plant stanols and sterols include certain vegetable oils (e.g., canola oil), nuts (e.g., walnuts), certain fruits, most beans, and vegetables. They are also found in cholesterol-lowering types of margarine, which are commonly advertised as "buttery spreads" (Arcangelo et al., 2022; MedlinePlus, 2024; National Center for Complementary and Integrative Health [NCCIH], 2024; Tangney & Rosenson, 2024).
Soy
Research has shown that soy-containing products can decrease cholesterol, but not as much as cholesterol-lowering medications. Dietary soy is a great protein source that is comprised of isoflavones (phytoestrogens). The isoflavones share characteristics with estrogen and might slightly influence cholesterol levels and inhibit LDL oxidation. Soy protein supplements were connected with a significant decrease of total cholesterol levels and an increase in HDL-C levels in a 2022 systematic review and meta-analysis. Most research has shown that soy foods have more benefits than supplements on cholesterol levels. Side effects include gastrointestinal upset, such as diarrhea and stomach pain (NCCIH, 2024; Tangney & Rosenson, 2024).
Flaxseed
Flaxseed supplements—including whole flaxseeds and flaxseed lignans, but not flaxseed oil—have been shown to lower cholesterol levels. They have been most beneficial in postmenopausal patients assigned female at birth. A systematic review and meta-analysis in 2022 reported that supplementation with whole flaxseed or lignans significantly lowered total cholesterol, and the lignan supplementation additionally lowered LDL-C and HDL-C. Flaxseed and supplements are well tolerated and confer minimal side effects when used in small amounts. Since flaxseed is a fiber supplement, it should be taken with a full glass of water to prevent constipation (NCCIH, 2024; Tangney & Rosenson, 2024).
Oats and Oat Bran
The consistent long-term dietary intake of whole oats or oat bran can decrease cholesterol levels. This is in part due to oat beta-glucan (β-glucan) found in the oat's cell wall, which elevates the excretion of bile acid that, in turn, stimulates metabolism and the elimination of cholesterol. A 2022 systematic review and meta-analysis of randomized controlled trials showed that the consumption of oats with β-glucan could significantly reduce total and LDL-C levels in patients with hypercholesterolemia. However, there was no evidence of triglyceride-lowering or beneficial effects on HDL-C levels. A large number of previous studies have demonstrated the benefits of oats since the mid-1980s. Oats are well tolerated, but may cause side effects like intestinal gas and bloating (MedlinePlus, 2024; NCCIH, 2024; Tangney & Rosenson, 2024; Yu et al., 2022).
References
Abdurahman, A. A., Bule, M., Shab-Bidar, S., Rezaei, S., & Djafarian, K. (2020). The association between sleep duration and risk of abnormal lipid profile: A systematic review and meta-analysis. Obesity Medicine, 18, 100236. https://doi.org/10.1016/j.obmed.2020.100236
American College of Cardiology. (n.d.). ASCVD risk estimator plus. Retrieved March 21, 2025, from https://tools.acc.org/ASCVD-Risk-Estimator-Plus/#!/calculate/estimate
American Heart Association. (2023). Depression, anxiety and stress linked to poor heart health in two new studies. https://newsroom.heart.org/news/depression-anxiety-and-stress-linked-to-poor-heart-health-in-two-new-studies
American Heart Association. (2024). What your cholesterol levels mean. https://www.heart.org/en/health-topics/cholesterol/about-cholesterol/what-your-cholesterol-levels-mean
American Heart Association. (2025a). Big differences found in heart attack and stroke risks among Asian American, Native Hawaiian and Pacific Islander groups. https://www.heart.org/en/news/2025/03/10/big-differences-found-in-heart-and-stroke-risks-among-asian-american
American Heart Association. (2025b). Cardiovascular risk factors and predicted risks across Asian American, Native Hawaiian and other Pacific Islander subgroups: The PANACHE study. https://epi.apprisor.org/epsAbstractEPI.cfm?id=1
Anni, N. S., Jung, S. J., Shim, J. S., Jeon, Y. W., Lee, G. B., & Kim, H. C. (2021). Stressful life events and serum triglyceride levels: The Cardiovascular and Metabolic Diseases Etiology Research Center cohort in Korea. Epidemiology and Health, 43, e2021042. https://doi.org/10.4178/epih.e2021042
Arcangelo, V. P., Peterson, A. M., Wilbur, V. F., & Kang, T. M. (2022). Pharmacotherapeutics for advanced practice. (5th ed.). Wolters Kluwer.
Bays, H. E., Bloedon, L. Brennan, D., Lei, L., Lincoff, A. M., Nicholls, S. J., Plutzky, J., Powell, H. A., & Nissen, S. E. (2025). Bempedoic acid for prevention of cardiovascular events in people with obesity: A CLEAR outcomes subset analysis. Journal of the American Heart Association, 14(4). https://doi.org/10.1161/JAHA.124.037898
Bays, H. E., Kirkpatrick, C. F., Maki, K. C., Toth, P. P., Morgan, R. T., Tondt, J., Christensen, S. M., Dixon, D. L., & Jacobson, T. A. (2024). Obesity, dyslipidemia, and cardiovascular disease: A joint expert review from the Obesity Medicine Association and the National Lipid Association 2024. Journal of Clinical Lipidology, 18, (3), E320-E350. https://doi.org/10.1016/j.jacl.2024.04.001
Cagen, L. (2023). Cholesterol. EBSCO. https://www.ebsco.com/research-starters/health-and-medicine/cholesterol
Centers for Disease Control and Prevention. (2024a). Adult activity: An overview. https://www.cdc.gov/physical-activity-basics/guidelines/adults.html
Centers for Disease Control and Prevention. (2024b). High cholesterol facts. https://www.cdc.gov/cholesterol/data-research/facts-stats/
Centers for Disease Control and Prevention. (2024c). Risk factors for high cholesterol. https://www.cdc.gov/cholesterol/risk-factors/?CDC_AAref_Val=https://www.cdc.gov/cholesterol/risk_factors.htm
Courtney, A. R. (2024). Lipids. EBSCO. https://www.ebsco.com/research-starters/health-and-medicine/lipids
Davidson, M. H., & Pradeep, P. (2024a). Dyslipidemia. MSD Manual Professional Version. https://www.msdmanuals.com/professional/endocrine-and-metabolic-disorders/lipid-disorders/dyslipidemia
Davidson, M. H., & Pradeep, P. (2024b). Overview of lipid metabolism. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/lipid-disorders/overview-of-lipid-metabolism
Devonish, J., Debnam, C., Furgurson, E., Sawa, H., Dahlquist, C., & Arendt, M. (2022). The role of all healthcare professionals in cessation. Tobacco Induced Diseases, 20, 01. https://doi.org/10.18332/tid/144766
Feingold, K. R. (2023). Approach to the patient with dyslipidemia. Endotext. https://www.ncbi.nlm.nih.gov/books/NBK326736/table/lipid_pat-dyslipid.T.drugs_that_increase/
Grundy, S. M., Stone, N. J., Bailey, A. L., Beam, C., Birtcher, K. K., Blumenthal, R. S., Braun, L. T., de Ferranti, S., Faiella-Tommasino, J., Forman, D. E., Goldberg, R., Heidenreich, P. A., Hlatky, M. A., Jones, D. W., Lloyd-Jones, D., Lopez-Pajares, N., Ndumele, C. E., Orringer, C. E., Peralta., C. E., … Yeboah, J. (2019). 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation, 139, e1082–e1143. https://doi.org/10.1161/CIR.0000000000000625
Gupta, A., Bera, K., Kikano, E., Pierce, J. D., Gan, J., Rajdev, M., Ciancibello, L. M., Gupta, A., Rajagopalan, S., Gilkeson, R. C. (2022). Coronary artery calcium scoring: Current status and future directions. RadioGraphics, 42(4), 947–967. https://doi.org/10.1148/rg.210122
Hill, M. F., & Bordoni, B. (2023). Hyperlipidemia. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK559182/
Ibrahim, M. A., Asuka, E., Jialal, I., & Corcione, J. (2023). Hypercholesterolemia (Nursing). StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/sites/books/NBK568722/
Jackson, E. A. (2025). Cardiovascular risk of smoking and benefits of smoking cessation. UpToDate. Retrieved March 24, 2025, from https://www.uptodate.com/contents/cardiovascular-risk-of-smoking-and-benefits-of-smoking-cessation
Jia, F., Shi, S. Y., Fei, S. F., Zhou, M., & Li, J. J. (2025). Association of insomnia, lipid profile, and lipid-lowering medications: A narrative review. Reviews in Cardiovascular Medicine, 26(1), 24978. https://doi.org/10.31083/RCM24978
July, M. (2024). Familial hypercholesterolemia. https://emedicine.medscape.com/article/121298-overview
Lloyd-Jones, D., Morris, P., Ballantyne, C. M., Birtcher, K. K., Covington, A. M., DePalma, S. M., Minissian, M. B., Orringer, C. E., Smith, S. C., Waring, A. A., & Wilkins, J. T. (2022). ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American College of Cardiology Solution Set Oversight Committee. JACC, 80(14), 1366–1418. https://doi.org/10.1016/j.jacc.2022.07.006
Majid, S., Keith, R. J., Fetterman, J. L., Weisbrod, R. M., Nystoriak, J., Wilson, T., Stokes, A. C., Blaha, M. J., Srivastava, S., Robertson, R. M., Bhatnagar, A., & Hamburg, N. M. (2021). Lipid profiles in users of combustible and electronic cigarettes. Vascular Medicine (London, England), 26(5), 483–488. https://doi.org/10.1177/1358863X211009313
MedlinePlus. (2024). How to lower cholesterol with diet. https://medlineplus.gov/howtolowercholesterolwithdiet.html
National Center for Complementary and Integrative Health. (2024). High cholesterol and natural products: What the science says. https://www.nccih.nih.gov/health/providers/digest/high-cholesterol-and-natural-products-science
National Heart, Lung, and Blood Institute. (n.d.). Therapeutic lifestyle changes (TLC) to lower cholesterol. National Institutes of Health, National Heart, Lung, and Blood Institute. Retrieved March 25, 2025, from https://www.nhlbi.nih.gov/education/TLC-Therapeutic-Lifestyle-Changes-Lower-Cholesterol#main-content
National Heart, Lung, and Blood Institute. (2024a). Blood cholesterol causes and risk factors. National Institutes of Health, National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health/blood-cholesterol/causes
National Heart, Lung, and Blood Institute. (2024b). Blood cholesterol diagnosis. National Institutes of Health, National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health/blood-cholesterol/diagnosis
National Heart, Lung, and Blood Institute. (2024c). Blood cholesterol treatment. National Institutes of Health, National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health/blood-cholesterol/treatment
National Institute of Diabetes and Digestive and Kidney Diseases. (2021). Diabetes, heart disease, and stroke. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/heart-disease-stroke
Pignone, M., & Cannon, C. P. (2024). Low-density lipoprotein cholesterol-lowering therapy in the primary prevention of cardiovascular disease. UpToDate. Retrieved March 21, 2025, from https://www.uptodate.com/contents/low-density-lipoprotein-cholesterol-lowering-therapy-in-the-primary-prevention-of-cardiovascular-disease
Prokopidis, K., Morgan, P. T., Veronese, N., Morwani-Mangnani, J., Triantafyllidis, K. K., Kechagias, K. S., Roberts, J., Hurst, C., Stevenson, E., Vlachopouos, D., & Witard, O. C. (2025). The effects of whey protein supplementation on indices of cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. Clinical Nutrition, 44, 109-121. https://doi.org/10.1016/j.clnu.2024.12.003
Regmi, M., & Rehman, A. (2023). Familial hyperchylomicronemia syndrome. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK551655/
Rogers, J. L. and Brashers, V. L. (Eds.). (2023). McCance and Huether's pathophysiology: The biologic basis for disease in adults and children (9th ed.). Elsevier.
Rosenson, R. S. (2024a). Low-density lipoprotein cholesterol lowering with drugs other than statins and PCSK9 inhibitors. UpToDate. Retrieved March 21, 2025, from https://www.uptodate.com/contents/low-density-lipoprotein-cholesterol-lowering-with-drugs-other-than-statins-and-pcsk9-inhibitors
Rosenson, R. S. (2024b). Statins: Actions, side effects, and administration. UpToDate. Retrieved March 24, 2025, from https://www.uptodate.com/contents/statins-actions-side-effects-and-administration
Rosenson, R. S., & Durrington, P. (2025). Familial hypercholesterolemia in adults: Overview. UpToDate. Retrieved March 21, 2025, from https://www.uptodate.com/contents/familial-hypercholesterolemia-in-adults-overview
Rosenson, R. S., & Eckel, R. H. (2025). Hypertriglyceridemia in adults: Management. UpToDate. Retrieved March 21, 2025, from https://www.uptodate.com/contents/hypertriglyceridemia-in-adults-management
Stroes, E. S. G., & Stiekema, L. C. A. (2025). PCSK9 inhibitors: Pharmacology, adverse effects, and use. UpToDate. Retrieved March 21, 2025, from https://www.uptodate.com/contents/pcsk9-inhibitors-pharmacology-adverse-effects-and-use
Sweeney, M. E. T. (2025). Hypertriglyceridemia. Medscape. https://emedicine.medscape.com/article/126568-overview#a4
Tangney, C. C., & Rosenson, R. S. (2024). Lipid management with diet or dietary supplements. UpToDate. Retrieved March 21, 2025, from https://www.uptodate.com/contents/lipid-management-with-diet-or-dietary-supplements
UpToDate Lexidrug. (n.d.-a). Bempedoic acid: Drug Information. Retrieved March 21, 2025, from https://www.uptodate.com/contents/bempedoic-acid-drug-information
UpToDate Lexidrug. (n.d.-b). Ezetimibe: Drug information. Retrieved March 24, 2025, from https://www.uptodate.com/contents/ezetimibe-drug-information
UpToDate Lexidrug. (n.d.-c). Icosapent ethyl (E-EPA): Drug information. Retrieved March 21, 2025, from https://www.uptodate.com/contents/icosapent-ethyl-e-epa-drug-information
UpToDate Lexidrug. (n.d.-d). Lomitapide: Drug information. Retrieved March 21, 2025, from https://www.uptodate.com/contents/lomitapide-drug-information
U.S. Food & Drug Administration. (2023). FDA completes final administrative actions on partially hydrogenated oils in food. https://www.fda.gov/food/hfp-constituent-updates/fda-completes-final-administrative-actions-partially-hydrogenated-oils-foods
US Department of Agriculture. (2020). Dietary guidelines for Americans, 2020-2025 (9th ed.). https://www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf
US Department of Health and Human Services. (2024a). Deaths: Leading causes for 2021. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. https://www.cdc.gov/nchs/data/nvsr/nvsr73/nvsr73-04.pdf
US Department of Health and Human Services. (2024b). HHS framework to support and accelerate smoking cessation 2024. https://www.hhs.gov/sites/default/files/hhs-framework-support-accelerate-smoking-cessation-2024.pdf
Vaezi, Z., & Amini, A. (2022). Familial hypercholesterolemia. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK556009/
Vijan, S. (2024). Screening for lipid disorders in adults. UpToDate. Retrieved March 21, 2025, from https://www.uptodate.com/contents/screening-for-lipid-disorders-in-adults
Wilson, P. W. F. (2024a). Atherosclerotic cardiovascular disease risk assessment for primary prevention in adults. UpToDate. Retrieved March 21, 2025, from https://www.uptodate.com/contents/atherosclerotic-cardiovascular-disease-risk-assessment-for-primary-prevention-in-adults
Wilson, P. W. F. (2024b). Overview of established risk factors for cardiovascular disease. UpToDate. Retrieved March 24, 2025, from https://www.uptodate.com/contents/overview-of-established-risk-factors-for-cardiovascular-disease
Yu, J., Xia, J., Yang, C., Pan, D., Xu, D., Sun, G., & Xia, H. (2022). Effects of oat beta-glucan intake on lipid profiles in hypercholesterolemic adults: A systematic review and meta-analysis of randomized controlled trials. Nutrients, 14(10), 2043. https://doi.org/10.3390/nu14102043
Zhang, H., Shao, M. M., Lin, X. D., Cheng, L. J., Ovlyakulov, B., Chen, B. B., & Chen, K. Y. (2021). A cross-sectional survey on occupational stress and associated dyslipidemia among medical staff in tertiary public hospitals in Wenzhou, China. Brain and Behavior, 11(3), e02014. https://doi.org/10.1002/brb3.2014
Zheng, Y. B., Huang, Y. T., Gong, Y. M., Li, M. Z., Zeng, N., Wu, S. L., Zhang, Z. B., Tian, S. S., Yuan, K., Liu, X. X., Vitiello, M. V., Wang, Y. M., Wang, Y. X., Zhang, X. J., Shi, J., Shi, L., Yan, W., Lu, L., & Bao, Y. P. (2024). Association of lifestyle with sleep health in general population in China: A cross-sectional study. Translational Psychiatry, 14(1), 320. https://doi.org/10.1038/s41398-024-03002-x
Powered by Froala Editor
Single Course Cost: $11.00
Add to CartSave while Futhering your Education
Cholesterol Management, Coaching, and Patient Education Nursing CE Course is offered in the packages below.- 27 ANCC Hours
- 10 Courses
- 530 ANCC Hours
- 190 Courses