About this course:
This learning activity reviews the disease process of chronic kidney disease (CKD) and the medical and nursing management of the affected individual.
Course preview
Chronic Kidney Disease
This learning activity reviews the disease process of chronic kidney disease (CKD) and the medical and nursing management of the affected individual.
This learning activity is designed to prepare the learner to:
- describe the pathophysiological changes that occur in CKD
- discuss the prevalence of CKD
- explain the proposed risk factors and preventative measures for CKD
- describe clinical manifestations of CKD
- review which interventions are appropriate when managing CKD
- differentiate between the stages of CKD
Background
Chronic kidney disease is characterized by kidney damage, which decreases function of the kidneys. Kidney disease is one of the leading causes of death in the United States. CKD is the preferred term when referencing the declining glomerular filtration rate (GFR). The terms renal insufficiency and chronic renal failure are still commonly used; however, these terms do not address the specific stages and guidelines recommended by the Kidney Disease Improving Global Outcomes (KDIGO) organization (2024). Approximately 14% of the population in the United States, or 35.5 million people, are estimated to have CKD, with many cases undiagnosed. In 2023, kidney diseases were listed as the eighth leading cause of death in the United States. Diabetes and hypertension are the leading causes of CKD, accounting for over 65% of all new cases. Every day in the United States, 360 people are diagnosed with CKD. Treating CKD can be costly. In 2023, Medicare paid out $156.7 billion to treat CKD, accounting for 1 in 4 Medicare dollars going to treat kidney disease (Centers for Disease Control and Prevention [CDC], 2024a, 2024b, 2024c; National Kidney Foundation [NKF], 2024a).
Anatomy and Physiology
Structures of the Renal System
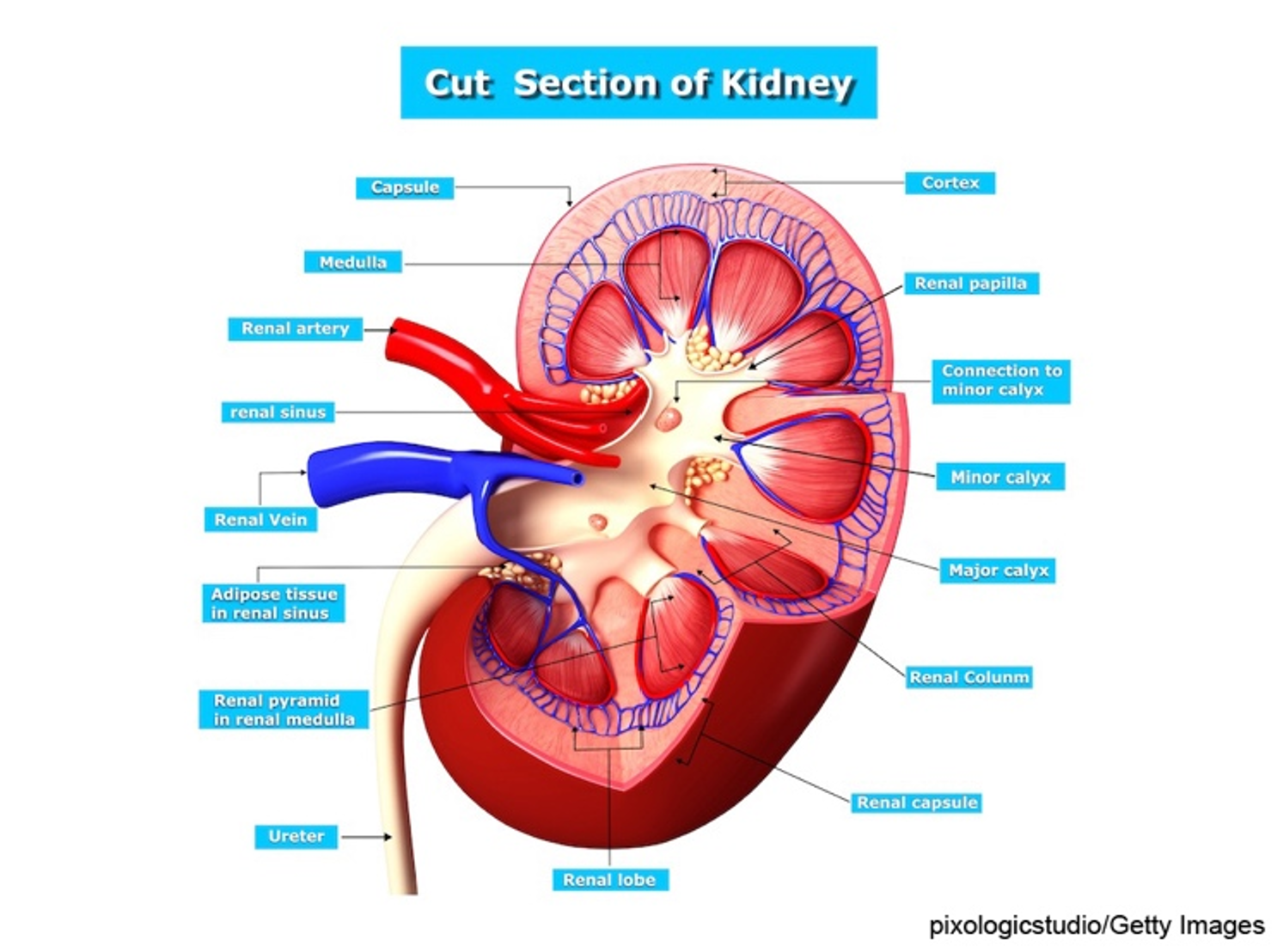
Six organs comprise the urinary system, including two kidneys, two ureters, the urinary bladder, and the urethra. The kidneys are in the posterior region of the abdominal cavity. They are located behind the peritoneum on either side of the vertebral column between T12 and L3. Due to liver placement, the right kidney is located slightly lower than the left kidney. Each kidney measures approximately 11 cm long and 6 cm wide. The kidneys are considered the body's purification system because they remove wastes, including toxins, from the blood. They are protected by the renal capsule and a fatty layer. The capsule and fatty layer are covered with a double layer of renal fascia that adheres the kidneys to the abdominal wall. The outer layer of the kidney is called the cortex (refer to Figure 1). The cortex contains all the glomeruli, most of the proximal tubules, and some distal tubule segments. The inner portion of the kidney is called the medulla and contains the tubules and collecting ducts that drain into the calyces. The calyces join and form the renal pelvis, which is continuous with the upper end of the ureter (Rogers & Brashers, 2023).
Figure 1
Kidney Structure
The nephron is the functional unit of the kidney and forms urine. The nephron comprises the glomerulus, proximal tubule, loops of Henle, distal tubule, and collecting duct (refer to Figure 2 and Table 1). Each kidney contains approximately 1.2 million nephrons. There are three types of nephrons: superficial cortical nephrons, which make up 85% of all nephrons; mid-cortical nephrons; and juxtamedullary nephrons, essential for urine concentration. The glomerulus contains loops of capillaries contained in the Bowman capsule. The walls of the capillaries serve as a filtration membrane for urine formation. An anion charge across the filtration membrane restricts the filtration of negatively charged molecules, such as proteins. Juxtaglomerular cells secrete renin and are located around the afferent arteriole. They are also connected to the sodium-sensing macula densa cells of the distal convoluted tubule. The proximal tubule is lined with microvilli to increase surface area and reabsorption. The loops of Henle selectively transport solutes and water, which contribute to the medulla's hypertonic state and are essential for concentrating urine. The collecting duct contains principal cells that facilitate sodium and water reabsorption and the excretion of potassium and intercalated cells that secrete hydrogen, bicarbonate, and potassium. Once the urine is produced and concentrated, it flows through the ureters into the bladder. Once the amount of urine in the bladder reaches 250 to 300 mL, mechanoreceptors respond to the stretching and stimulate the micturition (urination) reflex (Rogers & Brashers, 2023).
Figure 2
Significant Components of Nephron Structure
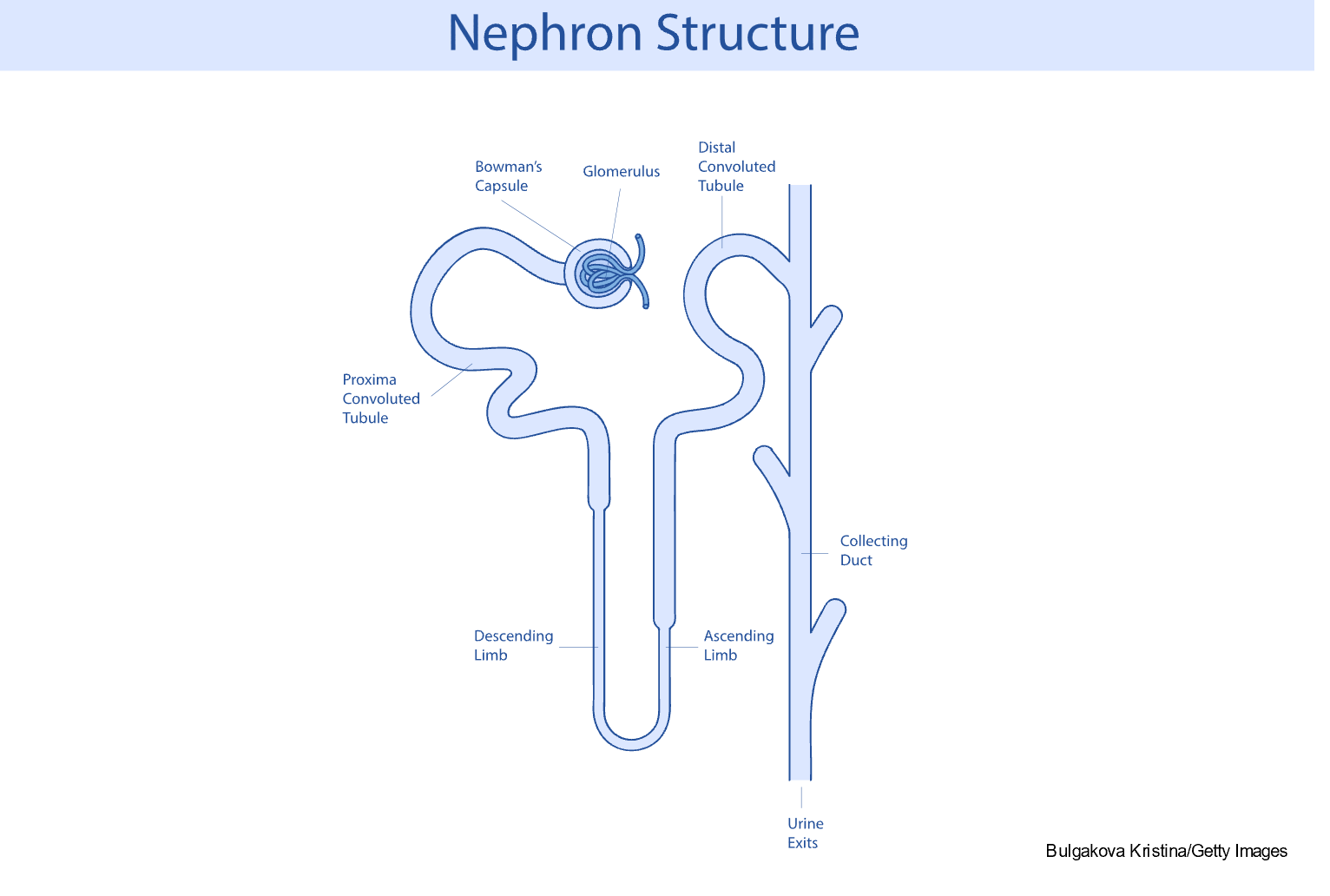
Table 1
Significant Functions of Nephron Segments
1. |
ft: none; border-bottom: 1pt solid windowtext; border-right: none; padding: 0in 5.4pt;"> Glomerulus: Forms an ultrafiltrate of plasma that filters blood components through three layers:
|
2. | Proximal convoluted tubule: The first segment of the renal tubule; extends from the Bowman space; absorbs and transports water, electrolytes, and other particles |
3. | Descending loop of Henle: Extends into the medulla and is highly permeable to water |
4. | Ascending loop of Henle: Is permeable to ions (especially sodium, potassium, calcium, and magnesium) but not water; passes urine into the distal convoluted tubule |
5. | Distal tubule: Important for maintaining fluid and electrolyte and acid-base balance |
6. | Collecting duct: Descends down the cortex and through the renal pyramids of the inner and outer medullae; drains urine into the minor calyx |
(Madrazo-Ibarra & Vaitla, 2023; Rogers & Brashers, 2023)
Renal Blood Flow
To fully understand CKD, it is critical to review the blood pathway through the kidneys (refer to Table 2). Renal blood flows at approximately 1000 to 1200 mL/min, or 20% to 25% of the total cardiac output. Blood flow through the glomerular capillaries is maintained at a set rate (i.e., autoregulation) despite various changes to systemic arterial pressures. The GFR is the plasma filtration per unit of time and is directly related to renal blood flow (i.e., perfusion pressure). The sympathetic noradrenergic nerves that regulate vasoconstriction innervate the renal blood vessels. The autoregulation of renal blood flow and sympathetic regulation of vasoconstriction maintain a constant GFR (Dalal et al., 2023; Rogers & Brashers, 2023).
There are hormones and other factors that also regulate renal blood flow. These hormones and other mediators alter the resistance of the renal vasculature by stimulating either vasodilation or vasoconstriction. The renin-angiotensin-aldosterone system (RAAS) is a central hormonal regulator of renal blood flow. Renin is an enzyme formed and stored in the granular cells of the afferent arterioles. Renin release is stimulated by decreased blood pressure (BP), reduced sodium chloride concentrations, and sympathetic nerve stimulation of beta-adrenergic receptors. When renin is released, it causes the production of angiotensin I, which is inactive. Angiotensin I is converted into angiotensin II by angiotensin-converting enzyme (ACE). Angiotensin II stimulates the adrenal cortex to release aldosterone, stimulates the release of antidiuretic hormone (ADH), and acts as a vasoconstrictor leading to increased blood pressure in the kidneys. Natriuretic peptides released by the myocardium inhibit the RAAS. When fluid volume expands, the heart dilates, and releases natriuretic peptides that inhibit sodium and water reabsorption, inhibit the secretion of renin and aldosterone, dilate the afferent arterioles, and constrict the efferent arterioles, which results in increased urine formation and decreased blood volume and blood pressure (Rogers & Brashers, 2023).
Table 2
Blood Flow Through the Kidneys
1. | Blood enters the kidney through the aorta to the right and left renal artery |
2. | Blood flows through renal arteries to the arterial branches |
3. | Blood flows into the afferent arterioles |
4. | Blood flows into the glomerulus |
5. | Blood leaves via efferent arterioles |
6. | Blood divides into two networks, which rejoin to form venous branches:
|
(Rogers & Brashers, 2023)
Kidney Function
The primary function of the nephron is urine formation, a complex process involving glomerular filtration, tubular reabsorption, and tubular secretion and excretion. In addition to urine formation, the kidneys have various secondary functions such as:
- Regulation of electrolytes, specifically calcium and phosphorus
- Regulation of fluid volume and blood pressure
- Control of acid-base balance
- Secretion of prostaglandins
- Erythropoiesis (the production of red blood cells [RBCs])
- Conversion of vitamin D into the active form
- Excretion of substances such as medications, poisons, and food additives (Rogers & Brashers, 2023)
Pathophysiology
CKD decreases filtration and tubular functions, with the effects manifested throughout the body. The kidneys can adapt to nephron loss. Systemic changes occur due to increased creatinine, urea, and potassium. Salt and water alterations are not apparent until less than 25% of kidney function remains. There are different hypotheses that explain how kidney function can be maintained despite nephron loss. One theory is the intact nephron hypothesis, which theorizes that nephrons are capable of compensatory hypertrophy and expansion or hyperfunction. Because of this, the kidneys can maintain the rate of filtration, reabsorption, and secretion to regulate solute and water levels despite a declining GFR. Although the urine of a patient with CKD may contain higher than normal protein levels and red and white blood cells, the overall urine concentration is like that of an individual without CKD. Since the kidney is a very complex organ, the location of the damage also influences the severity of the disease. Although the factors contributing to CKD are complex and involve the relation of many different processes, two factors are thought to advance CKD: increased levels of angiotensin II and proteinuria (the presence of excess protein in the urine). The extended pressure on the efferent arterioles from the increase of angiotensin II increases permeability, contributing to proteinuria. Proteinuria promotes tubulointerstitial injury when protein accumulates in the interstitial space, activating the immune response and leading to inflammation and progressive fibrosis. Angiotensin II may also promote inflammation and growth factors, further contributing to fibrosis and scarring (Rogers & Brashers, 2023).
Risk Factors
The most common diseases associated with CKD include diabetes and hypertension; however, many factors increase a patient's risk of developing CKD (National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK], n.d.). The risk factors associated with CKD are described in Table 3.
Table 3
Risk Factors for CKD
Diabetes | Excess blood glucose, often found with uncontrolled diabetes, damages the kidneys. Over time, repeated exposure and resulting kidney damage become irreversible and affect the kidneys' ability to filter waste and excess fluid from the blood. One of the first indications that uncontrolled diabetes is causing CKD is the presence of albumin and protein in the urine. CKD caused by uncontrolled diabetes is known as diabetic kidney disease. Both type 1 and type 2 diabetes mellitus (T1DM, T2DM) can lead to CKD when left unchecked. IDDM and NIDDM similarly impact the kidneys; however, since NIDDM is not usually diagnosed until later in life, CKD caused by IDDM usually occurs in younger individuals (Mottl & Tuttle, 2025; NIDDK, 2023). |
Hypertension | Prolonged, uncontrolled hypertension can cause damage to the blood vessels of the kidneys. Excess fluid and waste cannot be adequately filtered from the blood when the blood vessels are damaged and accumulate in the circulatory system, increasing blood volume and raising BP, perpetuating a vicious cycle (Mann, 2024b; NIDDK, 2023). |
Elevated BMI | Having a BMI of over 25 indirectly increases the lifetime CKD risk by up to 25% compared to individuals with a BMI less than 24.9. This is due to an increased risk of hypertension and diabetes, the two most significant causes of CKD (Friedman et al., 2021; Nawaz et al., 2022). |
Family History | There are genetic conditions that predispose an individual to CKD, including:
Certain ethnicities are also at an increased risk for developing CKD due to an increased prevalence of diabetes and hypertension:
|
Systemic Illness | Autoimmune diseases describe a group of illnesses that cause the immune system to attack the body's healthy cells. Certain autoimmune diseases attack the kidneys and lead to CKD, including:
Other systemic illnesses that can lead to CKD include:
|
Other Diseases and Conditions | Many diseases and conditions are not as common but can lead to CKD when not adequately managed. These include:
|
Age | Although CKD can develop anytime, individuals over 60 are at a greater risk. Kidney function naturally decreases with age. Hypertension and diabetes are also more prevalent in older adults. It is estimated that more than 50% of individuals over the age of 75 have some form of kidney disease (NKF, n.d.-a). |
Medications | Drug-induced nephrotoxicity occurs more often in patients with underlying cardiovascular disease. Due to their over-the-counter (OTC) availability, NSAIDs are among the most used nephrotoxic drugs (Rosenberg, 2024). |
Signs and Symptoms
Patients with CKD stages 1 to 3 are often asymptomatic. It is not until CKD reaches stages 4 or 5 that patients begin to have signs and symptoms related to metabolic acidosis, uremia, anemia, and alterations in the ability of the kidney to maintain water and electrolyte balance within the circulatory system. Some effects of CKD are included in Table 4. As CKD progresses, more symptoms may arise (Arora, 2025a; CDC, 2024a).
Table 4
Signs and Symptoms of CKD
Body System | Signs and Symptoms |
Neurological |
|
Cardiovascular |
|
Respiratory |
|
Hematological |
|
Gastrointestinal |
|
Renal and Urologic |
|
Integumentary |
|
Musculoskeletal |
|
Psychological |
|
Reproductive |
|
(Arora, 2025a; CDC, 2024; Fatehi & Hsu, 2024; Goel et al., 2021; Guerra et al., 2021; Jabbar et al., 2021; Karahan & Şahin, 2022; Ridgway et al., 2022; Rogers & Brashers, 2023; Safarpour et al., 2023; Vaidya & Aeddula, 2024)
Diagnosis
The decision to screen a patient for CKD should be individualized. Routine screening is not recommended in asymptomatic patients with a low risk of developing CKD. All patients diagnosed with hypertension or NIDDM should be screened. Evaluating GFR and albuminuria allows for a complete evaluation of CKD. If kidney disease is suspected, there are numerous testing options to determine the cause and severity of kidney disease. In addition to a thorough physical examination and medical history, there are diagnostic tests and procedures to help the healthcare provider (HCP) diagnose kidney disease (CDC, 2023d; KDIGO, 2024). Examples of diagnostic options to determine kidney function are discussed below.
Blood Tests
Blood Urea Nitrogen (BUN)
Urea nitrogen is a waste product of the breakdown of dietary protein. A typical blood urea nitrogen (BUN) result is between 7 and 20 mg/dL. As kidney function decreases, the BUN level in the blood increases. An increased BUN result can also be attributed to dehydration, urinary tract obstruction, severe burns, a high-protein diet, congestive heart failure, or a recent heart attack. Due to these other etiologies of increased BUN, it is not used alone to diagnose kidney failure. The ratio between BUN and creatinine can determine if BUN elevation is due to prerenal or renal causes. When renal disease is present, the ratio of BUN to creatinine is close to 10:1 (American Board of Internal Medicine [ABIM], 2024; CDC, 2023d; Gounden et al., 2024).
Serum Creatinine
Serum creatinine (SCr) measures the amount of creatinine in the blood. Creatinine is a waste product of creatine phosphate in the muscles. Creatinine is released constantly, but levels vary depending on age, sex, and body size (muscle bulk). Creatinine is cleared almost entirely by the kidneys. When kidney function decreases, creatinine begins to build up in the blood. A serum creatinine level above 1.2 for patients assigned female at birth or 1.4 for those assigned male can indicate that the kidneys are not functioning correctly. As kidney disease progresses and kidney function deteriorates, the creatinine level rises in the blood. The SCr is also used to calculate the estimated GFR. However, using the SCr level may not accurately reflect kidney function since it measures both urinary excretion and any SCr production from muscle turnover. Measuring cystatin C levels is an alternate method to determine estimated kidney function (ABIM, 2024; CDC, 2023d; Gounden et al., 2024).
Cystatin C
Cystatin C is a low-molecular-weight protein constantly produced by all cells in the body with a nucleus and filtered by the kidneys. Serum cystatin C levels are inversely related to GFR, like creatinine; however, the kidneys filter cystatin C using a different process than creatinine. Once the kidneys filter cystatin C, it is reabsorbed and metabolized by the proximal renal tubules, which differs from creatinine. The advantage of testing cystatin C is that the results are less impacted by patient age, sex, race, and muscle mass relative to SCr levels. Cystatin C results are affected by cancer, thyroid disease, and current smoking history. It is recommended that cystatin C be measured at least once to confirm a diagnosis of CKD or to help refine disease staging (Gounden et al., 2024; Inker & Perrone, 2024a).
Glomerular Filtration Rate (GFR)
Glomerular filtration rate (GFR) is accepted as the best overall index of kidney function. GFR measures how well the kidneys remove waste and excess fluid from the blood. It is a calculation based on SCr, cystatin C, or both, and considers the patient's age and sex. Since age is included in the GFR calculation, the rate can decrease as age increases. A GFR of less than 60 mL/min/1.73 m2 indicates that the kidneys are not functioning correctly. Once the GFR drops below 15, the patient is considered to have end-stage kidney disease (ESKD) (CDC, 2023d; Gounden et al., 2024). There are a variety of equations that HCPs can use to determine a patient's GFR. GFR value and staging of CKD are shown in Table 5.
Table 5
Stage of CKD-GFR Categories
Category | GFR mL/min/1.73 m2 | Description |
G1 | 90 or more | Normal or high |
G2 | 60–89 | Mildly decreased |
G3a | 45–59 | Mildly to moderately decreased |
G3b | 30–44 | Moderately to severely decreased |
G4 | 15–29 | Severely decreased |
G5 | 15 or less | End-stage kidney disease |
(Arora, 2025a; NKF, n.d.-b)
Urine Tests
Urinalysis
A urinalysis (UA) includes a gross assessment, a microscopic evaluation of the urine, and a dipstick test. The dipstick is chemically treated to react when in contact with specific components not typically found in urine, including protein, blood, pus, bacteria, and sugar. A UA is not a specific test for CKD; however, certain components in the urine can indicate a problem with the kidneys, indicating that further testing is warranted (CDC, 2023d; Fatehi & Hsu, 2024; Wald, 2024).
A gross assessment that includes color, turbidity, and odor can help detect hematuria, myoglobinuria, infection, or rare disorders such as maple syrup urine disease or phenylketonuria. The basic urine dipstick test detects specific gravity, pH, heme, leukocyte esterase, nitrite, protein, and glucose. The clinician places a dipstick, or strip of chemically treated paper, into the urine sample for this test. The protein test is not sensitive to non-albumin proteins and is most helpful in assessing for albuminuria (i.e., albumin in the urine). To assess for non-albumin proteins, a separate sulfosalicylic acid test should be done. Patients with diabetes or hypertension should have an albuminuria test or albumin-to-creatinine ratio (ACR) test (discussed below) at the time of diagnosis and then annually. Patients with other risk factors, including cardiovascular disease, advanced age, elevated BMI, and a family history of CKD, should be screened based on patient presentation. There is limited data on the benefits versus risks of screening for CKD in asymptomatic adults (CDC, 2023d; Fatehi & Hsu, 2024; Wald, 2024).
Albumin-to-Creatinine Ratio (ACR)
The albumin-to-creatinine ratio (ACR) evaluates kidney function by dividing the amount of albumin in the urine by the amount of creatinine in the urine. There are three stages of albuminuria: A1, A2, and A3 (refer to Table 6). The ACR and GFR are used to determine CKD staging (NKF, n.d.-b).
Table 6
Albuminuria Categories
Category | ACR | Description |
A1 | Less than 30 mg/g or 3 mg/mmol | Normal to mildly increased |
A2 | 30-300 mg/g or 3–30 mg/mmol | Moderately increased |
A3 | Greater than 300mg/g or 30mg/mmol | Severely increased |
(KDIGO, 2024; Rovin, 2025; Wald, 2024)
Creatinine Clearance
Creatinine clearance (CrCl) is the volume of blood cleared of creatinine per unit of time. This test determines how much waste products the kidneys filter out of the blood each minute. CrCl is measured by comparing the creatinine in the blood versus in the urine. A 24-hour urine collection and a single blood sample must be obtained to determine CrCl. Once the 24-hour urine results are analyzed, the creatinine level in the urine is compared to the level in the blood. The serum sample must be obtained within 24 hours of the urine collection. A normal CrCl is 90 to 140 mL/min/1.73m2 (ABIM, 2024; Corder et al., 2024; Inker & Perrone, 2024b; Rovin, 2025; Shahbaz et al., 2024).
Imaging Tests
Imaging tests such as ultrasounds and computed tomography (CT) scans can be used to visualize the kidneys. B-mode renal ultrasound is the preferred radiologic study for initial workup in patients with suspected CKD. An ultrasound can detect parenchymal or cystic disease and can also identify kidney obstruction due to a stone, cyst, or tumor. A CT scan may be combined with an ultrasound to assess structural abnormalities or obstructions. A CT scan is typically performed with contrast to get the highest quality images; however, contrast dye in patients with decreased kidney function may be contraindicated (Fatehi & Hsu, 2024; O'Neill, 2024).
Biopsy
There are many reasons why a kidney biopsy may be performed. A renal biopsy allows the HCP to identify the underlying disease process causing the renal impairment. This procedure also allows the HCP to identify and implement the most effective treatments. A kidney biopsy can also give the HCP a better understanding of the extent of the kidney damage, which may influence treatment options. For patients who have received a transplanted kidney, a biopsy can give the HCP information about why the organ is no longer functioning properly (Whittier & Korbet, 2024).
Guidelines
CKD staging is classified based on the cause (C), GFR category (G, refer to Table 8), and albuminuria category (A, refer to Table 9). This is collectively referred to as CGA staging. Diagnosis of CKD requires a GFR of less than 60 mL/min/1.73 m2 (stage G3a to G5) or markers of kidney damage that have been present for over 3 months. The duration criterion of 3 months is necessary to distinguish CKD from acute kidney injury (AKI) (KDIGO, 2024; Levey & Inker, 2025).
Treatment and Management
Non-Pharmacological Management
Dietary Modifications
Dietary modifications, known as medical nutrition therapy (MNT), are a cornerstone of CKD treatment. MNT for CKD includes a daily protein intake of less than 1 g/kg to slow CKD progression. Protein intake should be decreased further to 0.8 g/kg/day in adults with diabetes or those with a GFR less than 30 mL/min/1.73 m2 (stage G4 to G5). Salt intake should be limited to less than 2 g/day. When a patient has CKD, potassium and phosphorus can build up in the cardiovascular system. Calcium can be withdrawn from the bones to balance phosphorus levels when they are too high. This makes the bones weak, brittle, thin, and prone to fractures. High phosphorus levels may also cause pruritus and bone/joint pain. Therefore, patients should be instructed to avoid foods high in phosphorus, such as meat, poultry, fish, dairy, beans, beer, canned teas, dark sodas, and nuts. Instead, patients should be encouraged to eat fresh fruits and vegetables; bread, pasta, and rice; rice milk; corn and rice cereals; and light-colored sodas or homemade iced tea. Elevated potassium levels may result in profound cardiac implications, including arrhythmias. Potassium should be limited in the diet by avoiding oranges, bananas, and orange juice; potatoes and tomatoes; brown and wild rice; bran cereals; dairy foods; bran, whole-grain bread, and pasta; and beans and nuts. By contrast, foods low in potassium include apples and peaches; celery and lettuce; white bread and pasta; white rice; rice milk; and apple, grape, or cranberry juices. Because the kidneys also excrete magnesium, patients with CKD are at an increased risk of developing hypermagnesemia. In severe cases, patients should avoid foods high in magnesium, such as fortified breakfast cereals, dairy, spinach, leafy greens, and nuts (almonds, cashews, and peanuts) (Cupisti et al., 2020; KDIGO, 2024; Lightfoot et al., 2023; National Institutes of Health, 2020; NIDDK, 2023; NKF, 2023a, 2024b).
Consistent Physical Activity
Individuals with CKD should be encouraged to engage in physical activity appropriate for their cardiovascular health. The goal is 30 minutes of moderate activity five times per week. Patients with ESKD on dialysis should engage in aerobic exercises, improving fitness, strength, and quality of life. Resistance training can be beneficial in patients with earlier stages of CKD. Physical activity can also help patients maintain a BMI of 20 to 25 (KDIGO, 2024; Lightfoot et al., 2023; Navaneethan et al., 2025; NIDDK, 2023).
Adequate Sleep
Patients should aim for 7 to 8 hours of sleep per night. Sleep is an essential factor in overall physical and mental health. Adequate and restful sleep can also decrease BP and help patients achieve blood glucose goals (NIDDK, 2023).
Smoking Cessation
Smoking can further damage the kidneys, making CKD worse. Smoking cessation can also positively affect BP, which is beneficial to kidney function. Patients who successfully quit smoking also decrease their risk of experiencing a heart attack or stroke (NIDDK, 2023).
Pharmacological Management
Patients with CKD should not take herbal remedies and should consult their HCP or pharmacist before taking any OTC medications (KDIGO, 2024; Rosenberg, 2024).
Diabetes
The target hemoglobin A1c (HbA1c) to prevent or delay CKD progression is 7%. If a patient has comorbidities, a limited life expectancy, or is at increased risk for hypoglycemia, the target HbA1c may be above 7%. Metformin (Glucophage) is recommended as a first-line treatment to manage NIDDM in patients with CKD stages 1 to 3. Metformin (Glucophage) works by decreasing the hepatic production and intestinal absorption of glucose and increasing insulin sensitivity. Metformin (Glucophage) does not slow the progression of CKD. A sodium-glucose co-transporter 2 (SGLT2) inhibitor can be used as an add-on treatment for NIDDM in patients with CKD stages 1 to 3 to slow the progression of the disease and decrease the risk of cardiovascular events (this is discussed further below). Metformin (Glucophage) as monotherapy reduces the risk of hypoglycemia versus independent treatment with insulin. Metformin (Glucophage) is also easier for patients to administer, is less expensive, and is more convenient than insulin. Metformin (Glucophage) is contraindicated in patients with a GFR below 30 mL/min/1.73 m2. Metformin (Glucophage) can be utilized at the standard dose in patients with a GFR above 45 mL/min/1.73 m2. When the GFR is between 30 and 45 mL/min/m2, the HCP must determine if the benefits of continuing metformin outweigh the potential risks. Adverse effects of using metformin (Glucophage) in patients with decreased GFR include lactic acidosis and gastrointestinal effects such as diarrhea. Metformin (Glucophage) can cause hypoglycemia, nausea, diarrhea, vomiting, dizziness, weakness, and a slow and irregular heart rate (Berns & Glickman, 2024; KDIGO, 2024; Perkovic & Badve, 2024; UpToDate Lexidrug, n.d.-b).
In 2021, the US Food and Drug Administration (FDA) approved an SGLT2 inhibitor, dapagliflozin (Farxiga), to slow CKD progression. Dapagliflozin (Farxiga) also reduces the risk of death due to cardiovascular disease and hospitalization for heart failure in patients with CKD. The medication works by decreasing glucose absorption from the proximal renal tubule and increasing the amount of glucose excreted through the urine. Adverse effects include ketoacidosis, dehydration, urinary tract infections, hypoglycemia, and vaginal yeast infections (FDA, 2021; UpToDate Lexidrug, n.d.-a).
Hypertension
Managing hypertension is a priority when treating patients diagnosed with or at risk for CKD. Target BP results should be individualized according to patient demographics such as age, past medical history, and response to treatment. When treating hypertension in patients with CKD, it is essential to monitor the patient for postural dizziness and hypotension (Georgianos & Agarwal, 2023; KDIGO, 2024; Mann, 2024a; Perkovic & Badve, 2024).
ACE inhibitors prevent the production of angiotensin II, leading to vasodilation and decreased BP. ACE inhibitors include benazepril (Lotensin), captopril (Capoten), enalapril (Vasotec), and lisinopril (Prinivil, Zestril), to name a few. Common side effects of ACE inhibitors include a dry nagging cough, hyperkalemia, fatigue, dizziness, headaches, and loss of taste. In rare cases, ACE inhibitors may cause angioedema (swelling of the lips, tongue, or face), requiring discontinuation. ACE inhibitors are contraindicated in patients with hyperkalemia, bilateral renal artery stenosis, or angioedema (Arcangelo et al., 2022; Hedge et al., 2023; KDIGO, 2024; Mann, 2024a; Mann & Flack, 2024; Townsend, 2024).
Switching to an angiotensin receptor blocker (ARB) may be beneficial for patients who report a cough using ACE inhibitors. ARBs include, but are not limited to valsartan (Diovan), losartan (Cozaar), and candesartan (Atacand). ARBs decrease BP through competitive antagonist activity at angiotensin II receptor sites. All ARBs are dosed once daily; however, if BP is not controlled, losartan (Cozaar) may be dosed twice daily. Side effects include dizziness, headache, hyperkalemia, angioedema, nausea, vomiting, severe diarrhea, and weight loss. ARBs are contraindicated in patients with a life-threatening reaction to ARBs, SBP less than 80 mm Hg, hyperkalemia, or renal artery stenosis (Arcangelo et al., 2022; KDIGO, 2024; Townsend, 2024).
Anemia
Anemia is a complication associated with CKD due to inadequate erythropoietin production by the kidneys. Anemia in patients over 15 years of age with CKD occurs when the hemoglobin level drops below 13.0 g/dL in patients assigned male at birth and 12.0 g/dL in those assigned female. Folic acid and ferrous sulfate (iron) supplementation may increase healthy red blood cell production. Hgb that drops below 10 g/dL should be treated with an erythropoiesis-stimulating agent (ESA) such as epoetin alfa (Procrit, Epogen) or darbepoetin alfa (Aranesp). These medications stimulate the bone marrow to produce more RBCs (Arora, 2025b; Berns, 2024). Adverse effects of epoetin alfa (Procrit, Epogen) treatment include hypertension, arthralgia, muscle spasms, dizziness, dyspnea, peripheral edema, cough, upper respiratory infection, and an increased risk of seizures. Epoetin alfa (Procrit, Epogen) and darbepoetin alfa (Aranesp) are both contraindicated in patients with hypertension, pure red cell aplasia following treatment with ESAs, or a history of allergic reaction to the medication. Blood transfusions may be needed in patients with acute blood loss or severe, symptomatic anemia (Berns, 2024).
Bone Health
Paricalcitol (Zemplar) is a synthetic vitamin D analog used to prevent and treat secondary hyperparathyroidism associated with CKD stages 3 and 4 and patients with CKD stage 5 who are currently being treated with hemodialysis or peritoneal dialysis. It can also decrease the amount of protein excreted by the kidneys in patients with CKD stages 2 to 5. Adverse effects include diarrhea, vomiting, nausea, dizziness, hypertension, and peripheral edema (Arora, 2025b; UpToDate Lexidrug, n.d.-c).
Phosphate binders can be given with meals to eliminate excess phosphorus from the body. A phosphate binder works by binding the phosphorus in the stomach, preventing it from being absorbed into the bloodstream, and excreting it through the stool. These medications must be taken 5 to 10 minutes before or immediately after each meal, including snacks (Aurora, 2021b). There are different categories of phosphate binders, including the following (Hollier, 2021; Quarles & Kendrick, 2024; Shah et al., 2024; UpToDate Lexidrug, n.d.-d).
- Calcium-based phosphorus binders include calcium acetate (PhosLo) and calcium carbonate (Tums). Calcium-based phosphorus binders have widely replaced aluminum-based binders. They also function as a calcium supplement. Calcium acetate (PhosLo) coupled with dietary modifications is the first-line treatment of hyperphosphatemia. There is a risk of hypercalcemia due to the number of phosphate binders the patient must take throughout the day, requiring frequent monitoring of serum calcium levels. Adverse effects of calcium-based binders include nausea, diarrhea, vomiting, and constipation.
- Aluminum-free, calcium-free phosphorus binders trap phosphorus in the gastrointestinal tract through ion exchange and hydrogen bonding. These medications include sevelamer (Renagel) and sevelamer carbonate (Renvela). Common side effects include vomiting, nausea, diarrhea, abdominal pain, flatulence, and constipation. Serious adverse effects include gastrointestinal bleeding, ulcer, colitis, bowel obstruction, necrosis, or perforation.
Metabolic Acidosis
Managing metabolic acidosis in patients with CKD may improve protein levels and bone metabolism. Patients with CKD and a serum bicarbonate of less than 22 mmol/L should be treated with oral sodium bicarbonate to decrease the risk of CKD progression and death. Sodium bicarbonate, or baking soda, is an antacid commonly used to relieve heartburn and indigestion. Common side effects include dry mouth, increased thirst, and polyuria (KDIGO, 2024; Kovesdy, 2024; Navaneethan et al., 2025; Palevsky, 2024; UpToDate Lexidrug, n.d.-e).
End-Stage Kidney Disease
CKD may progress until the kidneys begin to permanently fail, known as end-stage kidney disease (ESKD) (CKD stage 5). More than 500,000 individuals in the United States are diagnosed with ESKD. When kidney function becomes severely impaired at this level, the patient will not survive without intervention. The HCP must initiate some type of renal replacement therapy (RRT) and, if a good candidate, seek a kidney donor for a transplant. Starting dialysis or receiving a kidney transplant can increase life expectancy in patients with ESKD. RRT can increase life expectancy by five to nine years. Receiving a kidney from a donor can increase life expectancy by 5 to 24 years. Supportive care and symptom management are the primary treatment goals for patients who choose not to pursue RRT or kidney donation. Due to the debilitating nature of ESKD and the time commitment and disruption in daily life that occurs with RRT, patients diagnosed with ESKD are eligible for Medicare coverage regardless of age (Centers for Medicare & Medicaid Services, 2024; Hashmi et al., 2023; NIDDK, n.d.; Rosenberg, 2024).
Patient Monitoring
Patients with ESKD must be monitored for hypertension, tachycardia, dysrhythmias, and tachypnea. Daily weights are a good indicator of fluid loss or gain, so patients should weigh themselves at the same time using the same scale every day. If 1 kg (2 to 3 lb) of weight gain is noted in a day, the patient is retaining approximately 1000 mL of extra fluid. Fluid restrictions may be required if the patient is experiencing symptoms of fluid volume overload. Further, patients should be monitored for metabolic acidosis, proteinuria, hematuria, and urinary casts (Hashmi et al., 2023; NKF, 2023b; Wouk, 2021).
Neurological assessment is critical, so the HCP should closely monitor the patient's level of consciousness, as uremia can lead to confusion or coma. Because patients with ESKD are also at higher risk of developing infections, the HCP must monitor the patient's vital signs for fever and CBC for leukocytosis (elevation of white blood cells [WBCs] above 11.0x109/L). Furthermore, patients with ESKD tend to be oliguric or anuric (refer to Table 4), so urine output and symptoms of fluid volume overload, such as wheezing, rhonchi, edema, peripheral swelling, hypertension, tachycardia, and jugular venous distention (JVD) should be monitored. Frequent oral care will help prevent stomatitis and decrease discomfort. A buildup of urea can lead to uremic frost and pruritus, so appropriate skincare with medicated creams and lotions may be required (Mank et al., 2024; Rosenberg, 2024).
Electrolyte Monitoring
Electrolyte imbalances are common in patients with ESKD. They frequently develop hyperkalemia, hypermagnesemia, or hyperphosphatemia as the kidneys can no longer excrete excess potassium, magnesium, and phosphorus (ABIM, 2024; Hashmi et al., 2023; Ikizler et al., 2020; Mount, 2024; Navaneethan et al., 2025; Rosenberg, 2024; Yu et al., 2024).
Renal Replacement Therapy
Renal replacement therapy (RRT) refers to the use of life-supporting treatment for ESKD. RRT can replace the nonendocrine–related functions of the kidneys. There are different types of RRT, including intermittent hemodialysis, continuous hemofiltration and hemodialysis, and peritoneal dialysis (Arora, 2025b; Hechanova, 2024b). RRT should be initiated when one or more of the following are uncontrollable in a patient with ESKD.
- Fluid overload (including refractory heart failure)
- Abnormal electrolyte levels
- Metabolic acidosis
- Pericarditis
- Uremic symptoms
- GFR of less than 10 mL/min/1.73 m2 without diabetes or less than 15 mL/min/1.73 m2 with diabetes (Arora, 2025b; Hechanova, 2024b; KDIGO, 2024)
Although the goal of RRT is to mimic the function of the kidneys and filter the patient's blood, no form of RRT is equivalent in efficiency to a healthy, functioning kidney (Hechanova, 2024b).
Hemodialysis
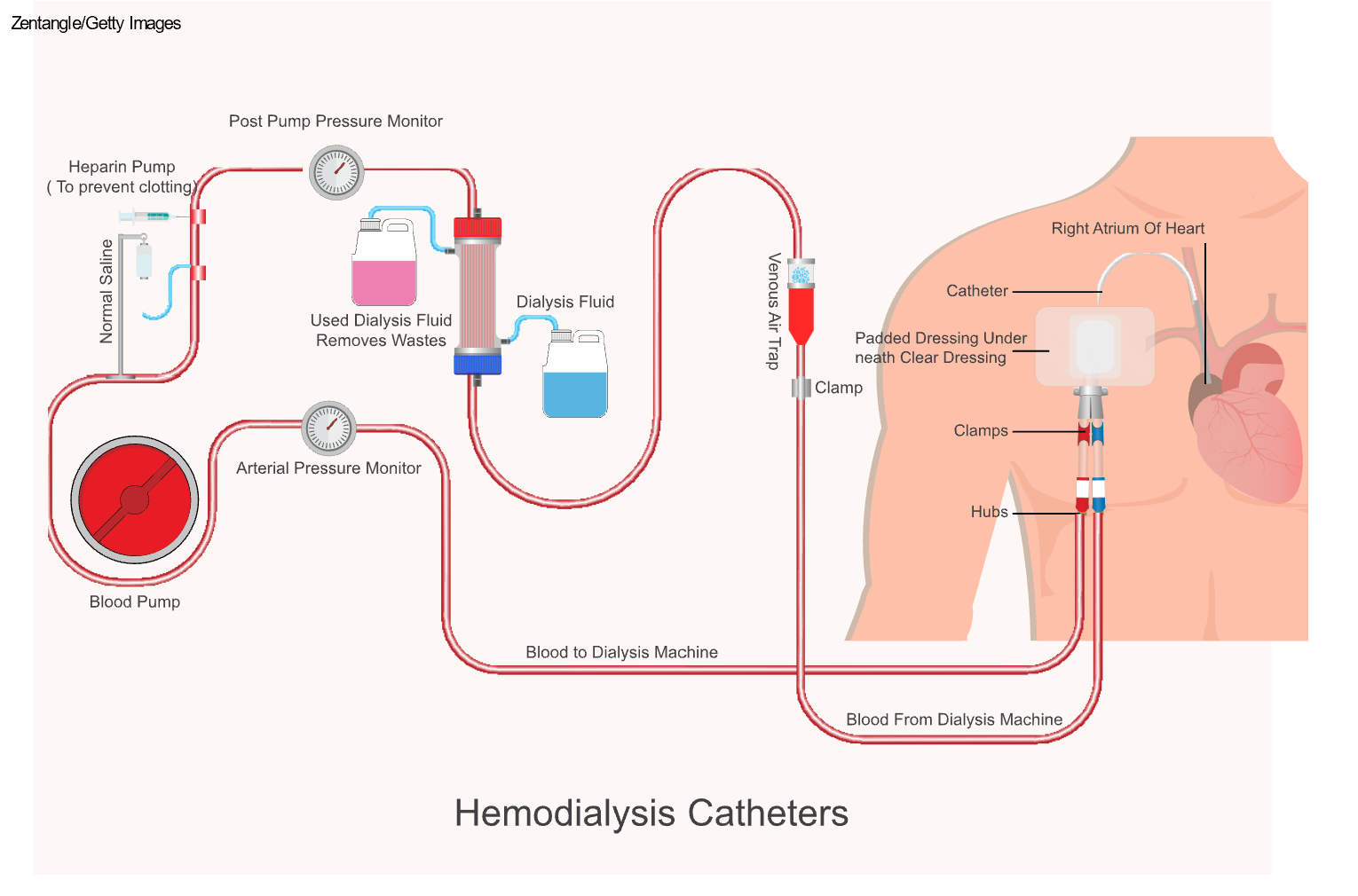
Hemodialysis is a treatment used for patients with ESKD to filter wastes and water out of the blood. Hemodialysis can help manage BP and electrolyte balance, including potassium, sodium, and calcium. As previously stated, hemodialysis increases the life expectancy of patients with ESKD, but it is not a cure. During hemodialysis, the patient's blood is removed from their body via an IV catheter, graft, or fistula, and then pumped through a filter known as a dialyzer, commonly referred to as an artificial kidney. For patients with a dialysis catheter, one lumen connects to an artery and the other to a vein. If the patient has a fistula or graft, one needle is inserted into the fistula or graft's arterial side and one into the fistula or graft's venous side. The dialysis machine pumps the blood through the dialyzer into very thin, hollow fibers and back into the patient's body (refer to Figure 3). While flowing through the fibers, a dialysis solution, known as dialysate, passes in the opposite direction, and waste products move from the blood into the dialysate via osmosis (Hechanova, 2024a; NIDDK, 2023).
Figure 3
Hemodialysis Circuit
Hemodialysis can be performed in different settings and at various frequencies. Many patients have hemodialysis performed at a dialysis center 3 days per week (Monday, Wednesday, and Friday, or Tuesday, Thursday, and Saturday). Some dialysis centers offer overnight hours to accommodate patients with other commitments during the day. Treatment occurs while the patient is sleeping, leaving their days free. Another option is home dialysis. This option allows patients to have longer or more frequent (i.e., 3 to 7 days per week) dialysis treatments, which better mimic the work of the kidneys. Completing more frequent treatments allows the patient to maintain a fluid intake that is closer to normal, decreases the need for antihypertensives, and reduces some side effects, including muscle cramps, BP fluctuations, and hyperphosphatemia (Hechanova, 2024a; NIDDK, 2023).
Peritoneal Dialysis
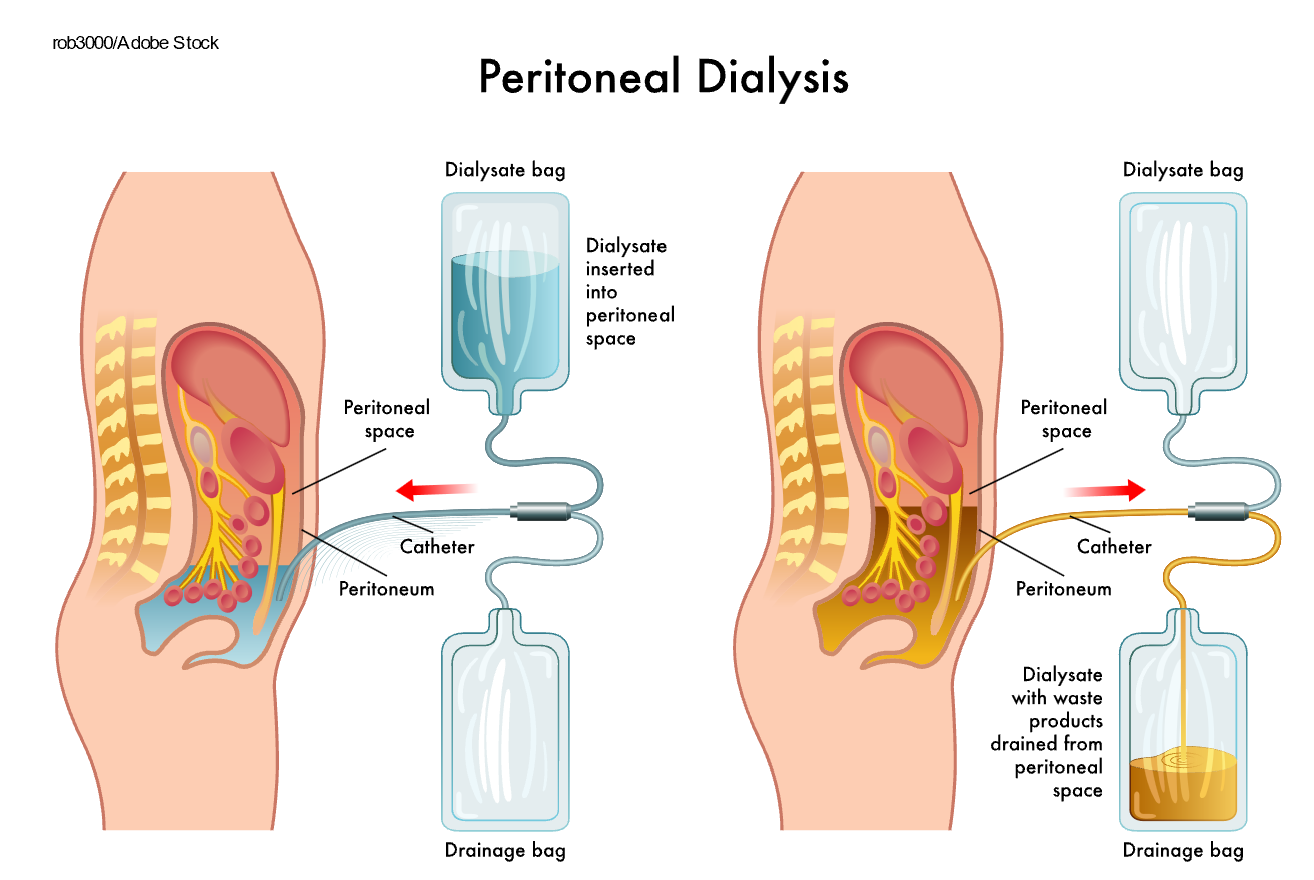
Peritoneal dialysis is a treatment for ESKD that uses the patient's peritoneum to filter the blood inside the body. A catheter is inserted surgically into the peritoneum. Then, a specifically formulated dialysate is instilled into the abdomen. Once the solution is instilled, the patient can disconnect, cap the catheter, and engage in regular daily activities. Peritoneal dialysis is completed on a schedule dictated by the patient's nephrologist, usually four to six times daily. Once the prescribed dwell time is reached, the patient drains the dialysate fluid into an empty drainage bag and instills fresh dialysate into the abdomen (refer to Figure 4). The patient exchanges the dialysate regularly throughout the day, known as continuous ambulatory peritoneal dialysis. Alternatively, they can use a machine known as a cycler to complete the process overnight while they sleep, known as automated peritoneal dialysis. Like hemodialysis, peritoneal dialysis increases the patient's life expectancy and decreases symptoms; however, it is not a cure for ESKD (Hechanova, 2024c; NIDDK, 2023).
Figure 4
Peritoneal Dialysis
One of the most severe complications related to peritoneal dialysis is infection. It is possible to get an infection of the skin surrounding the catheter site or the peritoneum (i.e., peritonitis). Signs of a catheter site infection include purulent drainage, erythema, swelling, tenderness, or bulging of the site. Prophylactic application of topical antibiotic cream or ointment is recommended. If drainage is not present, infection can be treated with topical antiseptics such as povidone iodine or chlorhexidine. For persistent infections or those with purulent drainage, vancomycin (Vancocin) is recommended empirically with subsequent therapy guided by culture results. Signs of peritonitis include abdominal pain, fever, nausea, and vomiting. The patient may also notice cloudiness or an unusual color of the used dialysate fluid or the cuff of the catheter beginning to emerge from the insertion site. When the clinical presentation suggests peritonitis, the outflowed peritoneal dialysis fluid should be collected for a cell count, differential count, gram stain, and culture. Antibiotics can be added directly to the dialysate to treat peritonitis. Cefazolin (Ancef) or vancomycin (Vancocin) are the treatments of choice for gram-positive bacterial infections. For gram-negative bacterial infections, third-generation cephalosporins or aminoglycosides are the treatments of choice. Another complication of peritoneal dialysis includes a hernia, an area of weakness in the abdominal muscles. Hernia risk is increased due to the surgical opening in the abdominal muscles and the considerable weight of the dialysate during indwelling. Hernias often occur in the umbilical area, near the catheter site, or in the groin (Hechanova, 2024c; Li et al., 2022; NIDDK, 2023; Rocco & Burkart, 2023).
Conservative Management
For patients not interested in RRT, conservative management should be considered. All health care team members should be able to assist the patient with advanced care planning and recognize when there is a need for end-of-life care. Palliative, end-of-life care should focus on symptom management and psychological and spiritual support for the patient and family members (KDIGO, 2024; Rosenberg, 2024).
Transplant
Of the over 103,000 Americans on the nation's organ transplant registry awaiting an organ transplant, more than 89,000 are awaiting a kidney. Due to the shortage of donor organs, not every patient on the transplant registry will receive a kidney transplant. Kidney donation can occur from deceased or living donors. Placing a patient on the organ transplant registry should be done preemptively for most patients with CKD G4 or G5 (GFR less than 30 mL/min/1.73 m2, with clinical data indicating irreversible progression of the CKD over the preceding 6 to 12 months). Transplantation from a living donor is the preferred treatment for transplant-eligible patients. Once the GFR drops below 10 mL/min/1.73 m2 (or earlier if symptoms are present), transplantation with either a living or deceased donor is recommended (Chadban et al., 2020; Health Resources & Services Administration, 2025; KDIGO, 2024; NKF, n.d.-a; Rossi & Cheng, 2024).
Patients with multiple myeloma, amyloidosis with extensive prerenal involvement, decompensated cirrhosis, severe irreversible obstructive or restrictive lung diseases, severe untreatable and symptomatic cardiac disease deemed by a cardiologist to preclude the patient from transplantation, and progressive central neurodegenerative disease should not be referred for kidney-alone transplant evaluation; those with cirrhosis should be considered for a combined liver-kidney transplant (Chadban et al., 2020; KDIGO, 2024; NKF, n.d.-a; Rossi & Cheng, 2024). Evaluation for kidney transplantation should be delayed in patients with the following conditions until they are appropriately managed.
- Unstable psychiatric conditions that affect decision-making
- Ongoing substance-use disorder
- Nonadherence to treatment
- Active infection (excluding hepatitis C)
- Active malignancy; except for low-grade cancers (i.e., prostate cancer)
- Active symptomatic cardiac disease (i.e., angina, arrhythmia)
- Active symptomatic peripheral arterial disease
- Recent stroke or transient ischemic attack (TIA); kidney transplantation should be delayed at least 6 months following a stroke and 3 months following a TIA
- Active symptomatic gastrointestinal disease (i.e., peptic ulcer disease, diverticulitis, pancreatitis, gallbladder disease or gallstones, inflammatory bowel disease)
- Acute hepatitis
- Tobacco use
- Bone and mineral disorders (i.e., severe hyperparathyroidism) (Chadban et al., 2020; Rossi & Cheng, 2024)
Testing of a potential transplant candidate is extensive and includes:
- Laboratory testing: blood chemistries; liver function tests; complete blood count (CBC); infectious profile (hepatitis A, B, and C; Epstein-Barr virus; Cytomegalovirus; Varicella-zoster virus; HIV); UA and urine culture
- Cardiac evaluation: 12-lead EKG; chest x-ray; exercise stress test; echocardiogram
- Immunologic evaluation: ABO blood group determination; human leukocyte antigen typing; crossmatching (Chhabra, 2022; Rossi & Cheng, 2024)
Following kidney transplantation, the patient requires lifelong immunosuppression therapy to prevent an alloimmune rejection response. The treatment goals are to prevent acute or chronic rejection and achieve the highest possible patient and graft survival rate. There are different categories of rejection. Hyperacute rejection occurs within hours of the transplant. Acute rejection occurs within the first 6 months of transplantation and accounts for 15% of rejection cases. Chronic rejection occurs more than a year following transplantation and results in allograft loss. Potential complications following transplantation include recurrence of kidney disease, renal artery thrombosis or stenosis, infection, and ureteral stenosis and obstruction (Chhabra, 2022; Rossi & Cheng, 2024). Refer to the Organ Donation Nursing CE course for more information on organ donation.
Future Research
Diabetes is the leading cause of kidney damage. However, recent research highlights that only half of primary HCPs discuss CKD with their patients with diabetes. Therefore, it is critical to increase awareness amongst primary care providers and encourage early screening for CKD in patients with diabetes. Future research on CKD prevalence in adults with NIDDM is needed to optimize their care. To this end, the NKF has launched a multi-site, cross-sectional study, Awareness, Detection, and Drug Therapy in Type 2 Diabetes Mellitus and Chronic Kidney Disease (ADD-CKD). The researchers aim to assess how CKD is identified and managed in NIDDM patients in the primary care setting, using a survey of 10,000 adult patients and their 500 primary HCPs (Navaneethan et al., 2025; NKF, n.d.-c).
There is also an NKF-funded project underway to create and analyze a large dataset of patient outcomes at each stage of CKD. Because CKD is a progressive spectrum disease, patients experience varying symptoms and complications within each stage. This research examines how these various complications at each stage of CKD impact patient prognosis (NKF, n.d.-c).
Further research is needed on how to predict the progression of CKD through the various stages and eventually ESKD. Although there are factors associated with CKD progression, and those that are modifiable should be addressed, there is no current ranking of known risk factors to determine which is the most predictive of CKD progression. In addition, further research is needed to determine which mathematical formula is most predictive of a patient's likelihood of experiencing complications, such as cardiovascular events and progressive disease (KDIGO, 2024).
References
American Board of Internal Medicine. (2024). ABIM laboratory test reference ranges—January 2024. https://www.abim.org/Media/bjijryql/laboratory-reference-ranges.pdf
Arcangelo, V. P., Peterson, A. M., Wilbur, V. F., & Kang, T. M. (2022). Pharmacotherapeutics for advanced practice (5th ed.). Wolters Kluwer.
Arora, P. (2025a). Chronic kidney disease (CKD). https://emedicine.medscape.com/article/238798-overview
Arora, P. (2025b). Chronic kidney disease (CKD) treatment & management. https://emedicine.medscape.com/article/238798-treatment
Berns, J. S. (2024). Treatment of anemia in nondialysis chronic kidney disease. UpToDate. Retrieved March 18, 2025, from https://www.uptodate.com/contents/treatment-of-anemia-in-nondialysis-chronic-kidney-disease
Berns, J. S., & Glickman, J. D. (2024). Management of hyperglycemia in patients with type 2 diabetes and advanced chronic kidney disease or end-stage kidney disease. UpToDate. Retrieved March 19, 2025, from https://www.uptodate.com/contents/management-of-hyperglycemia-in-patients-with-type-2-diabetes-and-advanced-chronic-kidney-disease-or-end-stage-kidney-disease
Centers for Disease Control and Prevention. (2024a). Chronic kidney disease basics. US Department of Health and Human Services, Center for Disease Control and Prevention. https://www.cdc.gov/kidney-disease/about/?CDC_AAref_Val=https://www.cdc.gov/kidneydisease/basics.html
Centers for Disease Control and Prevention. (2024b). Chronic kidney disease in the United States, 2023. US Department of Health and Human Services, Center for Disease Control and Prevention. https://www.cdc.gov/kidney-disease/media/pdfs/CKD-Factsheet-H.pdf
Centers for Disease Control and Prevention. (2024c). Mortality in the United States, 2023. National Center for Health Statistics, Vital Statistics System. https://www.cdc.gov/nchs/data/databriefs/db521.pdf
Centers for Medicare and Medicaid Services. (2024). End-stage renal disease (ESRD). https://www.cms.gov/Medicare/Coordination-of-Benefits-and-Recovery/Coordination-of-Benefits-and-Recovery-Overview/End-Stage-Renal-Disease-ESRD/ESRD
Chadban, S. J., Ahn, C., Axelrod, D. A., Foster, B. J., Kasiske, B. L., Kher, V., Kumar, D., Oberbauer, R., Pascual, J., Pilmore, H. L., Rodrigue, J. R., Segev, D. L., Sheerin, N. S., Tinckam, K. J., Wong, G., & Knoll, G. (2020). Transplantation, 104(4S1), S11–S103. https://doi.org/10.1097/TP.0000000000003136
Chhabra, D. (2022). Assessment and management of the kidney transplant patient. https://emedicine.medscape.com/article/429314-overview
Corder, C. J., Rathi, B. M., Sharif, S., & Leslie, S. W. (2024). 24-hour urinalysis. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK482482/
Cupisti, A., Gallieni, M., Avesani, C. M., D'Alessandro, C., Carrero, J. J., & Piccoli, G. B. (2020). Medical nutritional therapy for patients with chronic kidney disease not on dialysis: The low protein diet as a medication. Journal of Clinical Medicine, 9(11), 3644. https://doi.org/10.3390/jcm9113644
Dalal, R., Bruss, Z. S., & Shedev, J. S. (2023). Physiology, renal blood flow and filtration. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK482248/
Fatehi, P., & Hsu, C. Y. (2024). Chronic kidney disease (newly identified): Clinical presentation and diagnostic approach in adults. UpToDate. Retrieved March 18, 2025, from https://www.uptodate.com/contents/chronic-kidney-disease-newly-identified-clinical-presentation-and-diagnostic-approach-in-adults
Friedman, A. N., Kaplan, L. M., le Roux, C. W., & Schauer, P. R. (2021). Management of obesity in adults with CKD. Journal of the American Society of Nephrology, 32(4), 777–790. https://doi.org/10.1681/ASN.2020101472
Georgianos, P. I., & Agarwal, R. (2023). Hypertension in chronic kidney disease-treatment standard 2023. Nephrology Dialysis Transplantation, 38(12), 2694–2703. https://doi.org/10.1093/ndt/gfad118
Goel, V., Sil, A., & Das, A. (2021). Cutaneous manifestations of chronic kidney disease, dialysis and post-renal transplant: A review. Indian Journal of Dermatology, 66(1), 3–11. https://doi.org/10.4103/ijd.IJD_502_20
Gounden, V., Bhatt, H., & Jialal, I. (2024). Renal function tests. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK507821/
Guerra, F., Di Giacomo, D., Ranieri, J., Tunno, M., Piscitani, L., & Ferri, C. (2021). Chronic kidney disease and its relationship with mental health: Allostatic load perspective for integrated care. Journal of Personalized Medicine, 11(12), 1367. https://doi.org/10.3390/jpm11121367
Hashmi, M. F., Benjamin, O., & Lappin, S. L. (2023). End-stage renal disease. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK499861/
Health Resources & Services Administration. (2025). Organ donation statistics. https://www.organdonor.gov/learn/organ-donation-statistics
Hechanova, L. A. (2024a). Hemodialysis. MSD Manual Professional Version. https://www.msdmanuals.com/professional/genitourinary-disorders/renal-replacement-therapy/hemodialysis
Hechanova, L. A. (2024b). Overview of renal replacement therapy. MSD Manual Professional Version. https://www.msdmanuals.com/professional/genitourinary-disorders/renal-replacement-therapy/overview-of-renal-replacement-therapy
Hechanova, L. A. (2024c). Peritoneal dialysis. MSD Manual Professional Version. https://www.msdmanuals.com/professional/genitourinary-disorders/renal-replacement-therapy/peritoneal-dialysis
Hedge, S., Ahmed, I., & Aeddula, N. R. (2023). Secondary hypertension. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK544305/
Hollier, A. (Ed.). (2021). Clinical guidelines in primary care (4th ed.). Advanced Practice Education Associates.
Ikizler, T. A., Burrowes, J. D., Byham-Gray, L. D., Campbell, K. L., Carrero, J. J., Chan, W., Fouque, D., Friedman, A. N., Ghaddar, S., Goldstein-Fuchs, D. J., Kaysen, G. A., Kopple, J. D., Teta, D., Wan, A., Y. M., & Cuppari, L. (2020). KDOQI clinical practice guideline for nutrition in CKD: 2020 update. American Journal of Kidney Diseases, 76(3, suppl. 1), S1–S107. https://www.ajkd.org/article/S0272-6386(20)30726-5/fulltext
Inker, L. A., & Perrone, R. D. (2024a). Assessment of kidney function. UpToDate. Retrieved March 19, 2025, from https://www.uptodate.com/contents/assessment-of-kidney-function
Inker, L. A., & Perrone, R. D. (2024b). Calculation of the creatinine clearance. UpToDate. Retrieved March 19, 2025, from https://www.uptodate.com/contents/calculation-of-the-creatinine-clearance
Jabbar, A., Qureshi, R., Nasir, K., Dhrolia, M., & Ahmad, A. (2021). Transudative and exudative pleural effusion in chronic kidney disease patients: A prospective single-center study. Cureus, 13(10), e18649. https://doi.org/10.7759/cureus.18649
Karahan, D., & Şahin, İ. (2022). Comparison of gastrointestinal symptoms and findings in renal replacement therapy modalities. BMC Nephrology, 23(1), 261. https://doi.org/10.1186/s12882-022-02893-6
Kidney Disease Improving Global Outcomes. (2024). KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Supplement to Kidney International, 105(4S), 1–199. http://kdigo.org/wp-content/uploads/2024/03/KDIGO-2024-CKD-Guideline.pdf
Kovesdy, C. P. (2024). Pathogenesis, consequences, and treatment of metabolic acidosis in chronic kidney disease. UpToDate. Retrieved March 18, 2025, from https://www.uptodate.com/contents/pathogenesis-consequences-and-treatment-of-metabolic-acidosis-in-chronic-kidney-disease
Levey, A. S., & Inker, L. A. (2025). Definition and staging of chronic kidney disease in adults. UpToDate. Retrieved March 19, 2025, from https://www.uptodate.com/contents/definition-and-staging-of-chronic-kidney-disease-in-adults
Li, P. K., Chow, K. M., Cho, Y., Fan, S., Figueiredo, A. E., Harris, T., Kanjanabuch, T., Kim, Y. L., Madero, M., Malyszko, J., Mehrotra, R., Okpechi, I. G., Perl, J., Piraino, B., Runnegar, N., Teitelbaum, I., Wong, J. K. W., Yu, X., & Johnson, D. W. (2022). ISPD peritonitis recommendations: 2022 update on prevention and treatment. Peritoneal Dialysis International, 42(2). https://doi.org/10.1177/08968608221080586
Madrazo-Ibarra, A., & Vaitla, P. (2023). Histology, nephron. StatPearls [Internet]. Retrieved March 18, 2025, from https://www.ncbi.nlm.nih.gov/books/NBK554411/
Mank, V., Azhar, W., & Brown, K. (2024). Leukocytosis. StatPearls [Internet]. Retrieved March 18, 2025, from https://ncbi.nlm.nih.gov/books/NBK560882
Mann, J. F. E. (2024a). Antihypertensive therapy and progression of nondiabetic chronic kidney disease in adults. UpToDate. Retrieved March 19, 2025, from https://www.uptodate.com/contents/antihypertensive-therapy-and-progression-of-nondiabetic-chronic-kidney-disease-in-adults
Mann, J. F. E. (2024b). Overview of hypertension in acute and chronic kidney disease. UpToDate. Retrieved March 19, 2025, from https://www.uptodate.com/contents/overview-of-hypertension-in-acute-and-chronic-kidney-disease
Mann, J. F. E., & Flack, J. M. (2024). Choice of drug therapy in primary (essential) hypertension. UpToDate. Retrieved March 18, 2025, from https://www.uptodate.com/contents/choice-of-drug-therapy-in-primary-essential-hypertension
Mottl, A. K., & Tuttle, K. R. (2025). Diabetic kidney disease: Manifestations, evaluation, and diagnosis. UpToDate. Retrieved March 19, 2025, from https://www.uptodate.com/contents/diabetic-kidney-disease-manifestations-evaluation-and-diagnosis
Mount, D. B. (2024). Treatment and prevention of hyperkalemia in adults. UpToDate. Retrieved March 18, 2025, from https://www.uptodate.com/contents/treatment-and-prevention-of-hyperkalemia-in-adults
National Institute of Diabetes and Digestive and Kidney Diseases. (n.d.). End stage renal disease: Chapter 6 mortality. National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Retrieved March 19, 2025, from https://usrds-adr.niddk.nih.gov/2023/end-stage-renal-disease/6-mortality
National Institute of Diabetes and Digestive and Kidney Diseases. (2020). Anti-GBM (Goodpasture's) Disease. National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/kidney-disease/glomerular-disease/anti-gbm-goodpastures-disease
National Institute of Diabetes and Digestive and Kidney Diseases. (2023). Kidney disease for health professionals. https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/kidney-disease
National Institutes of Health. (2020). Magnesium fact sheet for consumers. National Institutes of Health, Office of Dietary Supplements. https://ods.od.nih.gov/pdf/factsheets/Magnesium-Consumer.pdf
National Kidney Foundation. (n.d.-a). Aging and kidney disease. Retrieved March 18, 2025, from https://www.kidney.org/news/monthly/wkd_aging
National Kidney Foundation. (n.d.-b). How to classify CKD. Retrieved March 18, 2025, from https://www.kidney.org/professionals/explore-your-knowledge/how-to-classify-ckd
National Kidney Foundation. (n.d.-c). Research. Retrieved March 18, 2025, from https://www.kidney.org/professionals/research
National Kidney Foundation. (2023a). Potassium In your CKD diet. https://www.kidney.org/kidney-topics/potassium-your-ckd-diet
National Kidney Foundation. (2023b). The dos and don’ts of fluid management for kidney disease. https://www.kidney.org/news-stories/dos-and-don-ts-fluid-management-kidney-disease
National Kidney Foundation. (2024a). Kidney disease: Fact sheet. https://www.kidney.org/about/kidney-disease-fact-sheet
National Kidney Foundation. (2024b). Phosphorus and your diet. https://www.kidney.org/kidney-topics/phosphorus-and-your-diet
Navaneethan, S. D., Bansal, N., Cavanaugh, K. L., Chang, A., Crowley, S., Delgado, C., Estrella, M. M., Ghossein, C., Ikizler, T. A., Koncicki, H., St. Peter, W., Tuttle, K. R., & William, J. (2025). KDOQI US commentary on the KDIGO 2024 clinical practice guideline for the evaluation and management of CKD. American Journal of Kidney Diseases, 85(2), 135–176. https://doi.org/10.1053/j.ajkd.2024.08.003
Nawaz, S., Chinnadurai, R., Al-Chalabi, S., Evans, P., Kalra, P. A., Syed, A. A., & Sinha, S. (2022). Obesity and chronic kidney disease: A current review. Obesity Science & Practice, 9(2), 61–74. https://doi.org/10.1002/osp4.629
Obrador, G. T. (2025). Epidemiology of chronic kidney disease. UpToDate. Retrieved March 18, 2025, from https://www.uptodate.com/contents/epidemiology-of-chronic-kidney-disease
O'Neill, W. C. (2024). Radiologic assessment of kidney disease. UpToDate. Retrieved March 18, 2025, from https://www.uptodate.com/contents/radiologic-assessment-of-kidney-disease
Palevsky, P. M. (2024). Kidney replacement therapy (dialysis) in acute kidney injury in adults: Indications, timing, and dialysis dose. UpToDate. Retrieved March 20, 2025, from https://www.uptodate.com/contents/kidney-replacement-therapy-dialysis-in-acute-kidney-injury-in-adults-indications-timing-and-dialysis-dose
Perkovic, V., & Badve, S. V. (2024). Treatment of diabetic kidney disease. UpToDate. Retrieved March 19, 2025, from https://www.uptodate.com/contents/treatment-of-diabetic-kidney-disease
Quarles, L. D., & Kendrick, J. (2024). Management of hyperphosphatemia in adults with chronic kidney disease. UpToDate. Retrieved March 18, 2025, from https://www.uptodate.com/contents/management-of-hyperphosphatemia-in-adults-with-chronic-kidney-disease
Ridgway, A., Cotterill, N., Dawson, S., Drake, M. J., Henderson, E. J., Huntley, A. L., Rees, J., Strong, E., Dudley, C., & Udayaraj, U. (2022). Nocturia and chronic kidney disease: Systematic review and nominal group technique consensus on primary care assessment and treatment. European Urology Focus, 8(1), 18–25. https://doi.org/10.1016/j.euf.2021.12.010
Rocco, M., & Burkart, J. M. (2023). Abdominal wall hernia and dialysate leak in peritoneal dialysis patients. UpToDate. Retrieved March 20, 2025, from https://www.uptodate.com/contents/abdominal-wall-hernia-and-dialysate-leak-in-peritoneal-dialysis-patients
Rogers, J. L. and Brashers, V. L. (Eds.). (2023). McCance and Huether's pathophysiology: The biologic basis for disease in adults and children (9th ed.). Elsevier.
Rosenberg, M. (2024). Overview of the management of chronic kidney disease in adults. UpToDate. Retrieved March 18, 2025, from https://www.uptodate.com/contents/overview-of-the-management-of-chronic-kidney-disease-in-adults
Rossi, A. P., & Cheng, X. S. (2024). Kidney transplantation in adults: Evaluation of the potential kidney transplant recipient. UpToDate. Retrieved March 20, 2025, from https://www.uptodate.com/contents/kidney-transplantation-in-adults-evaluation-of-the-potential-kidney-transplant-recipient
Rovin, B. H. (2025). Evaluation of proteinuria in adults. UpToDate. Retrieved March 18, 2025, from https://www.uptodate.com/contents/evaluation-of-proteinuria-in-adults
Safarpour, Y., Vaziri, N. D., & Jabbari, B. (2023). Restless legs syndrome in chronic kidney disease- a systematic review. Tremor and Other Hyperkinetic Movements (New York, N.Y.), 13, 10. https://doi.org/10.5334/tohm.752
Shah, A., Hashmi, M. F., & Aeddula, N. R. (2024). Chronic kidney disease-mineral bone disorder (CKD-MBD). StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK560742/
Shahbaz, H., Rout, P., & Gupta, M. (2024). Creatinine clearance. StatPearls. Retrieved March 18, 2025, from https://ncbi.nlm.nih.gov/books/NBK544228
UpToDate Lexidrug. (n.d.-a). Dapagliflozin: Drug information. Retrieved March 18, 2025, from https://www.uptodate.com/contents/dapagliflozin-drug-information
UpToDate Lexidrug. (n.d.-b). Metformin: Drug information. Retrieved March 18, 2025, from https://www.uptodate.com/contents/metformin-drug-information
UpToDate Lexidrug. (n.d.-c). Paricalcitrol: Drug information. Retrieved March 18, 2025, from https://www.uptodate.com/contents/paricalcitol-drug-information
UpToDate Lexidrug. (n.d.-d). Sevelamer: Drug information. Retrieved March 20, 2025, from https://www.uptodate.com/contents/sevelamer-drug-information
UpToDate Lexidrug. (n.d.-e). Sodium bicarbonate: Drug information. Retrieved March 20, 2025, from https://www.uptodate.com/contents/sodium-bicarbonate-drug-information
US Food and Drug Administration. (2021). FDA approves treatment for chronic kidney disease. https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-chronic-kidney-disease
Vaidya, S. R., & Aeddula, N. R. (2024). Chronic kidney disease. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK535404/
Wald, R. (2024). Urinalysis in the diagnosis of kidney disease. UpToDate. Retrieved March 18, 2025, from https://www.uptodate.com/contents/urinalysis-in-the-diagnosis-of-kidney-disease
Whittier, W. L., & Korbet, S. M. (2024). The kidney biopsy. UpToDate. Retrieved March 18, 2025, from https://www.uptodate.com/contents/the-kidney-biopsy
Wouk, N. (2021). End-stage renal disease: Medical management. American Family Physician, 104(5), 493–499. https://www.aafp.org/pubs/afp/issues/2021/1100/p493.html
Powered by Froala Editor