About this course:
This course aims to provide an overview of the most up-to-date science available regarding the pathogenesis, risk factors, clinical manifestations, disease severity, and evidence-based prevention and treatment approaches for patients with COVID-19.
Course preview
COVID-19
This course aims to provide an overview of the most up-to-date science available regarding the pathogenesis, risk factors, clinical manifestations, disease severity, and evidence-based prevention and treatment approaches for patients with COVID-19.
Upon completion of this course, the learner will be able to:
- describe COVID-19 mortality in the US, and discuss the pathophysiology of COVID-19, including risk factors, transmission, prevention, and the most common signs and symptoms
- review the clinical features of COVID-19 based on disease severity (mild, moderate, severe, critical), and common laboratory, biomarker, and radiographic findings
- outline the screening and testing for COVID-19 infections, including antibody testing
- discuss the management of COVID-19 based on the highest level of evidence available to date and provide an outline of the various treatment options, their clinical considerations, indications, side effects, and monitoring parameters
- compare mechanisms of COVID-19 vaccination, the types of COVID-19 vaccines available in the US, their mechanism of action, side effects, and indications for use in special populations (pregnancy, lactation, and immunocompromised conditions)
- summarize the most updated information available regarding long COVID-19
COVID-19 Mortality in the US
COVID-19 has become a leading cause of death in the US, affecting all ages across the lifespan, demonstrating that no group is ‘safe’ from contracting and ultimately dying from this virus. The National Center for Health Statistics (NCHS) releases provisional death data weekly in response to the global pandemic. As of July 15, 2023, 1,137,915 deaths have been reported within the 50 states and the District of Columbia, with COVID-19 as the underlying cause or a contributing factor. Of those deaths, approximately 65.4% (743,207) have occurred in a hospital or other inpatient healthcare setting, and 14.9% (169,764) in a nursing home or long-term care facility. The remaining are unspecified; they are presumed to have died in their homes or other indeterminate location. The distribution of COVID-19 deaths in the US varies according to race and Hispanic origin. Individuals who identify as non-Hispanic White comprise the highest percentage of deaths (66% or 751,057 deaths), followed by those who identify as Hispanic (15% or 171,301 deaths) and non-Hispanic Black (14% or 156,185 deaths). Native Hawaiian/Pacific Islanders and American Indian/Alaskan Natives have the lowest mortality rates (0.2% or 2,307 and 1% or 12,055 deaths, respectively). The most frequently listed comorbidities with COVID-19 deaths include influenza and pneumonia (46.5%), hypertension (18.6%), diabetes (14.7%), Alzheimer’s disease and other dementias (11.2%), and sepsis (10.4%; NCHS, 2023).
Background
Coronaviruses (CoV) are a large group of single-stranded ribonucleic acid (RNA) viruses. They have a central core of genetic material surrounded by a lipid envelope with protein spikes, which gives them the crown’s appearance shown in Figure 1. In Latin, a crown is called ‘corona,’ thus, the terminology coronavirus evolved (Azer, 2020; Parasher, 2020).
Figure 1
Structure of SARS-CoV-2 Virus
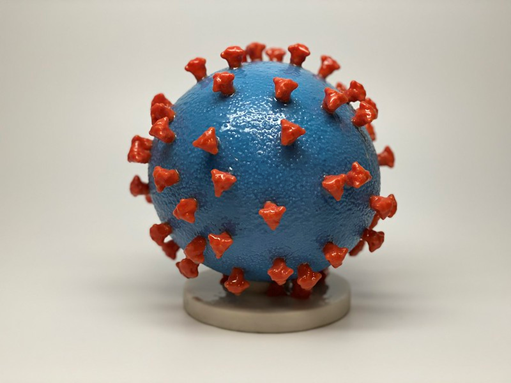
(NIH Image Gallery, 2020)
In humans, CoVs target the respiratory tract and most notably include severe acute respiratory syndrome (SARS-CoV), which emerged in 2003, Middle East respiratory syndrome (MERS-CoV) in 2012, and now the novel SARS-CoV-2 (COVID-19). COVID-19 has demonstrated a higher degree of lethality in humans when compared to these earlier outbreaks. While different types of CoV are known to cause illness in animals and humans, COVID-19 had never been detected in humans before December 2019 in Wuhan, China (Azer, 2020; Parasher, 2020; National Institute of Allergy and Infectious Diseases, 2022).
Immune System Overview
To understand how COVID-19 evades the body’s defense system and causes illness, it is first critical to ensure a foundational understanding of the immune system. The immune system is a collection of cells, tissues, and organs that work together to defend the body against attacks by pathogens or foreign invaders (such as microbes, viruses, bacteria, and parasites). The immune system strives to prevent invasion and protect against illness and infection by seeking and destroying pathogens. The key to a healthy immune system is distinguishing between the body’s cells (self) and foreign cells (non-self). The immune system cells launch an attack when they encounter anything that appears foreign. Any substance capable of triggering an immune response is called an antigen. An antigen can be a virus, bacteria, or any infectious organism, and all antigens carry marker molecules that identify them as foreign. White blood cells (WBCs) are the components of the immune system that fight infection and other illnesses. WBCs comprise five specific subtypes (neutrophils, monocytes, macrophages, eosinophils, and basophils). Each WBC functions in mediating the inflammatory and immune response to infection. WBCs have variable lifespans; while some may live for only 24 hours, the average WBC lifespan is 13 to 20 days. There are two main types of immune responses: innate and adaptive immunity (Longo, 2019; McCance & Heuther, 2019).
Innate Immunity
Also known as natural immunity, innate immunity is present at birth and is the first line of defense against pathogens. Innate immunity is activated immediately and rapidly in response to pathogen invasion and is always present and prepared to attack. It does not generate immunologic memory, meaning it does not remember past predators. The innate immune system responds nonspecifically every time a predator launches an attack. It includes physical barriers (skin and mucus membranes), mechanical barriers (coughing and sneezing), chemical barriers (tears and sweat), inflammatory responses, complement activation, and the production of natural killer (NK) cells (large granular lymphocytes). Redness and swelling surrounding a skin laceration is an example of innate immunity. Lymphocytes deploy to the wound site, infiltrating the area to keep microbes out and promote healing before further damage or infection ensues (McCance & Heuther, 2019).
Adaptive Immunity
Adaptive immunity or acquired immunity is the second line of defense and is highly specific, responding individually to each pathogen it encounters. The adaptive immune system is activated if an invading pathogen breaches innate immune mechanisms. Due to adaptation, the acquired immune system responds comparatively slower than the innate immune system. The adaptive immune system boasts immunologic memory and specificity, meaning it “remembers” prior antigens and can develop a repeat specified response. There are three types of adaptive immunity: humoral immunity, cell-mediated immunity, and T-regulatory cells. The immune system organs are positioned strategically throughout the body. They are called lymphoid organs because they house macrophages and lymphocytes, the two critical mediators of the adaptive immune system. Macrophages engulf and digest germs and dead cells, leaving antigens for the body to identify as dangerous, triggering the stimulation of antibodies. There are two main types of lymphocytes: B-lymphocytes (B-cells) and T-lymphocytes (T-cells). B-cells mediate the production of antibodies that attack antigens left behind by the...
...purchase below to continue the course
Pathogenesis of COVID-19
Structurally, SARS-CoV-2 is comprised of four proteins: the spike (S), membrane (M), envelop (E), and nucleocapsid (N). As demonstrated in Figure 1, the S protein protrudes furthest from the viral surface and is considered one of the central points for host attachment and penetration. The S protein contains two functional subunits (i.e., S1 and S2). S1 binds to the host cell receptor, and S2 facilitates the fusion between the viral and host cellular membranes. Based on scientific understanding, the virus’s high infectivity is related to mutations in its receptor binding and the acquisition of a furin cleavage (division or separation) site in the S protein. Furin is a protease (an enzyme) that breaks down proteins and peptides. It is widely expressed in various organs and tissues throughout the human body. Numerous studies have demonstrated that SARS-CoV-2 uses two chief host proteins to gain entry and activate the viral replication process: the angiotensin-converting enzyme 2 (ACE2) and the cell surface transmembrane protease serine 2 (TMPRSS2). TMPRSS2 activates the S protein and cleaves the ACE2 receptor to enable the attachment and entry of SARS-CoV-2 into the cell. The ACE2 receptors are the predominant binding receptors for the virus and are highly expressed throughout the upper and lower respiratory tracts, particularly the alveolar cells (e.g., bronchus, alveoli, mucosa). The respiratory tract is the main target of COVID-19, with post-mortem evidence of severe pulmonary histopathologic changes and diffuse lung damage. However, extra-pulmonary effects have also been well-established, as the virus can penetrate and infect the hepatic, renal, gastrointestinal (GI), cardiovascular, and nervous systems (Azer, 2020; McCance & Heuther, 2019; Parasher, 2020).
Disease Progression
Early-stage COVID-19 infection starts with the bronchial epithelial cells (type I and II pneumocyte [alveoli] cells) and the capillary epithelial cells. As shown in Figure 2, each sac of air (or alveolus) is the site of gas exchange. As depicted in Figure 3, the alveoli are wrapped with capillaries, the location where red blood cells release carbon dioxide (CO2) and acquire oxygen (O2). Type I cells are thinner and allow for the direct passage of O2, whereas type II cells secrete surface surfactant, a substance that lines the alveolus and prevents the air sacs from collapsing (Azer, 2020; McCance & Heuther, 2019; Parasher, 2020).
Figure 2
Alveoli and Bronchioles
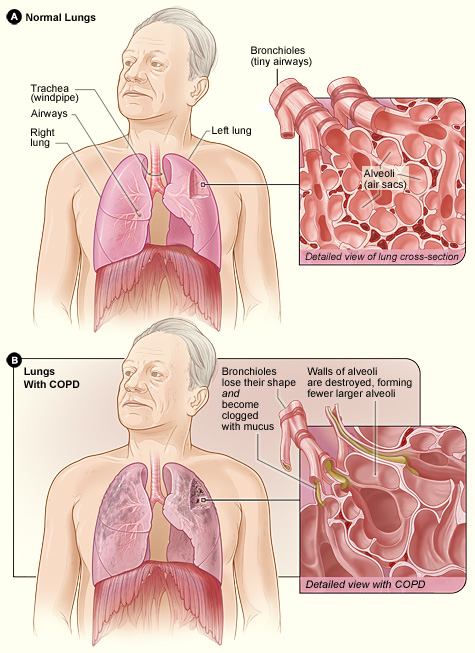
(NIH Image Gallery, 2017)
Figure 3
Cross Section of an Alveolus and Gas Exchange
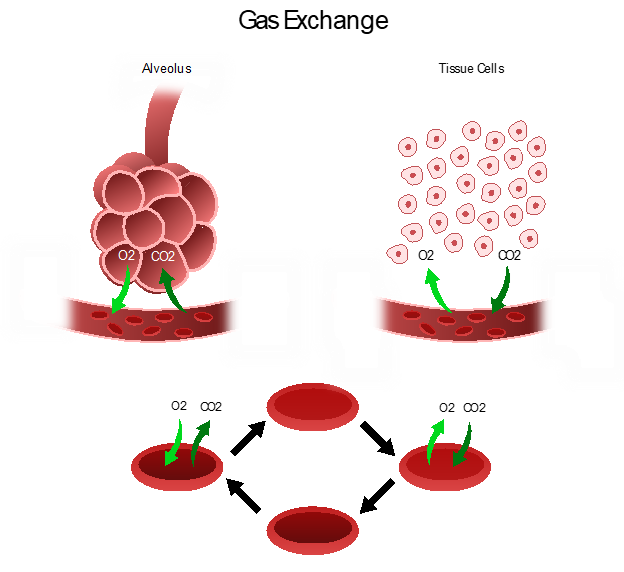
The S proteins primarily bind to the ACE2 receptors on the type II alveolar cells. Once the virus enters the host via this receptor, it releases its RNA, or viral material, into the cell, initiating replication. After they are infected, the type II cells respond by releasing inflammatory signals, which direct the recruitment of WBCs, particularly macrophages, and ignite the immune response. Since one infected host cell can generate hundreds of new virions, this process rapidly disseminates, infecting more cells and inducing widespread infection. Viral replication leads to the upregulation of cytokines (inflammatory mediators) as part of the host’s immune response. Interleukin (IL)-6 is a pro-inflammatory cytokine produced by various cell types, including lymphocytes and monocytes. Infection with SARS-CoV-2 triggers the bronchial epithelial cells to release IL-6, inducing inflammation, pyrexia (fever), vasodilation, and increased vascular permeability, recruiting more immune cells to the injury site. Fluid accumulates inside the alveolus, diluting the surface surfactant and triggering its collapse. Upon arrival at the infection site, recruited neutrophils release reactive oxygen species (ROS) to destroy the infected host cells, but not before the virions quickly replicate and disseminate to penetrate neighboring cells. Over time, the virus can infiltrate ACE2-bearing cells throughout extra-pulmonary organs, such as the blood vessels and kidneys (Azer, 2020; Johnson et al., 2021; Munkhdelger et al., 2023; Nikhra, 2020; Parasher, 2020; Peacock et al., 2021; Qiao et al., 2021; Xing et al., 2020).
As type I and II cells are destroyed, more alveoli collapse and gas exchange is impaired. Less O2 enters the bloodstream, and more fluid enters the alveolus, leading to hypoxemia and pulmonary edema. In severe cases, the hyperactivated immune response (cytokine storm) starts to damage non-infected cells and tissues. If enough type I and II cells are destroyed, systemic inflammatory response syndrome (SIRS) ensues, resulting in widespread inflammation and damage to various organ systems. COVID-19-associated SIRS can be associated with heightened cytokine release, as evidenced by elevated blood levels of IL-6, C-reactive protein (CRP), D-dimer, and ferritin, leading to a hypercoagulable state. Late-stage COVID-19 is characterized by alveolar interstitial thickening, increased vascular permeability, and vascular endothelial injury; this can prompt the activation of the coagulation cascade. Systemic infection or inflammation can impair healthy coagulation mechanisms, leading to microthrombus and venous thromboembolism (VTE; e.g., deep vein thrombosis [DVT] and pulmonary embolism [PE]) formation (Azer, 2020; Longo, 2019; Munkhdelger et al., 2023; Nikhra, 2020; Parasher, 2020).
A thrombus can develop anywhere within the cardiovascular system, with the majority classified as venous (VTE; within a vein) or arterial thrombosis (AT; within an artery). DVT is the most common type of VTE, typically arising in the large veins of a lower extremity. PE is the most severe complication of a DVT, in which the thrombus detaches from the vessel wall and circulates within the bloodstream. This circulating thrombus causes an abrupt blockage of a pulmonary vessel. PE can obstruct blood flow and induce sudden death in some patients. It can also cause hypoxia (low O2 levels in the tissues), permanently damaging the lungs or other organs due to insufficient O2 supply (Longo, 2019; McCance & Heuther, 2019). ATs predominantly arise from the heart (i.e., aorta or coronary arteries) but may also occur in the brain's cerebral arteries. ATs in patients diagnosed with COVID-19 commonly occur in the distal aorta, iliac, superficial femoral, tibial, and popliteal arteries. An AT or arterial emboli can cause tissues to become starved of blood and O2, leading to necrosis and infarction (Sanders et al., 2022).
For more information on the mechanisms of thrombosis and their complications, refer to the following NursingCE courses:
- Venous Thromboembolism
- Blood Clotting and Bleeding Disorders
- Stroke Prevention, Thrombolytic Therapy, and Rehab
Transmission
COVID-19 is a highly transmissible virus, infecting people across the lifespan. The latest epidemiology of COVID-19 indicates that most infections are spread through contact via respiratory droplets, in which the virus gains entry through the nose, mouth, or eyes (Centers for Disease Control and Prevention [CDC], 2021, 2023e; US Environmental Protection Agency [EPA], 2023). Table 1 summarizes the three major transmission factors: how, when, and where.
Table 1
The How, When, and Where of COVID-19 Transmission
How | Transmission is most common via respiratory droplets carrying infectious virus particles. Some sources state that infection can occur at distances greater than six feet with particles moving through an entire room, the CDC recommends distancing of greater than six feet, and the World Health Organization (WHO) recommends distancing of greater than three feet to prevent infection. Although less likely, the transmission may also occur through contaminated fomites (i.e., objects or materials likely to carry the infection) in the infected person’s immediate environment. |
When | Although individuals are most contagious while symptomatic, a significant proportion of COVID-19 infections are asymptomatic, demonstrating that the disease can be spread by individuals who show no signs or symptoms.
|
Where (Setting) | Transmission is most common among close contacts and is amplified in indoor, enclosed, and crowded spaces. |
(CDC, 2021, 2023e; EPA, 2023)
Respiratory droplets are produced through breathing and released upon exhalation (e.g., sneezing, speaking, coughing, singing). The droplet size is associated with the time it remains suspended in the air (i.e., larger droplets fall out of the air rapidly and stay close to the source, whereas smaller droplets can remain suspended for up to several hours and travel further). Airborne transmission occurs when smaller droplets and particles remain suspended in the air over long distances (i.e., greater than six feet) and time (i.e., typically hours). The WHO recommends using contact and droplet precautions to prevent the spread of COVID-19. In contrast, the CDC recommends airborne isolation precautions as laboratory testing has demonstrated the presence of the virus in air samples taken three hours after an individual with COVID-19 leaves the room (Bahl et al., 2022; EPA, 2023). Some circumstances facilitate the build-up of suspended small respiratory droplets, such as:
- aerosol-generating procedures (i.e., bronchoscopy or intubation)
- the presence of an infectious person producing respiratory droplets for an extended time (i.e., 15 minutes to multiple hours) in an enclosed space or area with inadequate ventilation
- enough viral particles were present to cause infections in people who were more than six feet away
- prolonged exposure to respiratory particles caused by expiratory exertion (i.e., by yelling, singing, exercising) in the absence of a mask (Bahl et al., 2022; CDC, 2021; EPA, 2023)
Risk Factors
Two major groups are at heightened risk of developing a more severe clinical course and poorer prognosis: older adults (older than 60 years, with mortality increasing incrementally alongside advancing age) and individuals with underlying comorbidities (CDC, 2023h). According to the CDC, adults (at any age) with the following comorbid conditions are at increased risk of severe illness from COVID-19:
- type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM)
- heart conditions, such as hypertension, heart failure, coronary artery disease, or cardiomyopathies
- cancer and other immunocompromised states (i.e., weakened immune system) from solid organ transplant or immunosuppressive therapy (such as long-term steroid use)
- chronic liver disease
- chronic kidney disease
- lung diseases such as chronic obstructive pulmonary disease (COPD), asthma, bronchiectasis, interstitial lung disease, PE, or pulmonary hypertension
- disabilities such as Down syndrome, cerebral palsy, spinal cord injuries, or intellectual or developmental disabilities
- overweight (body mass index [BMI]≥ 25 kg/m2) or obesity (BMI ≥30 kg/m2)
- hemoglobin blood disorders such as sickle cell disease or thalassemia
- cystic fibrosis
- dementia or Alzheimer's disease
- tobacco use (current or former)
- pregnancy (up to 42 days postpartum)
- human immunodeficiency virus (HIV)
- mental health disorders such as depression, substance use disorder (SUD), schizophrenia spectrum disorders, attention deficit hyperactivity disorder (ADHD), or mood disorders (CDC, 2023h)
Although over 90% of pregnant individuals recover from COVID-19 infection without needed hospitalization, COVID-19 infection during pregnancy does increase the need for intensive care unit (ICU) admission and mechanical ventilation and the risk for severe illness and death. Risk factors associated with more severe COVID-19 infections during pregnancy include age over 25, high BMI, an underlying medical condition such as chronic hypertension or pre-existing diabetes, and pregnancy-specific disorders (i.e., gestational diabetes or preeclampsia). While children and young adults have also been affected, they appear to be at a lower risk of a severe clinical course and mortality. Children with underlying disorders such as genetic, neurologic, metabolic conditions or congenital heart disease, diabetes, asthma, or are immunocompromised are at increased risk for severe illness compared to their healthy counterparts (Berghella & Hughes, 2023; CDC, 2022g, 2023h).
Risk Reduction
While much of the information surrounding COVID-19 has changed as the COVID-19 pandemic has evolved, best practices regarding protecting oneself and reducing the virus’s spread have remained relatively constant. The most important ways to slow the spread of COVID-19 are for all persons (over the age of 2 years) to wear a mask, stay at least six feet from people not in the same household, avoid crowds, and perform proper handwashing. Those who are able should also protect themselves and others from infection by getting vaccinated against the COVID-19 virus (CDC, 2023c; EPA, 2023). Figure 4 provides a brief overview of best practices regarding preventing the spread of COVID-19.
Figure 4
COVID-19 Prevention Strategies
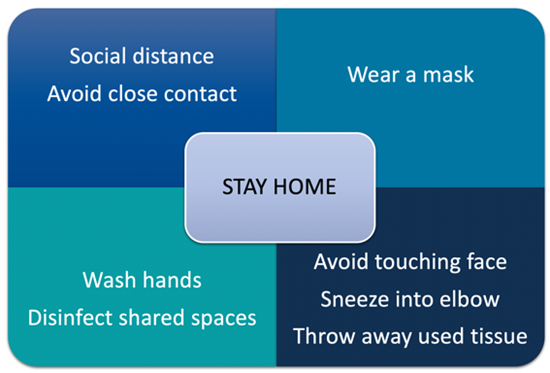
(CDC, 2023h; Selchick, 2021)
Clinical Features
The incubation period (the time between being exposed to the virus [becoming infected] and symptom emergence) varies based on the variant of the virus; however, there is a mean incubation period of approximately 6.5 days. The Delta variant has an incubation period of 4.3 days, and the Omicron variant has an incubation period of only 3-4 days (CDC, 2023a). While the signs and symptoms of COVID-19 can vary widely, the most common presenting symptoms include the following:
- fever or chills
- cough
- fatigue
- anorexia
- shortness of breath (SOB)
- myalgias
- ageusia (loss of taste)
- sore throat
- nasal congestion
- nausea or vomiting
- diarrhea
- headaches
- anosmia (loss of smell; CDC, 2023a)
Pregnant individuals are a high-risk group for severe COVID-19 infection that requires meticulous assessment due to the physiologic adaptations of pregnancy, which can overlap with symptoms of COVID-19, such as fatigue, GI distress, nasal congestion, and SOB. Pregnant individuals are also more likely to be asymptomatic than their non-pregnant counterparts of reproductive age; however, the likelihood of a pregnant individual presenting as asymptomatic is not well established. One systematic review demonstrated that 54% to 77% are asymptomatic, and another systematic review found that 95% of pregnant individuals present as asymptomatic. Lymphopenia and elevated C-reactive protein levels were the most common abnormal laboratory findings in infected pregnant women (Berghella & Hughes, 2023; McIntosh, 2023; WHO, 2023a).
Screening and Testing for COVID-19
Two kinds of diagnostic tests are available for COVID-19: viral tests and antibody tests. Viral tests evaluate for a current infection, and antibody tests identify a history of illness (CDC, 2022c, 2023g).
Viral Test
Two categories of viral tests are available: nucleic acid amplification tests (NAATs) and antigen tests. NAATs detect the presence of the virus’s RNA material. These tests are sensitive and readily available, with a 24-to-48-hour turnaround time. NAATs can be performed in a laboratory setting, but the most common type of NAAT used to detect COVID infection is rapid reverse transcriptase-polymerase chain reaction (RT-PCR) tests. These can be completed at the point of care. Rapid RT-PCR testing is comparable to laboratory NAATs. The viral RNA from the COVID-19 virus can remain in an individual's system for up to 90 days. When using NAAT within a few months following a known COVID diagnosis, any positive result is likely from continued RNA shedding, but a new infection cannot be ruled out as NAAT is unable to differentiate between the two (Caliendo & Hanson, 2022; CDC, 2023g).
Antigen tests detect specific proteins on the virus’s surface. Antigen tests are less expensive than NAATs and can give results in minutes. Antigen tests can also be performed in the home setting, point-of-care (POC), or laboratory. These tests have high specificity but are not as sensitive as NAATs. Due to the lower sensitivity, and a higher false-negative rate (i.e., a higher chance of missing an active infection), it is recommended by the US Food and Drug Administration (FDA) that any negative results be treated as preliminary results and do not necessarily rule out a COVID-19 infection. These tests can be repeated every 48 hours up to three times to verify the negative result. Antigen testing is preferred for individuals with a COVID-19 infection within the previous three months but with indications that warrant repeat testing, such as new onset symptoms (Caliendo & Hanson, 2022; CDC, 2023g). Most initial tests required collecting samples with nasopharyngeal (NP) swabs, as shown in Figure 5.
Figure 5
NP Specimen Collection
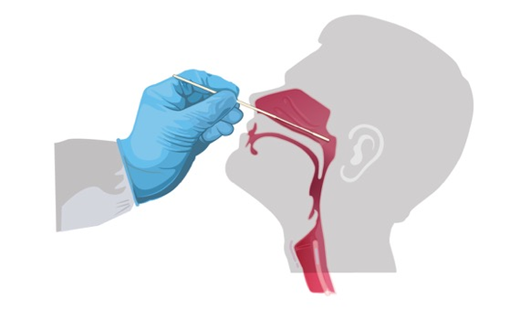
(CDC, 2022c)
Proper specimen collection is paramount to an accurate result and diagnosis of infection. Many viral upper respiratory tract tests use NP or oropharyngeal (OP) swabs. However, manufacturers have extended the sampling pool to allow for a sample collection from the anterior nostril, nasal mid-turbinate, or saliva. Lower respiratory tract specimens are collected via bronchoalveolar lavage, pleural fluid, tracheal aspirate, or sputum. Rapid, POC diagnostic tests are available as molecular and antigen tests. They use a mucus sample from the nose or throat; results may be available in minutes. At-home testing kits allow samples to be collected as an anterior nasal swab: the individual is advised to insert the absorbent tip of the swab no further than one inch (2 cm) into the nose. As demonstrated in Figure 6, the swab is then rotated in a circular path against the inside of the nose at least four times for 15 seconds. The procedure is then repeated in the other nostril using the same swab (CDC, 2022c, 2023g).
Figure 6
How to Collect a Nasal Mid-Turbinate Specimen
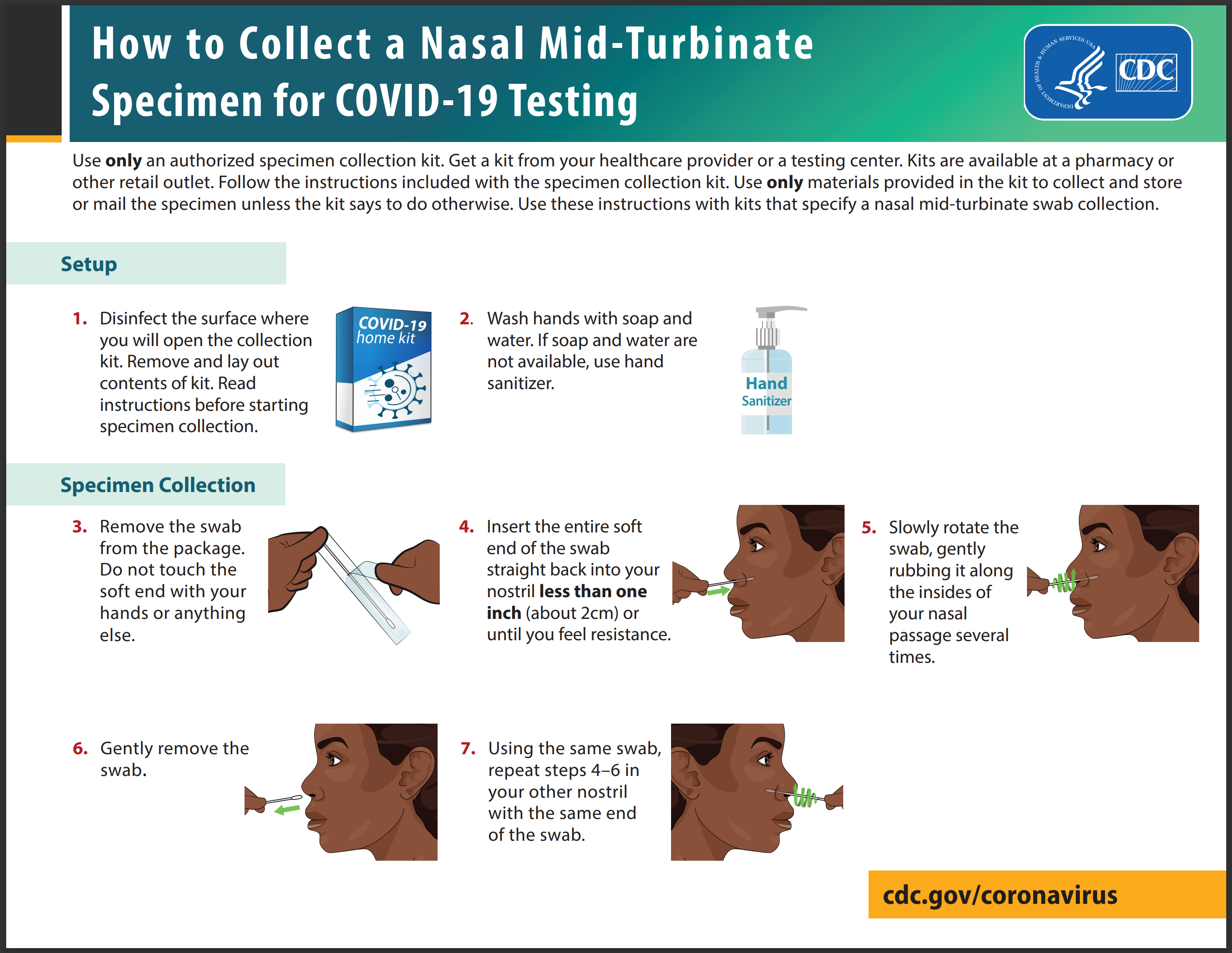
(CDC, n.d.)
A newer diagnostic test to detect COVID-19 is a breath test. This test utilizes rapid gas chromatography-mass spectrometry (GC-MS) to detect the presence of volatile organic compounds associated with COVID-19 in an individual's breath. Results of this test are available within minutes. A follow-up NAAT should be performed to confirm the positive test result if the test is positive. This type of testing was granted emergency use authorization (EUA) status in 2022 and must be administered under the supervision of a healthcare professional. The FDA issues a EUA to allow access to critical medications and medical products that may help during a public health emergency (CDC, 2022e; FDA 2023a, 2023f). A EUA is different from standard medical testing, medication, or vaccination approval and must meet the following criteria (FDA, 2023b):
- The product will be used for severe or life-threatening diseases or conditions.
- Based on the totality of scientific evidence available, it is reasonable to believe the product may be effective.
- The known and potential benefits of the product outweigh the known and potential risks.
- There are no adequate FDA-approved alternatives available.
Test selection differs based on various factors, including the purpose for testing, availability, setting (e.g., home, laboratory, clinic), cost, and turnaround time. For a diagnosis to be confirmed, a NAAT or antigen test should be performed (CDC, 2023g).
Viral Testing Recommendations
The recommendations for testing individuals for current infection with COVID-19 have evolved. There are various recommendations based on each individual’s level of risk, exposure, employment setting, test availability, and state and local guidelines (CDC, 2023g). According to the CDC recommendations, the following individuals should have a viral test:
- people who have symptoms of COVID-19
- people who have been exposed due to close contact (within 6 feet of an infected person for a cumulative total of 15 minutes or more over a 24-hour period) with someone with confirmed COVID-19; testing should occur at least five days following exposure for asymptomatic individuals or when symptoms develop
- individuals that require screening for COVID-19 infection, such as those living in close quarters (e.g., prisons or detention centers; homeless shelters), will become isolated from healthcare resources (e.g., cruise ships or shipping vessels), before starting immunosuppressive therapy, or before undergoing a surgical or aerosol-generating procedure
- people who have been asked or referred to get testing by their healthcare provider or local or state health department (Caliendo & Hanson, 2022; CDC, 2023g)
If testing results are positive at home or in a healthcare setting, it is essential to follow isolation guidelines. It is recommended that a NAAT should not be used in asymptomatic individuals that have had an infection with COVID-19 within the previous month or have tested positive for COVID-19 within the previous three months (Caliendo & Hanson, 2022; CDC, 2023g).
Antibody Testing
Antibody (or serology) tests do not diagnose an active infection; they instead detect the antibodies created by the immune system in response to a prior COVID-19 infection or vaccination. Antibody production following a COVID-19 infection varies, and it can take 7 to 14 days for a detectable amount of antibodies to develop. Most individuals will test positive for antibodies three weeks post-infection or vaccination. Antibody testing can differentiate between immunity caused by infection and immunity from vaccination. Positive antibody tests against the N protein, S protein, or receptor-binding domain (RBD) of the S protein indicate that the individual had a prior COVID-19 infection. A positive antibody test for the S protein only indicates antibody production due to vaccination. A positive antibody test can also help diagnose patients with COVID-19 complications such as post-acute sequelae of COVID-19. Antibodies can remain detectable for at least several months; however, it remains unknown exactly how long antibodies stay in the body following a COVID-19 infection (CDC, 2022d). The following studies remain the basis for NIH recommendations regarding antibody testing. Dan and colleagues (2021) analyzed antibody production in 188 patients with confirmed COVID-19 infections. They found durable immune responses in the majority, with immunoglobulin G (IgG) antibodies to the S protein relatively stable for up to 8 months. The memory B-cells were also more abundant at 6-months than at 1-month post-symptom onset, endorsing the concept that immunity takes time to develop. At 8 months, 95% of individuals were still positive for at least three of five SARS-CoV-2-specific immune memory responses (Dan et al., 2021). Choe and colleagues (2021) found similar findings eight months after asymptomatic or mild COVID-19 infection in a relatively younger (< 65 years) population. While this study was small (n= 58 individuals), the seropositive rates ranged from 69% to 91.4% at eight months post-infection (Choe et al., 2021). In a more extensive study including 12,219 healthcare workers, a prior COVID-19 infection that generated antibody responses offered protection from reinfection for most people in the 6 months following the illness (Lumley et al., 2021). More research is needed to determine the long-term durability of natural immunity via different strains of COVID-19 versus vaccination across populations (CDC, 2022d).
Infection Protection and Control (IPC)
Infection protection and control (IPC) is a critical and fundamental aspect of the clinical management of suspected or confirmed positive patients. Healthcare professionals (HCPs) should immediately implement appropriate infection control measures, including screening of all persons in healthcare facilities and proper use of personal protective equipment (PPE) based on local infection levels (CDC, 2023e). To uphold IPC measures, HCPs should follow the following guidelines, as outlined by the CDC:
- promote vaccination and follow vaccination schedule for repeat doses
- screen and triage for early recognition of suspected COVID-19 patients and rapid implementation of control measures
- post visual alerts in entrances and high-traffic areas outlining the most current IPC recommendations
- screen all individuals at the first point of contact in the healthcare facility by asking if they have symptoms, positive viral test for COVID-19, or have come into close contact with an individual with a current COVID-19 infection
- separate suspected or confirmed COVID-19 patients to a well-ventilated, isolated area away from other contacts, and maintain at least 1 m (~3 feet) distance between patients
- apply standard precautions for all patients at all times, which include (but are not limited to),
- hand and respiratory hygiene
- appropriate use of PPE
- proper environmental cleaning and safe waste management
- apply contact and droplet precautions for suspected or confirmed COVID-19 patients
- when caring for these patients, follow the appropriate transmission-based guidelines and wear gloves, a clean long-sleeved gown, a medical mask, and eye protection (i.e., goggles or face shield) and remove PPE when leaving the patient care area (see Figure 7; CDC, 2023e)
Figure 7
PPE Removal
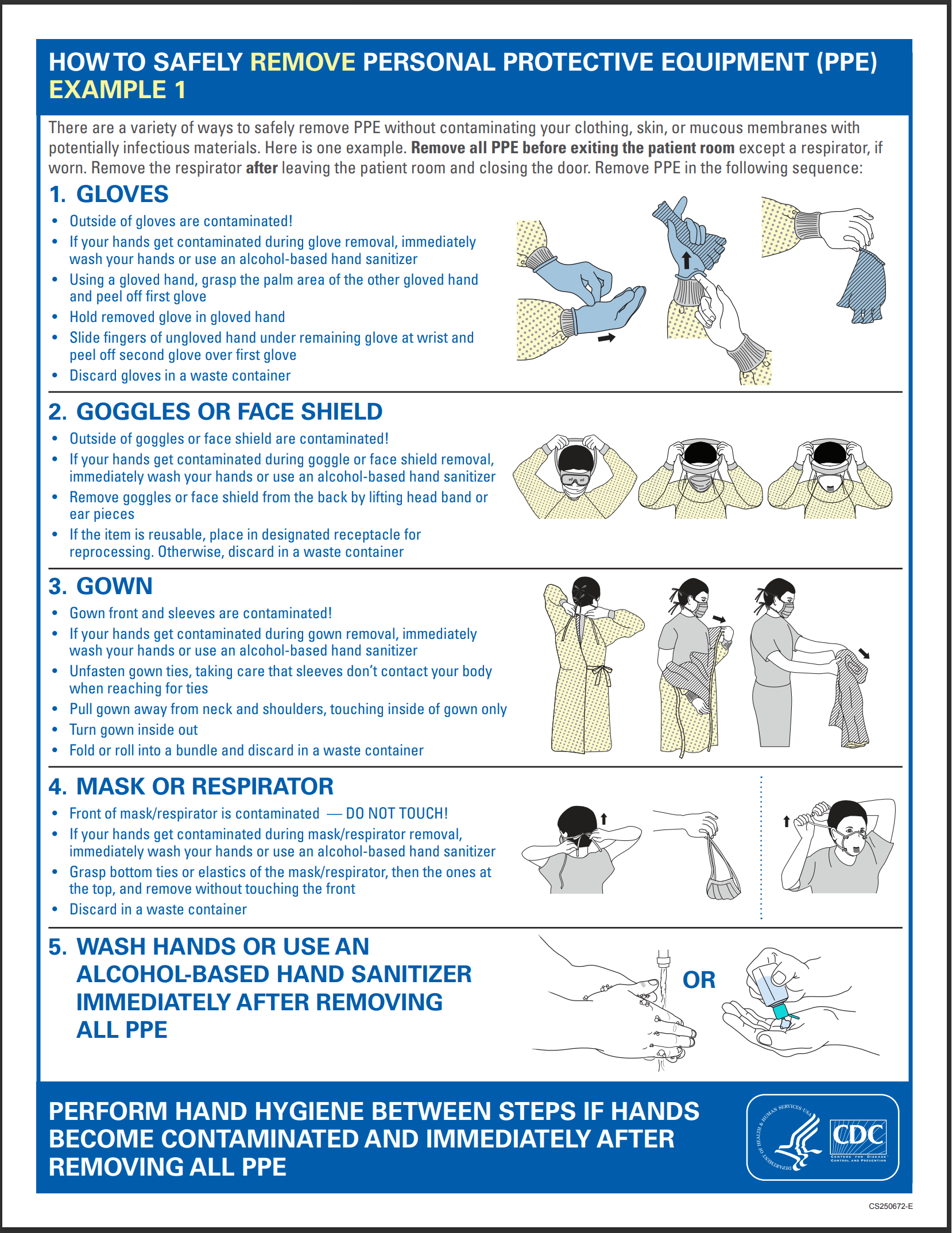
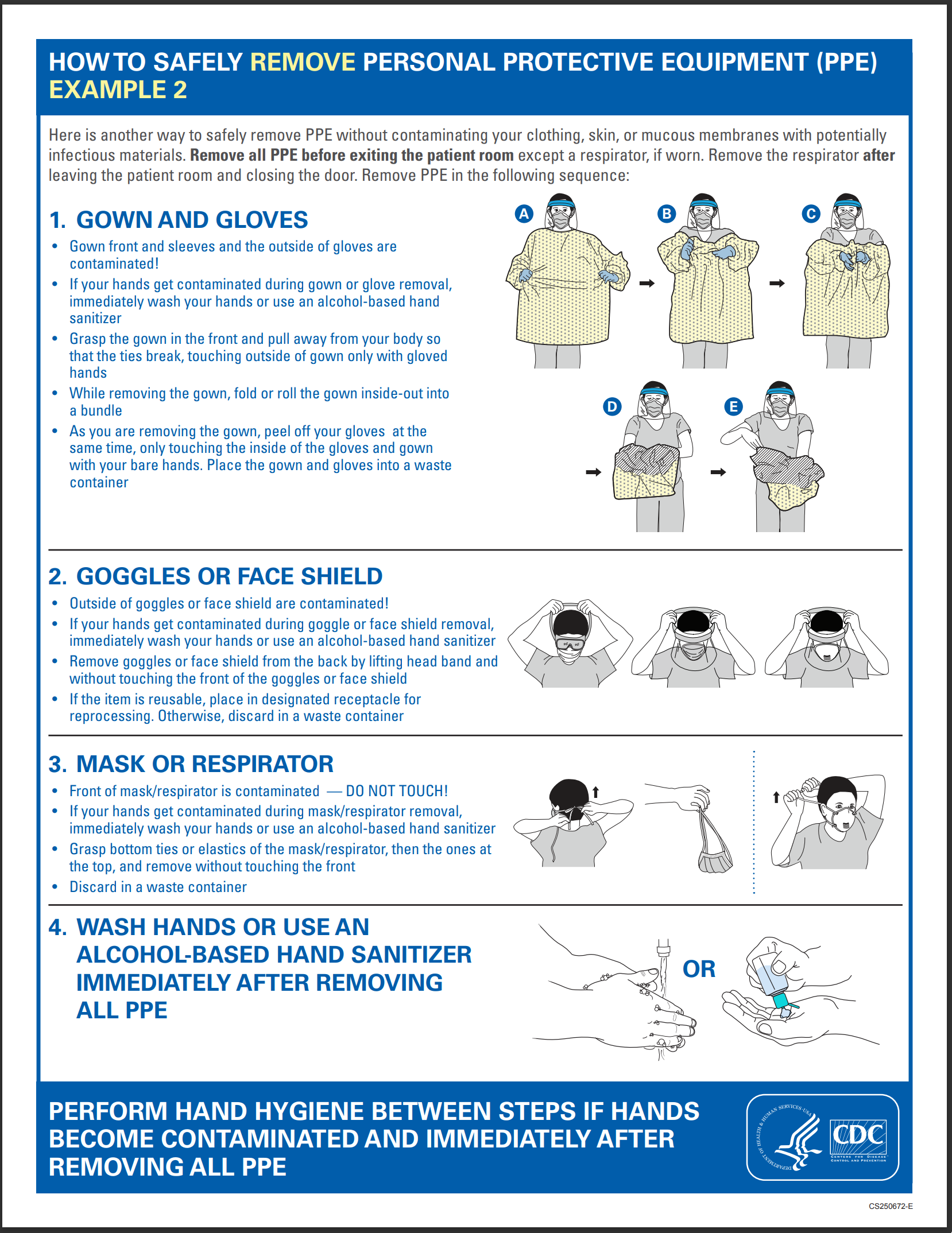
(CDC, 2023f)
For more information on PPE and standard procedures for donning and removing PPE, refer to the Personal Protective Equipment NursingCE course.
Isolation
Isolation is intended for persons who are COVID-19 positive and are advised to separate from others to prevent the spread of the virus. Those who test positive for COVID-19 should remain at home, in a separate space from household contacts (if possible), and use a separate bathroom (if available; CDC, 2023e). Isolation precautions can be lifted once the following criteria are met:
- patients that are asymptomatic or mildly ill should isolate until it has been 5 days since the onset of symptoms or the first positive test (this is day zero), the fever has resolved without the use of antipyretics, and other symptoms are improving
- these individuals should continue to wear a mask at home and in public until day 10
- if symptoms reappear or begin to worsen, isolation should restart at day zero
- if the individual is unable to wear a mask, they should isolate for 10 days
- patients that are moderate to critically ill and are not immunocompromised should isolate until 10 days (may be up to 20 days) have passed since symptoms first appeared, they are afebrile for at least 24 hours without using antipyretics, and symptoms such as cough or SOB have begun to improve
- patients that are immunocompromised should isolate for 20 days, and a test-based strategy should be used to determine when isolation can be discontinued; this includes being afebrile for 24 hours without using antipyretics, improvement of symptoms, and the results from two consecutive respiratory specimens using antigen testing or NAAT are negative; these specimens should be collected 48 hours apart (CDC, 2022a, 2023e)
Following recovery from a COVID-19 infection, some people may continue to test positive for 3 months without evidence of transmission to others. The CDC does not recommend routinely retesting individuals recovering from an acute infection. People should only be tested again if they develop new symptoms or have come in close contact with another person who tested positive for COVID-19 in the last 14 days (CDC, 2023e).
Quarantine
Quarantine is separating a person from other individuals after exposure to reduce the risk that the person exposed to COVID-19 by close contact may unknowingly transmit the infection to others by separating them (WHO, 2022). The WHO defines close contact as any of the following circumstances occurring during the infectious period (2 days before to 13 days after symptom onset or 2 days before and 10 days after a positive test):
- persons within 3 feet (6 feet per the CDC) of someone with a probable or confirmed COVID-19 infection for a total of 15 minutes or more
- persons who provided direct care to someone with a probable or confirmed case of COVID-19 without the use of PPE
- persons with direct physical contact with someone who has COVID-19 (i.e., hugged, kissed, or shared eating/drinking utensils)
- persons exposed to the respiratory droplets of someone with COVID-19 (i.e., sneezing, coughing; WHO, 2022).
Individuals included in high-risk groups (i.e., individuals that are pregnant, immunocompromised, over 60, working in a healthcare facility, or have multiple comorbidities) or living in high-risk settings (i.e., prison, long-term care facility, or group home) that have not completed the initial vaccination series or received a booster dose and have not been infected with COVID-19 within the previous 90 days should quarantine for 10 days. The quarantine period can be shortened to 5 days if the contact tests negative on day 5 and is asymptomatic (WHO, 2022).
Disease Severity
In addition to underlying comorbidities and the risk factors outlined earlier, the clinical manifestations of COVID-19 infection can vary between individuals. The severity of COVID-19 infection is based on the clinical characteristics of symptomatic individuals and has been subdivided into four major categories, as described in Table 2 (National Institutes of Health [NIH], 2023).
Table 2
COVID-19 Disease Severity
Severity | Description |
Mild disease |
|
Moderate disease |
|
Severe disease |
|
Critical disease |
|
(CDC, 2023a; NIH, 2023; WHO, 2023a)
Common Laboratory and Radiographic Findings
Many of the defining features of COVID-19 can be based on clinical grounds; however, several laboratory and radiologic findings common in patients with COVID-19 infections may help evaluate disease severity and guide treatment (McIntosh, 2023).
Labs and Biomarkers
Lymphopenia or lymphocytopenia (a lower-than-normal number of lymphocytes) is present in up to 83% of hospitalized patients with COVID-19 making it one of the most common laboratory findings. There is an association between several biomarkers, in particular, elevated liver function enzymes (LFTs), lactate dehydrogenase (LDH), procalcitonin, CRP, ferritin levels, D-dimer, prothrombin time, troponin, creatine phosphokinase, and inflammatory cytokines, and disease severity and poor patient outcomes (American College of Emergency Physicians [ACEP], 2021; Anesi, 2022; McIntosh, 2023). Table 3 provides an overview of well-cited markers correlating with disease severity.
Table 3
Biomarkers and Disease Severity
Indication | Biomarker | Normal Range |
Tissue damage | Elevated LDH | 80–225 U/L |
Inflammation | Elevated CRP | ≤0.8 mg/dL |
Elevated ferritin | Female: 24–307 ng/mL Male: 24–336 ng/mL | |
Liver damage | Elevated aspartate aminotransferase (AST) | 10–40 U/L |
Elevated alanine aminotransferase (ALT) | 10–40 U/L | |
Cardiac damage | Elevated cardiac troponin | I: ≤0.04 ng/mL T: ≤0.01 ng/mL |
Infection | Lymphocytopenia | 530–1570/μL |
Elevated procalcitonin | ≤0.10 ng/mL | |
Hypercoagulability (see below section) | Thrombocytopenia | 150,000–450,000/μL |
Elevated D-dimer | ˂ 0.5 μg/mL | |
Prolonged prothrombin time (PT) | 11-13 seconds | |
Fibrinogen | 200–400 mg/dL |
(American Board of Internal Medicine [ABIM], 2023; ACEP, 2021; Anesi, 2022; McIntosh, 2023)
Hypercoagulability
Coagulopathy is a well-documented clinical sequela of COVID-19 infections. Some patients may demonstrate signs of a hypercoagulable state, thereby predisposing them to potentially life-threatening thrombotic events. The most frequent signs of coagulopathy identified in hospitalized patients with COVID-19 include the following: elevated fibrinogen and D-dimer levels, mild prolongation of PT, mild thrombocytopenia (platelet count ~100,000μL), and a parallel rise in markers of inflammation including CRP and ferritin. Several studies have also demonstrated that elevated D-dimer levels (>1 μg/mL) are strongly associated with increased mortality (ACEP, 2021; Anesi, 2022). D-dimer is a fibrin degradation fragment produced from the lysis of fibrin. The D-dimer test measures the amount of the substance released into the bloodstream when fibrin proteins in a blood clot dissolve. Under physiologic conditions, the D-dimer level is typically not detectable or very low. These levels increase when there are a significant formation and breakdown of fibrin clots within the body. Therefore, this test is commonly used as a screening test for VTE, with a negative (low or undetectable) result indicating a low likelihood that a thrombus is present. However, the test is nonspecific, and an elevated D-dimer result cannot definitively predict whether a blood clot is present in the body. Further, this test historically has a high false-positive rate, particularly in postoperative and pregnant patients (COVID-19 Treatment Guidelines Panel, 2023; Pagana et al., 2022).
Fibrinogen is a soluble plasma protein essential to the coagulation cascade that helps control bleeding by assisting with fibrin formation. The conversion of fibrinogen into fibrin, a non-soluble plasma protein (blood clot), is the fourth reaction in the coagulation cascade. When an injury is detected, fibrinogen and platelets create a weak platelet plug that temporarily protects against further bleeding. Prothrombin time assesses how well the coagulation factors in the coagulation cascade are functioning collectively (Kennelly et al., 2023; Pagana et al., 2022). Studies have demonstrated varying rates of thromboembolism among hospitalized patients with COVID-19. A meta-analysis of hospitalized patients with COVID-19 found an overall prevalence of thromboembolism following prophylactic treatment of 14.1%, with that number increasing to 40.3% when screening was conducted using ultrasound. In comparison, before COVID-19, the incidence of thromboembolism in hospitalized patients that had received prophylactic treatment was 2.8% to 5.6%. While DVT and PE are the most common thrombotic complications of COVID-19 infections, other manifestations associated with hypercoagulability have been reported, such as clotting of implanted catheter devices, microvascular thrombosis of the toes (i.e., “COVID toes”), cerebral vascular ischemia, and myocardial injury (ACEP, 2021; COVID-19 Treatment Guidelines Panel, 2023).
Radiographic Findings
Chest imaging (i.e., chest x-ray [CXR] and computed tomography [CT]) may help obtain information regarding disease severity but have variable findings and should not be used to screen or diagnose COVID-19 infection. While some patients have unremarkable chest imaging early in the illness, classic features of COVID-19 on CXR include bilateral airspace consolidation, peripheral bilateral ground-glass opacities, interstitial thickening of the lung(s), lung consolidation zones, or in rare cases, pleural effusions. Abnormal CT findings are not specific to COVID-19 and may overlap with other respiratory infections. CT imaging should be reserved for symptomatic, hospitalized patients with specific pulmonary indications. CT findings may include ground glass opacities with or without mixed consolidation, pleural thickening, or interlobular septal thickening (ACEP, 2021; CDC, 2023a; McIntosh, 2023).
Management
Data surrounding the treatment of COVID-19 continues to evolve and is premised on the severity of the disease and individual patient factors. Various treatment algorithms have been devised to help clinicians navigate the most effective treatment options for patients in each category. There is variation between guidelines based on the evolving evidence and the availability of resources across geographic regions and institutions. Various organizations, including The American Society of Health System Pharmacists (ASHP) and WHO, maintain online resources outlining the most current recommendations, which are updated as needed. In addition, the NIH (2023) compiled the COVID-19 Treatment Guidelines, an electronic resource for clinicians to ensure timely access to the most updated clinical evidence regarding treatment strategies. The NIH guidelines are updated frequently by a panel of experts appointed based on their clinical experiences and prior expertise in developing treatment-based guidelines. All guideline recommendations are based on scientific evidence and expert opinion and are endorsed by most panel members (NIH, 2023).
The NIH (2023) guidelines support the therapeutic management of patients with COVID-19 based on their proposed understanding that two primary processes drive the pathogenesis of the disease: viral replication early in the course of illness, followed by an exaggerated immune/inflammatory response to the virus that leads to tissue damage. Based on these concepts, the guidelines endorse using therapies that directly target the COVID-19 virus early in the course of the disease and immunosuppressive and anti-inflammatory agents in later stages. The primary treatment categories for COVID-19 infections include monoclonal antibodies, convalescent plasma, antiviral therapies, corticosteroids, and immunomodulatory agents (NIH, 2023). It is well-established that antibiotics are ineffective against viral infections, yet continue to be prescribed for patients with COVID-19. Although antibiotics do not treat COVID-19, there is an increased risk of developing bacterial pneumonia in patients infected with COVID-19. One randomized study found that 57% of hospitalized patients with COVID-19 received an antibiotic within the first 48 hours of admission. However, only 4% of those were found to have a co-infection with bacteria. Inappropriate use of antibiotics can have detrimental effects on antibiotic resistance. Since the beginning of the COVID-19 pandemic, the resistance to bacterial and fungal infections has increased, with the resistance of Staphylococcus aureus to erythromycin (Erythrocin), clindamycin (Cleocin), and oxacillin (Bactocil) increasing by 90%. Due to the growing public health concern of antibiotic resistance, established treatment guidelines must be followed (Dueñas-Castell et al., 2022).
Treatment Overview Based on Disease Severity
Management of asymptomatic infection is based on monitoring since it is indeterminate what percentage of individuals will progress to clinical disease. Some asymptomatic individuals have been reported to demonstrate objective radiographic findings consistent with COVID-19 pneumonia; however, most do not. Patients with mild illness may exhibit various signs and symptoms as outlined earlier but are stable for outpatient (ambulatory) or telemedicine follow-up. Treatment is primarily centered on the alleviation of symptoms using supportive therapies. No specific imaging or laboratory evaluations are routinely indicated in otherwise healthy patients with mild COVID-19 treated on an ambulatory basis. However, older patients and those with underlying comorbidities are at higher risk for disease progression and require heightened monitoring and surveillance. When caring for this population, it is also important to consider possible cognitive impairment, increased risk for falls, and polypharmacy. As noted in Table 2, pulmonary disease can progress rapidly in patients with moderate illness; therefore, more cautious surveillance is advised. Empiric antibiotics are recommended only if secondary bacterial pneumonia is suspected. However, the patient should be monitored closely, and antibiotics should be de-escalated and discontinued as soon as there is no clinical evidence of bacterial infection. Patients with severe and critical illness require hospitalization, as they have more prominent symptoms and are at higher risk for rapid clinical deterioration, necessitating inpatient monitoring. Oxygen therapy should be administered immediately for any respiratory distress signs, using a nasal cannula or a high-flow oxygen device according to individual patient requirements. As with moderate illness, empiric antibiotics are indicated only if bacterial pneumonia or sepsis is suspected. However, patients should still be evaluated daily, and antibiotics should be de-escalated and discontinued as soon as there is no clinical evidence of bacterial infection. Critically ill patients require ICU care and are at the highest risk for severe complications and fatal outcomes, as described in Table 2. Further, these patients are at increased risk for exacerbating underlying comorbidities and cardiac, hepatic, renal, central nervous system, or thrombotic sequelae (NIH, 2023). Table 4 briefly overviews the NIH (2023) panel recommendations for pharmacologic therapies based on disease severity. Each category will be explored at greater length in the upcoming sections.
Table 4
NIH Pharmacologic Management-Based Disease Severity
Clinical Scenario | NIH Panel Pharmacologic Recommendations |
Non-hospitalized, mild to moderate COVID-19 infection |
|
Hospitalized, does not require supplemental oxygen |
|
Hospitalized, requires supplemental oxygen (but not oxygen delivered via high-flow device or mechanical ventilation) |
|
Hospitalized, requires oxygen via a high-flow device or non-invasive ventilation |
|
Hospitalized, requires mechanical ventilation or extracorporeal membrane oxygenation (ECMO) |
|
(NIH, 2023)
Monoclonal Antibodies
Monoclonal antibodies are widely used to manage cancer, infectious diseases, and autoimmune conditions. They are synthetically engineered versions of antibodies that attach to a specific target site. Scientists analyze particular antigens on infected cells' surfaces in the laboratory to find a protein (target) to match the antigen. Using proteins from animals or humans, they create an antibody that can attach to the target antigen, similar to how a key fits into a lock. This technology allows treatment to focus on the specified cells, thereby limiting harm to healthy cells. Monoclonal antibody therapy can only be performed for diseases with identified antigens (and the respective antibodies). These drugs work on infected cells like natural antibodies, identifying and binding to the target cells and then alerting the immune system to their presence (Malik & Ghatol, 2022). Monoclonal antibodies have been designed to target and bind to the S protein of SARS-CoV-2, thereby preventing it from interacting with the ACE2 receptor. This action precludes the virus from entering the host cells and replicating. Since the beginning of the COVID-19 pandemic, monoclonal antibodies such as bamlanivimab plus etesevimab, casirivimab plus imdevimab (REGN-CoV), sotrovimab (Xevudy), bebtelovimab (LY-CoV1404), and tixagevimab plus cilgavimab (Evusheld) have received EUA from the FDA; however, none of these monoclonal antibodies are currently authorized for use in the US due to the current variants present not being susceptible to these products (NIH, 2023). The WHO (2023b) has issued a strong recommendation against the use of casirivimab plus imdevimab (REGN-CoV), sotrovimab (Xevudy). Vilobelimab (Gohibic) is an anti-C5a monoclonal antibody and the only monoclonal antibody approved through EAU to treat COVID-19 infection. As discussed previously, there is insufficient evidence to recommend for or against its use in patients with COVID-19 receiving mechanical ventilation or ECMO. In a trial including 368 participants, there were no statistically significant differences in 28-day mortality rates between those that received vilobelimab (Gohibic) or a placebo (NIH, 2023).
Vilobelimab (Gohibic) is administered via intravenous (IV) infusion using a dedicated line over 30 to 60 minutes. The EUA dosage for vilobelimab (Gohibic) is 800 mg once daily for a maximum of 6 doses on days 1, 2, 4, 8, 15, and 22 while the patient remains hospitalized (even if transferred out of the ICU) with treatment beginning within 48 hours of intubation or the initiation of ECMO. Before administration, vilobelimab (Gohibic) must be diluted using 170 mL of 0.9% sodium chloride. This is accomplished by removing 80 mL of 0.9% sodium chloride from a 250 mL fluid bag and instilling 80 mL of vilobelimab (Gohibic) from the manufacturer's vials. The most common adverse reactions associated with vilobelimab (Gohibic) include pneumonia, sepsis, PE, DVT, hypertension, pneumothorax, urinary tract infection, hypoxia, thrombocytopenia, supraventricular tachycardia, constipation, respiratory tract infection, and rash. Serious fungal, viral, and bacterial infections are also associated with vilobelimab (Gohibic) use and should be included when assessing the benefit versus risks of treatment. Hypersensitivity reactions (HSRs) are common with the administration of monoclonal antibodies and have been observed in patients treated with vilobelimab (Gohibic; FDA, n.d.-b). An HSR occurs when a foreign substance overstimulates the immune system and creates antibodies that cause an exaggerated response. While data is limited to clinical trials, HSRs have been reported with varying severity. Early manifestations of HSR can include hives, urticaria, pruritis, swelling, back pain, facial flushing, rhinitis, abdominal cramping, chills, and anxiety. Symptoms may suddenly progress to life-threatening hypotension, bronchospasm, angioedema (swelling of the oral cavity, lips, and tongue), and anaphylaxis. Therefore, HCPs must remain hypervigilant for signs of HSR and ensure they are prepared to intervene immediately. If an HSR is suspected, the infusion should be stopped immediately. In addition to closely monitoring patients, clinical interventions may include applying supplemental O2, administering normal saline, and other emergency medications as indicated. If life-threatening symptoms are present, epinephrine 0.1-0.5 mg (1:10,000 solution for adult patients) may need to be administered by IV push or subcutaneous (SC) injection until emergency personnel arrives (Nettina, 2019).
Antiviral Treatments
Antiviral therapies have been a source of interest among researchers as they are proposed to inhibit viral entry via the ACE2 receptor and TMPRSS2 and block viral membrane fusion. Since research has demonstrated that viral replication is most active early, antiviral therapy seems to have the most significant therapeutic impact when administered before the onset of a hyperinflammatory state (NIH, 2023).
Ritonavir-boosted Nirmatrelvir (Paxlovid)
Ritonavir-boosted nirmatrelvir (Paxlovid) is an oral protease inhibitor effective against all coronaviruses that infect humans. Ritonavir-boosted nirmatrelvir (Paxlovid) was approved by the FDA in May 2023 for the treatment of nonhospitalized patients at least 12 years old and 40 kg with mild to moderate COVID-19 with a high risk of progressing to severe disease. Treatment should be initiated immediately and no later than five days after symptom development. For patients that are hospitalized for a non-COVID diagnosis but are COVID-positive, ritonavir-boosted nirmatrelvir (Paxlovid) can be used if the patient has mild to moderate disease, is at high risk of progressing to severe COVID-19, does not require the use of supplemental oxygen, and is within five days of symptom onset. Initial dosing is 300 mg of nirmatrelvir plus 100 mg of ritonavir administered orally twice daily for five days. The most common adverse effects include dysgeusia, diarrhea, HSRs, and hepatotoxicity. Renal impairment can prolong the excretion of ritonavir-boosted nirmatrelvir (Paxlovid). Due to this, dosing should be adjusted based on the estimated glomerular filtration rate (eGFR). Ritonavir-boosted nirmatrelvir (Paxlovid) should not be used in patients with an eGFR of less than 30 mL/min/1.73m2 or those with severe hepatic disease. Treatment with ritonavir-boosted nirmatrelvir (Paxlovid) is recommended in pregnant and breastfeeding individuals that meet the prescribing criteria. The WHO strongly recommends its use in those with non-severe COVID infection at high risk of disease progression and hospitalization but conditionally recommends against its use in those at low risk. Since ritonavir-boosted nirmatrelvir (Paxlovid) is a strong CYP3A4 inhibitor and P-glycoprotein inhibitor, many drug-drug interactions must be considered before initiating treatment with this medication (FDA, 2023d; NIH, 2023; WHO, 2023b). Table 5 outlines common medications that should be discontinued during treatment and those that require an alternative COVID-19 treatment plan.
Table 5
Ritonavir-boosted Nirmatrelvir (Paxlovid) Drug Interactions
Contraindicated Medications - Use Alternative Treatment Options | |
Anticonvulsants | Carbamazepine (Tegretol) Phenobarbital (Donnatal) Phenytoin (Dilantin) Primidone (Mysoline) |
Anti-Infectives | Glecaprevir/pibrentasvir (Mavyret) Rifampin (Rifadin) Rifapentine (Priftin) |
Cardiovascular drugs | Amiodarone (Cordarone) Clopidogrel (Plavix) Disopyramide (Norpace) Eplerenone (Inspra) Flecainide (Tambocor) |
Neuropsychiatric drugs | Clozapine (Clozaril) Lurasidone (Latuda) Midazolam (Versed) |
PDE5 inhibitors (when used for pulmonary hypertension) | Sildenafil (Viagra) Tadalafil (Cialis) Vardenafil (Levitra) |
Miscellaneous drugs | Ergot derivatives St. John's wort |
Drugs to Hold During Treatment and at Least 2-3 days After Treatment Completion | |
Anticoagulants | Rivaroxaban (Xarelto) |
Anti-Infectives | Erythromycin (Erythrocin) |
Benign prostatic hyperplasia (BPH) drugs | Alfuzosin (Uroxatral) Silodosin (Rapaflo) |
Immunosuppressants | Everolimus (Afinitor) Sirolimus (Rapamune) Tacrolimus (Prograf) |
Lipid-modifiers | Atorvastatin (Lipitor) Lomitapide (Juxtapid) Lovastatin (Mevacor) Rosuvastatin (Crestor) Simvastatin (Zocor) |
Respiratory | Salmeterol (Serevent Diskus) |
Miscellaneous drugs | Colchicine (Colcrys) Finerenone (Kerendia) Naloxegol (Movantik) |
(NIH, 2023)
Remdesivir (Veklury)
Remdesivir (Veklury) works by binding to the viral RNA and inhibiting viral replication. Remdesivir (Veklury), when administered intravenously, has been approved for use in patients at least 28 days old and 3 kg. In nonhospitalized patients with mild to moderate COVID-19 at an increased risk of progression to severe disease, treatment should start within seven days of symptom onset and continue for three days. Hospitalized patients not requiring mechanical ventilation or ECMO should be treated with remdesivir (Veklury) for five days or until discharge from the hospital, whichever occurs first. Per the FDA guidelines, if improvement is not seen after five days, the treatment may be extended for another five days. Dosing for adults and children at least 40 kg is 200 mg on day one and 100 mg once daily starting on day two until treatment discontinuation. Dosing for children weighing 3 to 40 kg is 5 mg/kg on day one, followed by 2.5 mg/kg once daily from day two until treatment discontinuation. Adverse effects include nausea, elevated ALT and AST, increased prothrombin time, and HSRs. Before initiating treatment, the patient's liver function, renal function, and prothrombin time should be evaluated with repeat testing as indicated based on clinical indications. Remdesivir (Veklury) is contraindicated in patients with an eGFR less than mL/min/1.73m2. If ALT increases to more than ten times the upper limit of normal or if the ALT elevation is accompanied by signs or symptoms of liver inflammation (e.g., abdominal distention, hepatomegaly dark urine, anorexia, jaundice, etc.), treatment should be discontinued. Due to the risk of HSRs, treatment should be administered in a setting with close patient monitoring. The patient should be monitored for at least one hour following the infusion. Slower infusion rates, with a maximum infusion time of up to 120 minutes, can be considered to prevent HSR (FDA, 2023e; NIH, 2023). The WHO (2023b) conditionally recommends using remdesivir (Veklury) in those with non-severe COVID-19 at high risk of hospitalization or those with severe COVID-19. It conditionally recommends against its use in those with critical COVID-19 (WHO, 2023b)
Molnupiravir (Lagevrio)
Molnupiravir (Lagevrio) is a nucleoside analogue that inhibits the replication of SARS-CoV-2. It is approved for emergency use in patients over 18 with mild to moderate COVID-19 who are at high risk of progression to severe illness within five days of symptom onset and when other treatment options are unavailable or contraindicated. Recommended dosing is 800 mg orally twice daily for five days. Molnupiravir (Lagevrio) should not be used during pregnancy, and those of reproductive age should utilize birth control methods when engaging in sexual activity while being treated. Adverse effects include diarrhea, nausea, dizziness, and HSRs. No drug interactions have been identified, and dosing adjustments are not indicated in patients with hepatic or renal impairment (FDA, 2023c; NIH, 2023). The WHO (2023b) conditionally recommends using molnupiravir (Lagevrio) in those with non-severe COVID-19 at high risk of hospitalization.
Chloroquine (Aralen) or Hydroxychloroquine (Plaquenil)
Since early in the COVID-19 pandemic, much information has circulated regarding the potential efficacy of chloroquine (Aralen) and hydroxychloroquine (Plaquenil) in treating COVID-19 infections. Chloroquine (Aralen) is FDA-approved for the treatment of malaria, and hydroxychloroquine (Plaquenil) is FDA-approved for the treatment of malaria, lupus erythematosus, and rheumatoid arthritis (RA). Researchers hypothesized that these agents demonstrated antiviral activity in some in vitro systems. Based on preliminary evidence, the FDA issued an EUA for both agents. However, several randomized trials have failed to demonstrate any clinical benefit in COVID-19 patients prescribed chloroquine (Aralen) or hydroxychloroquine (Plaquenil). Neither agent has been shown to lessen the severity or reduce upper or lower respiratory tract viral loads, and some reports demonstrated an increase in the risk of experiencing mild adverse effects. At this time, neither chloroquine (Aralen) nor hydroxychloroquine (Plaquenil) are approved for use in COVID-19 infection (NIH, 2023). The WHO (2023b) strongly recommends against using hydroxychloroquine to treat COVID-19.
Ivermectin (Stromectol)
Ivermectin (Stromectol) is an anthelmintic (anti-parasitic) agent used to treat various conditions, such as scabies. Ivermectin (Stromectol) is widely used in animals to prevent heartworm disease and treat parasitic infections. This medication was evaluated as a potential treatment for COVID-19, as it has been shown to inhibit the replication of SARS-CoV-2 in cell cultures. It has also demonstrated anti-inflammatory properties in vivo animal studies; however, pharmacokinetic and pharmacodynamic studies showed that the dose needed to elicit in vitro antiviral efficiency is 100 times higher than those approved for human use. Due to this, the NIH and WHO recommend against using ivermectin (Stromectol) as a treatment for COVID-19 infection (NIH, 2023; WHO, 2023b).
Convalescent Plasma
Convalescent plasma therapy has been used to treat several viral diseases with varying degrees of efficacy. Plasma is the largest portion of the blood and comprises water, salt, enzymes, proteins, and antibodies. The plasma of many patients who have recovered from COVID-19 contains antibodies against the virus. Administering COVID-19 antibody-rich convalescent plasma to those currently infected with the virus is hypothesized to provide short-term passive immunity (possibly by viral neutralization), thereby contributing to a faster recovery. Currently, the FDA has approved an EUA for high-titer convalescent plasma (contains high levels of anti-SARS-CoV-2 antibodies) in the treatment of patients with COVID-19 that have an immunosuppressive disease or are being treated with immunosuppressive drugs (not including those used to treat COVID-19 such as systemic corticosteroids. According to the FDA, COVID-19 convalescent plasma administration should begin with one high-titer COVID-19 plasma unit (about 200 mL). The administration of additional convalescent plasma units should be based on the prescribing provider's medical judgment and the patient’s clinical response. HCPs are advised to administer the COVID-19 convalescent plasma according to standard hospital procedures and institutional medical and nursing practices for other plasma transfusions. Patients with impaired cardiac function and underlying heart failure should receive a smaller volume or a more prolonged transfusion time. Adverse effects of convalescent plasma do not occur frequently; when they do occur, they often mimic the risks associated with plasma infusion for alternative indications. These risks include transfusion-transmitted infections, allergic reactions including anaphylaxis, transfusion-related acute lung injury (TRALI), transfusion-associated cardiac overload (TACO), hemolytic reactions, febrile nonhemolytic reactions, post-transfusion purpura, and hypothermia. Currently, the NIH panel states there is insufficient data to recommend for or against using convalescent plasma in immunocompromised patients with COVID-19. There is also insufficient data to determine the safety and efficacy of convalescent plasma use in pregnant patients (FDA, 2021; NIH, 2023). The WHO (2023b) issued a strong recommendation in December of 2021 against using convalescent plasma in COVID-19 patients except in severe or critical cases within the context of a clinical trial.
Dexamethasone (Decadron) and Other Corticosteroids
Dexamethasone (Decadron) is a long-acting, systemic glucocorticoid with potent anti-inflammatory effects. Despite initial concerns regarding the potential for prolonged viral replication, dexamethasone (Decadron) has emerged as an effective treatment option in COVID-19 patients requiring supplemental oxygen. Dexamethasone (Decadron) has demonstrated efficacy in preventing an extended cytokine response and accelerating the resolution of pulmonary and systemic inflammation in patients with pneumonia that can lead to lung injury and multisystem organ failure. No benefit was shown when dexamethasone (Decadron) was used in hospitalized patients not requiring supplemental oxygen, and mortality rates increased. The NIH recommends dexamethasone (Decadron) 6 mg PO or IV daily for up to 10 days of treatment or at hospital discharge (whichever is sooner). An oral formulation of dexamethasone (Decadron) achieves up to 86% bioavailability; however, IV should be considered in patients unable to tolerate oral intake or those with impaired gastric absorption. If dexamethasone (Decadron) is unavailable, alternative glucocorticoids may be substituted. The equivalent dose of 6 mg of dexamethasone (Decadron) should be used when these drugs are utilized. For prednisone (Deltasone), the equivalent dose is 40 mg, methylprednisolone (Solumedrol) is 32 mg, and hydrocortisone (Cortef) is 160 mg. Due to differences in half-life, prednisone (Deltasone) and methylprednisolone (Solumedrol) should be administered daily or twice daily, and hydrocortisone (Cortef) must be administered two to four times daily. Adverse effects of dexamethasone (Decadron) include hyperglycemia, avascular necrosis, psychiatric changes, and the development of secondary infections (e.g., mucormycosis or aspergillosis). Since dexamethasone (Decadron) does not have mineralocorticoid properties, there is minimal effect on sodium levels and fluid volume. Dexamethasone (Decadron) is safe to use during pregnancy and lactation and should be administered to those patients that meet the criteria for use (ACEP, 2021; NIH, 2023). The WHO (2023b) makes a conditional recommendation against systemic steroids in non-severe COVID-19 cases and a strong recommendation for their use in those with severe COVID-19.
Immunomodulators
Immunomodulators, including interferons (IFNs) and interleukin (IL)-inhibitors, have been proposed as potential treatment modalities for COVID-19 infections (NIH, 2023).
IFNs
IFNs are a family of cytokines with antiviral properties approved to treat hepatitis B, hepatitis C, and multiple sclerosis. Interferon alfa and beta have been evaluated for the treatment of COVID-19; however, most of the research was conducted in 2020 before more effective treatments were available. Due to more recent studies demonstrating no benefit of utilizing interferon alfa or beta or poor outcomes and patient harm due to treatment with interferon beta, these are no longer recommended. The NIH recommends against using interferon alfa or beta in nonhospitalized or hospitalized patients (NIH, 2023).
Interleukin-6 (IL-6) Inhibitors
Since COVID-19 infections are associated with heightened cytokine release, it is hypothesized that modulating the levels of IL-6 or its effects may alter the course of the disease. Therefore, IL-6 inhibitors have been a source of interest for researchers, particularly sarilumab (Kevzara) and tocilizumab (Actemra). These drugs are approved for several indications, such as moderate to severe RA, polyarticular juvenile idiopathic arthritis (PJIA), and systemic juvenile idiopathic arthritis (SJIA). The FDA has approved tocilizumab (Actemra) for the treatment of COVID-19 in adults that are hospitalized and require supplemental oxygen, mechanical ventilation, or ECMO; however, its use in pediatric patients between 2 and 18 continues to be approved under EUA. The NIH panel recommends adding tocilizumab (Actemra) in hospitalized patients receiving dexamethasone (Decadron) and remdesivir (Veklury) with increasing oxygen needs and systemic inflammation or that require supplemental oxygen use either via nasal cannula or HFNC, NIV, or mechanical ventilation. The recommended dosage of tocilizumab (Actemra) for individuals under 30 kg is 12 mg/kg; for those above 30 kg, the dosing is 8 mg/kg with a maximum dose of 800 mg. Tocilizumab (Actemra) should be administered intravenously as a single infusion over 60 minutes. If tocilizumab (Actemra) is unavailable, the NIH recommends utilizing sarilumab (Kevzara) as an alternative. Sarilumab (Kevzara) is FDA-approved as an SC injection for treating polymyalgia rheumatica; however, when used to treat COVID-19, the medication is administered intravenously. The dosing of sarilumab (Kevzara) is 400 mg reconstituted in 100 mg of 0.9% normal saline and administered over 60 minutes. Adverse effects of tocilizumab (Actemra) and sarilumab (Kevzara) include constipation, diarrhea, nausea, anxiety, insomnia, hypertension, neutropenia, thrombocytopenia, transaminitis, lipid abnormalities, and HSRs. Due to the effects of tocilizumab (Actemra) and sarilumab (Kevzara), liver function, platelet, and neutrophil levels should be assessed before initiating treatment. Severe and potentially fatal infections (including tuberculosis, invasive fungal, bacterial, viral, protozoal, and other opportunistic infections) have been reported in patients receiving tocilizumab (Actemra). Further, reactivation of prior infections such as HIV, hepatitis B, and tuberculosis has also been reported. Tocilizumab (Actemra) and sarilumab (Kevzara) should not be used in patients with a GI perforation or those that are at an increased risk, those with a history of HSR to either drug, patients with ALT or AST results that are five times the upper limit (the FDA cut off is ten times the upper limit), have a concurrent infection, neutrophil count less than 500 cells/µL, or platelet count less than 50,000 cells/µL (FDA, n.d.-a, 2017; NIH, 2023). The WHO (2023b) has issued a strong recommendation for the use of IL-6 blockers tocilizumab (Actemra) and sarilumab (Kevzara) in severe or critical COVID-19 cases.
Baricitinib (Olumiant)
Baricitinib (Olumiant) is an oral Janus kinase (JAK) inhibitor used to treat moderate to severe RA. Its mechanism in treating COVID-19 infections is premised on the belief that it disrupts regulators of endocytosis, which may help reduce viral entry and inflammation. It is also thought to interfere with intracellular virus particle assembly and have antiviral activity. The FDA has approved baricitinib (Olumiant) for the treatment of COVID-19 in combination with remdesivir (Veklury) in adults that are hospitalized and require supplemental oxygen, mechanical ventilation, or ECMO; however, its use in pediatric patients between 2 and 18 continues to be approved under EUA. It is preferred over tocilizumab (Actemra) in patients that require oxygen via HFNC or non-invasive mechanical ventilation. If baricitinib (Olumiant) or tocilizumab (Actemra) is unavailable, oral tofacitinib (Xeljanz) can be used as a substitute. Baricitinib (Olumiant) is administered orally once daily for 14 days or until hospital discharge, whichever comes first. Dosing for adults with an eGFR of at least 60 mL/min/1.73m2 is 4 mg, with dosing adjustments made based on eGFR results. Baricitinib (Olumiant) is not recommended for patients with an eGFR less than 15 mL/min/1.73m2. Dosing for children ages 9 to 17 follows the dosing for adults. Dosing for children aged 2 to 9 with an eGFR above 60 mL/min/1.73m2 is 2 mg. Potential adverse effects include lymphoma, thrombosis, pulmonary embolism, urinary tract infection, neutropenia, and elevated liver enzymes. There is also an increased risk of opportunistic infections, malignancies including lung cancer and lymphomas, and cardiovascular events such as myocardial infarction or stroke (FDA, 2022; NIH, 2023). The WHO (2023b) strongly recommends using the JAK inhibitor baricitinib (Olumiant) in severe or critical COVID-19 cases. They also endorse combining this with an IL-6 blocker and systemic corticosteroids. They conditionally recommend against the use of the JAK inhibitors ruxolitinib (Jakafi) and tofacitinib (Xeljanz) in severe or critical COVID-19 cases (WHO, 2023b).
Anticoagulation (AC) in Patients with COVID-19
Various organizations, including the NIH, CDC, and the American Society of Hematology (ASH), have published interim guidance for AC management in hospitalized patients with COVID-19. Several studies have shown that prophylactic dosing of AC in critically ill patients with COVID-19 is associated with a lower risk of VTE but a higher risk of major bleeding events. The consensus amongst the guidelines is that if a patient is being treated with AC due to an underlying disease, this treatment should be continued unless bleeding occurs. The NIH panel and the ASH recommend using prophylactic-dose AC over therapeutic-dose AC in patients with COVID-19-related critical illness who do not have suspected or confirmed VTE; however, in patients that are critically ill and require ECMO or continuous renal replacement therapy, anticoagulation protocols related to those interventions should be followed. Per the ASH, the optimal AC therapy, such as low-molecular-weight heparin (LMWH) versus unfractionated heparin (UFH; see Table 6), and its effect on clinical outcomes is uncertain; however, the NIH recommends using LMWH over UFH. It is recommended that LMWH or UFH be used over oral AC agents due to their shorter half-lives, ability to be administered intravenously or SC, fewer drug interactions, and quick reversal process. The NIH panel does not recommend routine screening for VTE in patients unless signs and symptoms are present. For hospitalized COVID-19 patients who experience rapid deterioration of pulmonary, cardiac, or neurological function or the sudden, localized loss of peripheral perfusion, the possibility of thromboembolic disease should be urgently evaluated and considered (ASH, 2022; NIH, 2023).
The ASH and NIH panel recommend initiating heparin at a therapeutic dose for acutely ill patients with an elevated D-dimer requiring supplemental oxygen unless contraindicated. Contraindications include a platelet count less than 50 x 109/L, hemoglobin less than 8 g/dL, a history of a bleeding disorder, or bleeding within the previous 30 days that required an emergency room visit. If a patient is started on a therapeutic dose of heparin, the treatment should continue for 14 days or until they are transferred to a higher level of care or discharged from the hospital, whichever event occurs first. In patients that do not require hospitalization, the NIH and the ASH recommend against utilizing ACs such as aspirin or P2Y12 inhibitors (e.g., clopidogrel [Plavix]) to prevent VTE. In pregnant patients hospitalized with severe COVID-19, prophylactic-dose AC is recommended if there are no contraindications to its use. If AC therapy is prescribed before diagnosing COVID-19 in a pregnant patient, it should be continued (ASH, 2022; NIH, 2023).
Table 6
Anticoagulation
AC Type | Description |
LMWH | LMWHs are SC injections that inhibit thrombin and factor Xa, two primary components of the coagulation cascade and healthy blood clotting. Some of the most common LMWH agents include enoxaparin (Lovenox), dalteparin (Fragmin), and tinzaparin (Innohep).
|
UFH | UFH is a rapidly acting agent that binds to antithrombin and quickly enhances its ability to inhibit FXa and FIIa. UFH is administered via SC injection or as a continuous IV infusion. Dosing is determined by body weight, and patients require frequent monitoring while on treatment with UFH to ensure safety. UFH has been the preferred treatment in patients at heightened risk for bleeding due to its short half-life and reversibility. While the definitive half-life of UFH depends on the dose, the average half-life is about 30 to 90 minutes in most healthy adults. Protamine sulfate (Prosulf) is the reversal agent in the event of life-threatening bleeding.
|
(Badireddy & Mudipalli, 2023; Douketis, 2022; Longo, 2019; Witt et al., 2018)
Adjunctive Therapies and Concomitant Medications
Vitamin C
Vitamin C (ascorbic acid) is a water-soluble and potent antioxidant believed to benefit patients with severe and critical illness based on its anti-inflammatory properties, influences on cellular immunity, and vascular integrity. Research demonstrates that people may need more vitamin C in states of oxidative stress. Therefore, the role of vitamin C supplementation has been evaluated in numerous disease states, including sepsis and cancer. Since severe and critical COVID-19 infections can cause sepsis and ARDS, the potential role of high doses of vitamin C in lessening inflammation and vascular injury in patients with COVID-19 has been a topic of interest that remains under investigation. No controlled trials have demonstrated a statistically significant benefit of using vitamin C to treat COVID-19. According to the NIH panel, there is insufficient data to recommend either for or against using vitamin C to treat COVID-19 in hospitalized or nonhospitalized patients. Further clinical trials with large sample sizes are needed to modify the treatment guidelines (NIH, 2023; NIH Office of Dietary Supplements, 2021; Olczak-Pruc et al., 2022).
Vitamin D
Vitamin D (calciferol) is a fat-soluble vitamin found in certain foods and is also produced endogenously when ultraviolet rays from the sunlight trigger its synthesis through the skin. Vitamin D enables normal bone mineralization by promoting calcium absorption and maintaining adequate serum calcium and phosphate concentrations. It also acts as an anti-inflammatory, modulating various cellular processes, such as cell growth, neuromuscular and immune function, and glucose metabolism. The rationale for vitamin D use is its immunomodulatory effects, which could protect against COVID-19 infection or decrease illness severity. Many studies have been published on vitamin D use in COVID-19; however, these studies had limitations such as small sample sizes or lack of randomization of participants. Despite the findings that vitamin D deficiency increases an individual's risk of COVID-19 infection and poor patient outcomes, there is insufficient evidence that vitamin D supplementation is beneficial for preventing infection or decreasing illness severity and improving patient outcomes, with some studies revealing no reduction in the length of the hospital stay or the mortality rate compared to placebo. Further, high vitamin D levels are linked to adverse effects of hypercalcemia and nephrocalcinosis. The definitive role of vitamin D supplementation in preventing or treating COVID-19 remains unknown, and observational studies continue to evaluate the role of vitamin D in preventing and treating COVID-19. The NIH panel states there is insufficient data to recommend either for or against using vitamin D to prevent or treat COVID-19 (NIH, 2023; NIH Office of Dietary Supplements, 2022a).
Zinc
Zinc is an essential mineral naturally found in food and can be taken as a dietary supplement. Zinc plays a role in cellular metabolism, including the catalytic activity of enzymes, improved immune function, wound healing, and protein and DNA synthesis. Zinc has been used to treat the common cold, childhood pneumonia, and age-related macular degeneration. For other viruses, increased intracellular zinc levels have been shown to impair viral replication and induce apoptosis; however, the effects of zinc on the severity of COVID-19 infection and its impact on patient outcomes are still being studied. Long-term zinc supplementation is associated with risks, as it can cause copper deficiency, which can induce potentially irreversible neurologic manifestations (e.g., myelopathy, paresthesia, ataxia, and spasticity) and hematologic effects (e.g., anemia and leukopenia). Currently, the NIH panel does not recommend either for or against zinc use for the treatment of COVID-19. Moreover, the panel recommends against using zinc supplementation above the recommended dietary allowance (11 mg daily for men and 8 mg for non-pregnant women; NIH, 2023; NIH Office of Dietary Supplements, 2022b).
Reinfection and Breakthrough Infection
Like other viral infections of the respiratory tract, there is a risk of reinfection with SARS-CoV-2. The prevalence, risk factors, and severity of reinfection with COVID-19 are highly based on the variant of initial and reinfection. The omicron variant has shown the lowest level of protection against reinfection at 36% at 40 weeks post-initial infection versus other variants, with an average protection level against reinfection of over 85% at 40 weeks post-initial infection. Reinfection occurs when the immune response gained from the initial infection begins to lessen. NAAT should be used to diagnose individuals that begin to have symptoms of COVID-19 after initial infection. There is also evidence of breakthrough COVID-19 infections in those individuals that have been vaccinated with or without receiving booster doses; however, breakthrough infection in those that have been vaccinated is less likely to lead to severe or critical illness (Cohen & Pulliam, 2023; NIH, 2023).
Vaccination
To slow the spread of COVID-19 and decrease the severity of illness, a large proportion of the world needs to develop immunity to the virus. The safest and most effective way to accomplish this is through vaccination. It is recommended that all eligible individuals get vaccinated and follow guidelines related to required booster doses to maintain immunity. Vaccines work by impersonating the infectious bacteria or viruses that inflict disease to prompt the body to create antibodies that fight the condition upon exposure. Vaccination is a prime example of adaptive immunity, as it stimulates the creation of antibodies against a pathogen to prevent acquiring the illness later. If that pathogen tries to invade the body, the adaptive immune system responds more quickly, producing additional antibodies to address the infection and thwart illness. Vaccination stimulates the body’s immune system to build defenses against the infectious organism without inducing the disease. The most common types of vaccines contain a weakened version or inactivated parts of a particular organism to trigger an immune response. Typically, the vaccine comprises a minuscule, weakened, and non-dangerous fragment of the organism that includes specific parts of the antigen. This tiny amount is enough for the body to learn how to build the particular antibody in the event of an encounter with the actual antigen later. More novel vaccines contain the blueprint for producing antigens rather than the antigen itself. Regardless of whether the vaccine is made up of the antigen itself or the blueprint so that the body will produce the antibodies, the recipient does not acquire the illness from the vaccine. Some vaccines require multiple doses, given weeks or months apart. This is sometimes needed to allow for the production of long-lived antibodies and the development of long-standing memory cells. Table 7 compares the major types of vaccines used throughout the US (CDC, 2023b; McCance & Huether, 2019; WHO, 2020).
Table 7
Major Types of Vaccines
Type | Description and Example |
Inactivated |
|
Live/attenuated |
|
Toxoid |
|
Conjugate |
|
Subunit |
|
(CDC, 2023b)
COVID-19 Vaccines
Vaccine development is traditionally a prolonged, multi-step, and intricate process that takes at least ten years of research and testing before the vaccine is marketed. The FDA is the regulatory authority in the US that oversees the safety, effectiveness, and quality of vaccines before approving them for use. The year 2020 was marked by significant uncertainty, turmoil, and tragedy brought forth by the COVID-19 virus; however, it was also characterized by the fastest vaccine developed in the US. Currently, three COVID-19 vaccine options are available in the US: Pfizer-BioNTech, Moderna, and Novavax (CDC, 2023d; FDA, 2023b).
Pfizer/BioNTech and Moderna mRNA Vaccines
Pfizer and Moderna not only developed the first two COVID-19 vaccines in the US, but their chemical structure, technology, and mechanism of building immunity are very similar. Both vaccines use mRNA to generate immunity to COVID-19, which scientists have studied and broadly tested for over a decade but have never been brought to market before. Immunity induced from mRNA vaccines is distinct from traditional vaccines as they do not contain a weakened or inactivated version of the COVID-19 virus; thus, there is no potential risk of infection or insertional mutagenesis. These vaccines contain only the genetic material for a specific protein, and no genetic material enters the nucleus of the cells. When the vaccine is injected, the mRNA is taken up by macrophages near the injection site. The mRNA provides a blueprint (i.e., instructions) to the immune system cells, teaching them how to create the S protein unique to SARS-CoV-2. The S protein subsequently appears on the macrophages' surface, triggering the body’s immune response and directing the production of antibodies that recognize and react to the S protein. Once the mRNA has completed its task, it is rapidly degraded by enzymes and removed from the body. This enzymatic degradation occurs naturally in the body to remove dead, unwanted, or damaged cells (MedlinePlus, 2022).
Originally these vaccines were monovalent; however, FDA approval for the monovalent form of these vaccines has been removed, and the bivalent versions of these vaccines are now available and FDA-approved for use. Since this occurred in April 2023, the original monovalent versions of these vaccines can no longer be used. These vaccines are considered bivalent because they have two mRNA components: one that protects against the original strain of the COVID virus, which provides broad immunity, and another that protects against the omicron variant. Due to the approval and release of the bivalent vaccine, the dosing and administration guidelines for initial vaccination have also been updated. These guidelines are outlined in Table 8. Following the process established to determine the targeted strain of the flu vaccine each year, the FDA met in June 2023 to determine which strains and variants are most likely to affect individuals during the winter months, with manufacturers adapting the formulation to be more effective against these identified strains and variants (FDA, 2023a).
Table 8
Pfizer and Moderna COVID-19 Vaccine Dosing
Age | Bivalent Vaccine | Bivalent Doses Indicated | Dosage Interval | Dosage |
6 months- 4 years | Pfizer | 3 | 3-8 weeks between doses 1 and 2; 8 weeks between doses 2 and 3 | 0.2 mL/3 ug |
Moderna | 2 | 4-8 weeks between doses | 0.25 mL/25 ug | |
5 years | Pfizer | 1 | N/A | 0.2 mL/10 ug |
Moderna | 2 | 4-8 weeks between doses | 0.25 mL/25 ug | |
6-11 years | Pfizer | 1 | N/A | 0.2 mL/10 ug |
Moderna | 1 | N/A | 0.25 mL/25 ug | |
12 and older | Pfizer | 1 | N/A | 0.3 mL/30 ug |
Moderna | 1 | N/A | 0.5 mL/50 ug | |
Individuals older than 65 can receive an additional dose of either bivalent mRNA vaccine at least four months after the first dose. | ||||
(CDC, 2023d)
Although found to be generally safe, a history of a severe allergic reaction (e.g., anaphylaxis) after a previous dose of an mRNA COVID-19 vaccine or any of its components warrants using a different type of vaccine. If myocarditis or pericarditis develops after receiving a dose of any COVID-19 vaccine, no further doses should be administered (CDC, 2023d). There are also a few contraindications to mRNA vaccines, which include the following:
- a history of an immediate allergic reaction of any severity to a prior dose of an mRNA COVID-19 vaccine or any of its components
- a history of anaphylaxis after any vaccination or injected therapy
- the presence of acute illness that is severe, with or without the presence of a fever (CDC, 2023d)
The CDC defines an immediate allergic reaction as any HSR or symptoms consistent with urticaria, angioedema, respiratory distress (e.g., wheezing, stridor), or anaphylaxis. These symptoms often occur within 15-30 minutes of vaccination but may take up to 4 hours after administration to develop. Patients meeting any of the above criteria should not be vaccinated with a second dose of the same type of vaccine. Anaphylactic reactions in persons receiving mRNA COVID-19 vaccines outside clinical trials have been reported. The CDC recommends that HCPs become familiar with identifying immediate-type allergic reactions and have a plan to respond to these emergencies (CDC, 2022b). The following medications and equipment should be readily available at all sites where mRNA vaccines are administered:
- at least three prefilled syringes of age-appropriate epinephrine or autoinjectors
- antihistamines
- histamine-1 (H1) blocker, such as diphenhydramine (Benadryl)
- histamine-2 (H2) blocker, such as famotidine (Pepcid)
- antihistamines may be given as adjunctive therapy but should not be used as the initial or sole treatment for suspected anaphylaxis
- pulse oximeter
- blood pressure monitor
- supplemental oxygen
- bronchodilators (e.g., albuterol [ProAir])
- intravenous fluids
- intubation kit (CDC 2022b)
Both the Pfizer and Moderna bivalent COVID-19 vaccines have a risk for adverse effects. Adverse effects are either localized or systemic. In general, symptoms were more severe following the second dose of either vaccine and were more prevalent in adolescents and children. Systemic adverse effects were mild in severity, appearing 1-2 days following vaccine administration and resolving after 1-2 days (CDC, 2023d). Table 9 compares the side effect profiles of the Pfizer and Moderna vaccines.
Table 9
mRNA Vaccine Side Effect Profiles
Pfizer | Moderna |
|
|
(ModernaTX, 2023; Pfizer, 2023)
Receipt of the Pfizer or Moderna vaccine will not affect the results of viral COVID-19 tests performed after vaccination. These tests detect active disease and not immunity; the vaccine does not cause the virus or induce viral replication. Therefore, if a patient who has previously received the vaccine subsequently has a positive viral test, they should be treated as having an active COVID-19 infection. Since the vaccine is intended to induce an immune response, antibody tests may be positive in vaccinated patients (CDC, 2023d).
Novavax Vaccine
The Novavax is a recombinant nanoparticle vaccine created by Novavax that is approved for EUA for individuals 12 years or older. It is a protein-based subunit vaccine that works differently than the other COVID-19 vaccines. It comprises a synthetically engineered version of the S protein and an adjuvant (i.e., saponin-based Matrix-M). The adjuvant signals the immune system to act, enhancing the immune response to produce large quantities of neutralizing antibodies. This technology is not new, as it has been effectively used for the hepatitis B (HepB) and human papillomavirus (HPV) vaccines, but it does take longer to develop. The Novavax vaccine does not contain live virus particles; thus, it cannot replicate or cause infection with COVID-19. Primary dosing of Novavax includes two 0.5 mL doses administered three weeks apart. Novavax administration is contraindicated in individuals with a history of severe allergic reactions to any vaccine component. Adverse effects of Novavax include pain and tenderness at the injection site, fatigue, muscle pain, nausea and vomiting, headaches, fever, chills, myocarditis, and pericarditis. As the use of Novavax becomes more widespread, additional adverse effects may be identified. There is currently no information regarding using Novavax in conjunction with other vaccines (CDC, 2023d; Novavax, 2023).
Clinical Considerations for Vaccine Use in Special Populations
Prior or Current COVID-19 Infection
Exposure to COVID-19 does not prohibit an individual from being vaccinated. If the individual is asymptomatic, they may receive one of the COVID-19 vaccines. It is important to educate individuals that vaccination is not used as post-exposure prophylaxis but will prevent future severe COVID-19 infections. Vaccination is also recommended for individuals with a history of asymptomatic or symptomatic COVID-19. This also includes those individuals that have prolonged post-COVID-19 symptoms. If the individual has an active COVID-19 infection, vaccination should be postponed until symptoms resolve and the criteria to discontinue isolation have been met; however, in these individuals, vaccination may be delayed until three months after symptom onset (if symptomatic) or positive test. Evidence has shown that this delay between active infection and vaccination may improve immune response. This delay is considered safe as there is a low risk of reinfection in the weeks to months following active infection (CDC, 2023d; NIH, 2023).
Persons who Previously Received Antibody-Based Therapy
Individuals that have received antibody products for post-exposure prophylaxis or treatment may be vaccinated at any time. The administration of monoclonal antibodies or convalescent plasma does not require that vaccination be delayed; however, there may be a decrease in antibody titers in these individuals. The significance of this reduction in antibody titers is currently unknown, and the benefit of vaccination outweighs the risks of diminished vaccine effectiveness (CDC, 2023d)
Pregnancy and Lactation
Pregnancy alone places individuals in a high-priority group and thereby eligible for vaccination. Pregnancy has been identified as a condition that increases the risk of serious illness with a COVID-19 infection, including hospitalization, ventilation requirement, and death. The American College of Obstetricians and Gynecologists (ACOG), the CDC, and the Society for Maternal-Fetal Medicine collectively support and recommend vaccination for pregnant or lactating individuals or those actively trying to become pregnant or that may become pregnant sometime in the future. Evidence has shown that the risks of vaccination for this population do not outweigh the benefits. Although there are three vaccines available, ACOG recommends the use of a bivalent mRNA for the primary vaccination in this population due to limited studies of the use of Novavax in pregnant and lactating individuals (ACOG, 2023; CDC, 2023d; NIH, 2023).
Cancer and Other Immunocompromised Conditions
Vaccination is the most effective way to prevent COVID-19 infection. This includes those individuals with active cancer and those receiving cancer treatment. Since the available COVID-19 vaccines do not contain live viruses, they are safe for immunocompromised patients. Individuals older than six months and moderately to severely immunocompromised are eligible to receive an additional dose of either mRNA vaccine. Patients and HCPs should follow the vaccine administration guideline geared toward the patient's immune status at the time of vaccination. Individuals with planned chemotherapy should undergo vaccination at least two weeks before the start of treatment. Individuals that have received a stem cell transplant should wait three months after treatment to undergo COVID-19 vaccination. Due to the risk of developing severe Covid if infected, all family members and close contacts of an immunocompromised individual should undergo vaccination (CDC, 2023d; NIH, 2023). Table 10 outlines the vaccination schedule for unvaccinated and moderately to severely immunocompromised individuals.
Table 10
Pfizer and Moderna COVID-19 Vaccine Dosing
Age | Bivalent Vaccine | Bivalent Doses Indicated | Dosage Interval | Dosage |
6 months- 4 years | Pfizer | 3 | 3 weeks between doses 1 and 2; 8 weeks between doses 2 and 3 | 0.2 mL/3 ug |
Moderna | 3 | 4 weeks between doses 1 and 2; at least 4 weeks between doses 2 and 3 | 0.25 mL/25 ug | |
5 years | Pfizer | 3 | 3 weeks between dose 1 and 2 and at least 4 weeks between dose 2 and 3 | 0.2 mL/10 ug |
Moderna | 3 | 4 weeks between doses 1 and 2; at least 4 weeks between doses 2 and 3 | 0.25 mL/25 ug | |
6-11 years | Pfizer | 3 | 3 weeks between doses 1 and 2; at least 4 weeks between doses 2 and 3 | 0.2 mL/10 ug |
Moderna | 3 | 4 weeks between doses 1 and 2; at least 4 weeks between doses 2 and 3 | 0.25 mL/25 ug | |
12 and older | Pfizer | 3 | 3 weeks between doses 1 and 2; at least 4 weeks between doses 2 and 3 | 0.3 mL/30 ug |
Moderna | 3 | 4 weeks between doses 1 and 2; at least 4 weeks between doses 2 and 3 | 0.5 mL/50 ug |
(CDC, 2023d)
Post-Covid Conditions
Post-COVID conditions is a blanket term for a range of COVID-19 symptoms that persist for at least four weeks or a lack of return to the individual's baseline state of health before contracting COVID-19. It is also called long-COVID, post-acute COVID-19, or chronic COVID. For the remainder of the section, the term long-COVID will be used to describe this condition. It is estimated that long-COVID affects 10% to 30% of nonhospitalized and 50% to 70% of hospitalized individuals that have experienced a COVID-19 infection. The severity of the original illness does not determine whether a patient may be affected by long-COVID, and some individuals that were asymptomatic during the initial acute infection may experience symptoms related to long-COVID. Long-COVID is characterized by persistent symptoms, the development of symptoms in asymptomatic individuals, symptoms that reappear after a period of improvement, or worsening symptoms of COVID-19 or those of a preexisting condition. Factors that may influence the development of long-COVID include an underlying health condition (e.g., asthma, cancer, or chronic kidney disease), physical or mental changes as a result of illness (e.g., depression, anxiety, or deconditioning), or socioeconomic challenges as a result of illness. Although the cause of long-COVID is unknown, it is possibly caused by organ damage, a prolonged inflammatory state, autoimmunity, or an inadequate response of antibodies to the infection. Laboratory and imaging studies may be conducted to assess the patient or eliminate other underlying conditions; however, the lack of abnormal results does not eliminate the presence of or minimize the effects of long-COVID on the individual's quality of life. Other conditions that may mimic long-COVID include chronic fatigue syndrome, fibromyalgia, and Lyme disease. Common symptoms experienced with long-COVID include dyspnea, abdominal pain, diarrhea, insomnia, impaired ability to complete daily activities, fatigue, cognitive impairment, headaches, tachycardia, tinnitus, and anosmia. Long-COVID may also result in the development of chronic conditions such as cardiovascular disease, type 2 diabetes mellitus, or postural orthostatic tachycardia syndrome (POTS). Long-COVID is typically treated on an outpatient basis, focusing on symptom management. More research is needed on this phenomenon, as understanding what causes long-COVID and how to manage it effectively is incomplete. To address the need for more research and support patients affected, long-COVID clinics have been established at medical centers across the US, bringing together individuals affected and HCPs from various specialties to conduct thorough research (CDC, 2022f; Davis et al., 2023)
References
American Board of Internal Medicine. (2023). ABIM laboratory test reference ranges – July 2023. https://www.abim.org/Media/bfijryql/laboratory-reference-ranges.pdf
American College of Emergency Physicians. (2021). ACEP covid-19 field guide. https://www.acep.org/corona/covid-19-field-guide/cover-page
American College of Obstetricians and Gynecologists. (2023). COVID-19 vaccination considerations for obstetric-gynecologic care. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care
American Society of Hematology. (2022). ASH guidelines on the use of anticoagulation in patients with COVID-19. https://www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines/ash-guidelines-on-use-of-anticoagulation-in-patients-with-covid-19
Anesi, G. L. (2022). COVID-19: Epidemiology, clinical features, and prognosis of the critically ill adult. UpToDate. Retrieved August 1, 2023, from https://www.uptodate.com/contents/covid-19-epidemiology-clinical-features-and-prognosis-of-the-critically-ill-adult
Azer, S. A. (2020). COVID-19: Pathophysiology, diagnosis, complications, and investigational therapeutics. New Microbes and New Infections, 37(100738), 1-8. https://doi.org/10.1016/j.nmni.2020.100738
Badireddy, M., & Mudipalli, V. R. (2023). Deep venous thrombosis prophylaxis. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK534865
Bahl, P., Doolan, C., de Silva, C., Chughtai, A. A., Bourouiba, L., & MacIntyre, C. R. (2022). Airborne or droplet precautions for health workers treating coronavirus disease 2019? The Journal of Infectious Diseases, 225(9), 1561-1568. https://doi.org/10.1093/infdis/jiaa189
Berghella, V., & Hughes, B. L. (2023). COVID-19: Overview of pregnancy issues. UpToDate. Retrieved July 24, 2023. from https://www.uptodate.com/contents/covid-19-overview-of-pregnancy-issues#H53441334
Caliendo, A. M., & Hanson, K. E. (2022). COVID-19: Diagnosis. UpToDate. Retrieved July 27, 2023, from https://www.uptodate.com/contents/covid-19-diagnosis#H4291362019
Centers for Disease Control and Prevention. (n.d.). How to collect a nasal mid-turbinate specimen for COVID-19 testing [Image]. Retrieved July 28, 2023, from https://www.nihb.org/covid-19/wp-content/uploads/2021/07/How-to-Collect-a-Nasal-Mid-Turbinate-Specimen-for-COVID-19-Testing.pdf
Centers for Disease Control and Prevention. (2021). Scientific brief: SARS-CoV-2 transmission. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/sars-cov-2-transmission.html
Centers for Disease Control and Prevention. (2022a). Ending isolation and precautions for people with COVID-19: Interim guidance. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html
Centers for Disease Control and Prevention. (2022b). Interim considerations: Preparing for the potential management of anaphylaxis after COVID-19 vaccination. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html
Centers for Disease Control and Prevention. (2022c). Interim guidelines for collecting and handling of clinical specimens for COVID-19 testing. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html
Centers for Disease Control and Prevention. (2022d). Interim guidelines for COVID-19 antibody testing. https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing/antibody-tests-guidelines.html
Centers for Disease Control and Prevention. (2022e). Other diagnostic tests for SARS-CoV-2. https://www.cdc.gov/coronavirus/2019-ncov/lab/testing/other-diagnostic-tests-sars-cov2.html
Centers for Disease Control and Prevention. (2022f). Post-COVID conditions: Information for healthcare providers. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html
Centers for Disease Control and Prevention. (2022g). Pregnant and recently pregnant people: At increased risk for severe illness from COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/pregnant-people.html
Centers for Disease Control and Prevention. (2023a). Clinical care considerations: Clinical considerations for care of children and adults with confirmed COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/clinical-considerations-index.html
Centers for Disease Control and Prevention. (2023b). Explaining how vaccines work. https://www.cdc.gov/vaccines/hcp/conversations/understanding-vacc-work.html
Centers for Disease Control and Prevention. (2023c). Infection control guidance. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html
Centers for Disease Control and Prevention. (2023d). Interim clinical considerations for use of COVID-19 vaccines in the United States. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
Centers for Disease Control and Prevention. (2023e). Interim infection prevention and control recommendations for healthcare personnel during the Coronavirus Disease 2019 (COVID-19) pandemic. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html
Centers for Disease Control and Prevention. (2023f). Occupationally-acquired infections in healthcare settings. https://www.cdc.gov/infectioncontrol/oai-hcp.html
Centers for Disease Control and Prevention. (2023g). Overview of testing for SARS-CoV-2, the virus that causes COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html
Centers for Disease Control and Prevention. (2023h). People with certain medical conditions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
Choe, P. G., Kim, K.-H., Kang, C. K., Suh, H. J., Kang, E., Lee, S. Y., Kim, N. J., Yi, J., Park, W. B., & Oh, M. (2021). Antibody responses 8 months after asymptomatic or mild SARS-CoV-2 infection. Emerging Infectious Diseases, 27(3). https://doi.org/10.3201/eid2703.204543
Cohen, C., & Pulliam, J. (2023). COVID-19 infection, reinfection, and the transition to endemicity. The Lancet, 401(10379), 798-800. https://doi.org/10.1016/S0140-6736(22)02634-4
COVID-19 Treatment Guidelines Panel. (2023). Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf
Dan, J. M., Mateus, J., Kato, Y., Hastie, K. M., Yu, E. D., Falithi, C. E., Grifoni, A., Ramirez, S. I., Haupt, S., Frazier, A., Nakao, C., Rayaprolu, V., Rawlings, S. A., Peters, B., Krammers, F., Simon, V., Saphire, E. O., Smith, D. M., Weiskopf, D., . . . Crotty, S. (2021). Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science, 371(6529), 1-23. https://doi.org/10.1126/science.abf4063
Davis, H. E., McCorkell, L., Vogel, J. M., & Topol, E. J. (2023). Long COVID: Major findings, mechanisms, and recommendations. Nature Reviews Microbiology, 21, 133-146. https://doi.org/10.1038/s41579-022-00846-2
Douketis, J. D. (2022). Drugs for deep venous thrombosis. Merck Manual Professional Version. https://www.merckmanuals.com/professional/cardiovascular-disorders/peripheral-venous-disorders/drugs-for-deep-venous-thrombosis
Dueñas-Castell, C., Polanco-Guerra, C. J., Martinez-Ávila, M. C., Hurtado, A. J. A., Yanez, T. R., Gutierrez-Ariza, J. C., & Rico-Fontalvo, J. (2022). When to use antibiotics in COVID-19: A proposal based on questions. Cureus, 14(7), e27398. https://doi.org/10.7759/cureus.27398
Johnson, B. A., Xie, X., Bailey, A. L., Kalveram, B., Lokugamage, K. G., Muruato, A., Zou, J., Zhang, X., Juelich, T., Smith, J. K., Zhang, L., Bopp, N., Schindewolf, C., Vu, M., Vanderheiden, A., Winkler, E. S., Swetnam, D., Plante, J. A., Aguilar, P., . . . Menachery, D. (2021). Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis. Nature, 591, 293-299. https://doi.org/10.1038/s41586-021-03237-4
Kennelly, P. J., Botham, K. M., McGuinness, O., Rodwell, V. W., & Weil, P. A. (2023). Harper’s illustrated biochemistry (32nd ed.). McGraw-Hill.
Longo, D. L. (2019). Harrison’s hematology and oncology (3rd ed.). McGraw-Hill Education.
Lumley, S.F., O’Donnell, D., Stoesser, N. E., Matthews, P. C., Howarth, A., Hatch, S. B., Marsden, B. D., Cox, S., James, T., Warren, F., Peck, L. J., Ritter, T. G., de Toledo, Z., Warren, L., Axten, D., Cornall, R., Jones, E. Y., Stuart, D. I., Screaton, G., . . . Eyre, D. W. (2021). Antibody status and incidence of SARS-CoV-2 infection in health care workers. The New England Journal of Medicine, 284, 533-540. https://doi.org/10.1056/NEJMoa2034545
Malik, B., & Ghatol, A. (2022). Understanding how monoclonal antibodies work. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK572118
McCance, K. L., & Huether, S. E. (2019). Pathophysiology: The biologic basis for disease in adults and children (8th ed.). Elsevier.
McIntosh, K. (2023). COVID-19: Clinical features. UpToDate. Retrieved July 24, 2023, from https://www.uptodate.com/contents/covid-19-clinical-features
MedlinePlus. (2022). What are mRNA vaccines, and how do they work? National Library of Medicine. https://medlineplus.gov/genetics/understanding/therapy/mrnavaccines
ModernaTX. (2023). Fact sheet for healthcare providers administering vaccine: Emergency use authorization of Moderna COVID-19 vaccine, bivalent. https://www.fda.gov/media/167208/download?attachment
Munkdelger, J., Yoshikawa, A., & Bychkov, A. (2023). Infectious viral covid-19. PathologyOutlines.com, Inc. https://www.pathologyoutlines.com/topic/lungnontumorcovid.html
National Center for Health Statistics. (2023). COVID-19 mortality overview: Provisional death counts for COVID-19. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/covid19/mortality-overview.htm
National Institute of Allergy and Infectious Diseases. (2022). Origins of coronaviruses. https://www.niaid.nih.gov/diseases-conditions/origins-coronaviruses
National Institutes of Health. (2023). Clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum
National Institutes of Health Image Gallery. (2017). COPD (chronic obstructive pulmonary disease) [Image]. https://www.flickr.com/photos/nihgov/32486640184
National Institutes of Health Image Gallery. (2020). Novel Coronavirus SARS-CoV-2 [Image]. https://www.flickr.com/photos/nihgov/49643893178/in/photolist
National Institutes of Health Office of Dietary Supplements. (2021). Vitamin C: Fact sheet for health professionals. https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional
National Institutes of Health Office of Dietary Supplements. (2022a). Vitamin D: Fact sheet for health professionals. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional
National Institutes of Health Office of Dietary Supplements. (2022b). Zinc: Fact sheet for health professionals. https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional
Nettina, S. M. (2019). Lippincott manual of nursing practice (11th ed.). Wolters Kluwer
Novavax. (2023). Fact sheet for healthcare providers administering vaccine (vaccination providers): Emergency use authorization (EUA) of the Novavax COVID-19 vaccine, adjuvanted to prevent coronavirus disease 2019 (COVID-19). https://www.fda.gov/media/159897/download?attachment
Nikhra, V. (2020). Exploring pathophysiology of COVID-19 infection: Faux espoir and dormant therapeutic options. International Journal of Clinical Virology, 4, 34-40. https://doi.org/10.29328/journal.ijcv.1001013
Olczak-Pruc, M., Swieczkowski, D., Ladny, J. R., Pruc, M., Juarez-Vela, R., Rafique, Z., Peacock, F. W., & Szarpak, L. (2022). Vitamin C supplementation for the treatment of COVID-19: A systematic review and meta-analysis. Nutrients, 14(19), 4217. https://doi.org/10.3390/nu14194217
Pagana, K. D., Pagana, T. J., & Pagana, T. N. (2022). Mosby's manual of diagnostic and laboratory tests (7th ed.). Elsevier.
Parasher, A. (2020). COVID-19: Current understanding of its pathophysiology, clinical presentation, and treatment. Postgraduate Medical Journal, 97(1147), 312-320. https://doi.org/10.1136/postgradmedj-2020-138577
Peacock, T. P., Goldhill, D. H., Zhou, J., Baillon, L., Frise, R., Swann, O. C., Kugathasan, R., Penn, R., Brown, J. C., Sanchez-David, R. Y., Braga, L., Kavanagh Williamson, M., Hassard, J. A., Staller, E., Hanley, B., Osborn, M., Giacca, M., Davidson, A. D., Matthews, D. A., & Barclay, W. S. (2021). The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nature Microbiology, 6, 899-909. https://doi.org/10.1038/s41564-021-00908-w
Pfizer. (2023). Fact sheet for healthcare providers administering vaccine: Emergency use authorization of Pfizer-BioNTech COVID-19 vaccine, bivalent. https://www.fda.gov/media/167211/download?attachment
Qiao, Y., Wang, X-M., Mannan, R., Pitchiaya, S., Zhang, Y., Wotring, J. W., Xiao, L., Robinson, D. R., Wu, Y-M., Tien, J. C., Cao, X., Simko, S. A., Apel, I. J., Bawa, P., Kregel, S., Narayanan, S. P., Raskind, G., Ellison, S. J., Parolia, A., . . . Chinnaiyan, A. M. (2021). Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2. PNAS, 118(1), e2021450118. https://doi.org/10.1073/pnas.2021450118
Sanders, I., Stather, P., Sivagangan, P., & Al-Jundi, W. (2022). The mysterious risk of arterial thrombosis with COVID-19: A case series and systematic review of acute limb ischaemia. Cureus, 14(5), e25080. https://doi.org/10.7759/cureus.25080
Selchick, F. (2021). COVID-19 prevention strategies [Image].
US Environmental Protection Agency. (2023). Indoor air and coronavirus (COVID-19). https://www.epa.gov/coronavirus/indoor-air-and-coronavirus-covid-19
US Food & Drug Administration. (n.d.-a). Fact sheet for healthcare providers: Emergency use authorization for Actemra. Retrieved August 4, 2023, from https://www.fda.gov/media/150321/download?attachment
US Food & Drug Administration. (n.d.-b). Fact sheet for healthcare providers: Emergency use authorization for Gohibic. Retrieved August 2, 2023, from https://www.fda.gov/media/166824/download?attachment
US Food & Drug Administration. (2017). Highlights of prescribing information: Kevzara (sarilumab). https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761037s000lbl.pdf
US Food & Drug Administration. (2021). Fact sheet for health care providers: Emergency use authorization (EUA) of COVID-19 convalescent plasma for treatment of coronavirus disease 2019 (COVID-19). https://www.fda.gov/media/141478/download?attachment
US Food & Drug Administration. (2022). Highlights of prescribing information: Olumiant (baricitinib). https://pi.lilly.com/us/olumiant-uspi.pdf
US Food & Drug Administration. (2023a). Coronavirus (COVID-19) update: FDA authorizes changes to simplify use of bivalent mRNA COVID-19 vaccines. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-changes-simplify-use-bivalent-mrna-covid-19-vaccines
US Food & Drug Administration. (2023b). Emergency use authorization. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
US Food & Drug Administration. (2023c). Fact sheet for healthcare providers: Emergency use authorization for Lagevrio (molnupiravir) capsules. https://www.fda.gov/media/155054/download?attachment
US Food & Drug Administration. (2023d). Fact sheet for healthcare providers: Emergency use authorization for Paxlovid. https://www.fda.gov/media/155050/download?attachment
US Food & Drug Administration. (2023e). Highlights of prescribing information: Veklury (remdesivir). https://www.gilead.com/-/media/files/pdfs/medicines/covid-19/veklury/veklury_pi.pdf
US Food & Drug Administration. (2023f). In vitro diagnostics EUAs - other tests for SARS-CoV-2. https://www.fda.gov/medical-devices/covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-other-tests-sars-cov-2
Witt, D. M., Nieuwlaat, R. Clark, N. P., Ansell, J., Holbrook, A., Skov, J., Shehab, N., Mock, J., Myers, T., Dentali, F., Crowther, M.A., Agarwal, A., Bhatt, M., Khatib, R., Riva, J. J., Zhang, Y., & Guyatt, G. (2018). American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Advances, 2(22). 3257-3291. https://doi.org/10.1182/bloodadvances.2018024893
World Health Organization. (2020). How do vaccines work? https://www.who.int/news-room/feature-stories/detail/how-do-vaccines-work
World Health Organization. (2022). Contact tracing and quarantine in the context of COVID-19: Interim guidance. https://www.who.int/publications/i/item/WHO-2019-nCoV-Contact_tracing_and_quarantine-2022.1
World Health Organization. (2023a). Clinical management of COVID-19. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2023.1
World Health Organization. (2023b). Therapeutics and COVID-19: Living guideline. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2023.1
Xing, Y., Li, X., Gao, X., & Dong, Q. (2020). Natural polymorphisms are present in the furin cleavage site of the SARS-CoV-2 spike glycoprotein. Frontiers in Genetics, 11(783). https://doi.org/10.3389/fgene.2020.00783