About this course:
This learning activity aims to increase nurses' knowledge of health care-associated infections (HAIs), such as Clostridioides difficile, central line–associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated pneumonia, and antibiotic-resistant organisms such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. This includes understanding the epidemiology and pathophysiology, risk and protective factors, signs and symptoms, diagnosis, treatment and management, and nursing implications of HAIs.
Course preview
Health Care-Associated Infections
This learning activity aims to increase nurses' knowledge of health care-associated infections (HAIs), such as Clostridioides difficile, central line–associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated pneumonia, and antibiotic-resistant organisms such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. This includes understanding the epidemiology and pathophysiology, risk and protective factors, signs and symptoms, diagnosis, treatment and management, and nursing implications of HAIs.
By completing this educational module, learners should be able to:
- define important terminology in infection control, discuss the elements of the chain of infection, and identify the modes and mechanisms of pathogen transmission in the health care environment
- describe the pathophysiology of HAIs
- outline the causes, prevention, treatment, and nursing care of patients with HAIs
- explain factors that influence the transmission of HAIs, identify strategies for infection prevention and control to reduce patient and healthcare provider (HCP) exposure, and minimize the opportunity for the transmission of pathogens
- distinguish multidrug-resistant organisms (MDROs) from other infections
- identify practices to reduce the opportunity for patient exposure to potentially infectious materials in hospital settings
Terminology
Many terms are associated with HAIs, the organisms that cause them, and associated treatment and prevention techniques. A summary of those terms is listed as follows:
- antibody: a protein the immune system produces to neutralize a threat of some kind, such as an infecting organism, a chemical, or some other foreign body
- antimicrobial: able to destroy or suppress the growth of pathogens and other microorganisms
- antiseptic: a substance that reduces the number of pathogens on a surface
- asepsis: method(s) used to ensure an environment is as pathogen-free as possible
- aseptic: as pathogen-free as possible
- chlorhexidine: an antibacterial compound with a substantial residual activity that is used as a liquid antiseptic and disinfectant
- cleaning: the process of removing all foreign material (e.g., dirt, body fluids, lubricants) from objects by using water and detergents or soaps and washing or scrubbing the object
- common vehicle: contaminated material, product, or substance that serves as an intermediate means by which an infectious agent is introduced into a susceptible host through a suitable portal of entry
- contact precautions: measures taken to prevent the spread of diseases transmitted by the physical transfer of pathogens to a susceptible host's body surface
- contamination: the process of becoming unsterile or unclean
- disinfectant: any chemical agent used to destroy or inhibit the growth of harmful organisms
- empiric antibiotic therapy: antibiotics administered before receiving a culture and sensitivity test result
- endemic: prevalent in or characteristic of a particular environment
- endogenous: produced within an organism or a system rather than externally caused
- exogenous: externally caused, rather than produced within an organism or a system
- flora: the aggregate of bacteria, fungi, and other microorganisms typically found in a specific environment, such as the gastrointestinal tract or the skin
- rgin-top-alt:0in;mso-add-space:auto; line-height:normal;mso-list:l7 level1 lfo2;">immunosuppression: the inhibition of the body's protective response to a pathogenic invasion, usually due to disease, drug therapy, or surgery
- infection: invasion and proliferation of pathogens in body tissues
- isolation: the separation of an infected person from others for the period of communicability of a particular disease
- medical asepsis: infection-control practices common in health care, such as hand hygiene
- pathogen: a biological, physical, or chemical entity capable of causing disease, such as bacteria, viruses, fungi, protozoa, helminths, or prions
- personal protective equipment (PPE): devices used to protect employees from workplace injuries or illnesses resulting from biological, chemical, radiological, physical, electric, mechanical, or other workplace hazards
- pneumococcal: pertaining to or caused by pneumococci, organisms of the species Streptococcus pneumoniae (S. pneumoniae), a common cause of pneumonia and other infectious diseases
- portal of exit: the route by which microorganisms exit the reservoir on their way to a susceptible host
- portal of entry: the route by which microorganisms enter a susceptible host
- reservoir: a place in which an infectious agent can survive but may or may not multiply (e.g., HCPs may be reservoirs for nosocomial organisms)
- sepsis: the presence of pathogens or their toxins in blood or tissues
- standard precautions: a group of infection prevention and control strategies that combine the significant features of universal precautions and body substance isolation, based on the principle that all blood, body fluids, secretions, excretions (except sweat), non-intact skin, and mucous membranes may contain transmissible infectious agents
- staphylococcus: a genus of gram-positive bacteria that are potential pathogens, causing local lesions and severe opportunistic infections
- surgical asepsis: techniques used to destroy all pathogenic organisms, also called sterile technique
- susceptible host: a person or animal not possessing sufficient resistance to a particular infectious agent to prevent contracting an infection or a disease when exposed to the agent
- transmission: any mechanism by which a source or reservoir spreads a pathogen to a susceptible host
- transmission-based precautions: measures taken to prevent the spread of diseases from people suspected of being infected or colonized with highly transmissible pathogens that require protections beyond standard precautions to interrupt transmission (i.e., airborne, droplet, and contact precautions
- virulence: the ability of a microorganism to cause disease (American Nurses Association [ANA], n.d.-b; Centers for Disease Control and Prevention [CDC], 2024e; Ernstmeyer & Christman, 2023; The National Institute for Occupational Safety and Health [NIOSH], 2022; Rogers & Brashers, 2023)
The Infectious Process
Transmission of infection in health care consists of six major elements that occur in order, as displayed in Figure 1. If these elements are not all present or do not all happen in sequence, an infection will not develop. Understanding the chain of infection allows HCPs to disrupt the cycle and prevent infection (NIOSH, 2022).
Figure 1
Chain of Infection

(NIOSH, 2022)
Current Context
HAIs, previously referred to as hospital-acquired infections, are nosocomial infections that manifest in a health care setting while the patient is receiving care for another condition. This terminology does not imply that an infection was solely caused by the health care services rendered, only that it manifested following admission to the health care facility. HAIs can develop in susceptible hosts in any health care facility, including hospitals, ambulatory clinics, surgical centers, inpatient rehabilitation facilities, and long-term care facilities (LTCFs). Common types of HAIs include central line–associated bloodstream infections (CLABSI), catheter-associated urinary tract infections (CAUTI), surgical site infections (SSI), hospital-acquired pneumonia (HAP), ventilator-associated pneumonia (VAP), Clostridioides difficile (C. difficile) infection, methicillin-resistant S. aureus (MRSA) infection, and vancomycin-resistant enterococci (VRE) infection (CDC, n.d.-b; Marchaim & Kaye, 2023; Monegro et al., 2023; National Healthcare Safety Network [NHSN], 2025).
HAIs can be endogenous (developing from the patient's flora) or exogenous (originating outside the patient's body). Reservoirs known for transmitting exogenous HAIs include the hands of HCPs, other patients, equipment (e.g., blood pressure cuffs, urine collection devices), and the environment (e.g., contaminated surfaces, toilets, sinks, doorknobs). Annually in the United States, HAIs affect approximately 687,000 persons, leading to more than 72,000 deaths (Cagle et al., 2022). According to the CDC (2024f), approximately 1 in 31 hospitalized patients and 1 in 43 residents of skilled nursing facilities report at least one HAI on any given day. HAIs are associated with high morbidity and mortality with devastating impacts, including prolonged hospitalization, increased suffering, lost productivity, and substantial costs to the health care system and society (Iwu, 2024).
HAIs are monitored closely by agencies such as the NHSN, the most widely used HAI-tracking system. The NHSN collects data to identify problematic areas and standardize infection rates to measure, track, and evaluate HAI prevention modalities. This monitoring allows for a more accurate and direct comparison of infection rates between health care facilities and trends over time. The US Department of Health and Human Services (HHS) has set national targets for reducing HAIs by 2028, using 2022 as the baseline year. The goals are to achieve a 40% reduction in CLABSIs, a 25% reduction in CAUTIs, a 40% reduction in MRSA bacteremia cases, and a 20% reduction in C. difficile infections. Between 2022 and 2023, the United States saw significant progress in reducing infection rates across most categories (Blot et al., 2022; Cagle et al., 2022; CDC, n.d.-a, n.d.-b; HHS, n.d., 2024; Marchaim & Kaye, 2023; Monegro et al., 2023; NHSN, 2025). The infection rates and percentage changes are as follows:
- CLABSI decreased by 13% to 20,032 cases
- CAUTI decreased by 11% to 17,370 cases
- SSI had mixed results, with 9,635 total cases (hysterectomy cases increas by 8%, while colon surgery cases remained unchanged)
- C. difficile infections decreased by 13% to 102,354 cases
- MRSA infections decreased by 16% to 8,107 cases (CDC, 2024d; HHS, n.d., 2024)
The risk for HAIs depends on multiple influences, such as the infection control practices of the health care facility, the prevalence of pathogens within the community or health care setting, and individual patient factors (e.g., compromised immune system, increased length of stay, and comorbidities such as heart disease, COPD, and diabetes mellitus [DM]). Across health care settings, the risk for HAIs is highest among patients admitted to intensive care units (ICUs), accounting for 20–30% of all nosocomial infections. A study involving 231,459 patients across 947 hospitals revealed that 19.5% of patients admitted to the ICU had at least one HAI. The most common HAIs are described below; while not representative of all HAIs, they are among the most common and are associated with severe complications. Most are preventable with appropriate infection prevention strategies. Since October 2008, the Centers for Medicare and Medicaid Services (CMS) no longer reimburse for HAIs, including CLABSI (Blot et al., 2022; CDC, n.d.-b; CMS, 2024; Iwu, 2024; Marchaim & Kaye, 2023; NHSN, 2025; Rajesh et al., 2021).
Types of Health Care–Associated Infections
Clostridioides Difficile
Epidemiology and Pathophysiology
C. difficile is a gram-positive, spore-forming anaerobic bacterium responsible for a spectrum of C. difficile infections (CDIs), including uncomplicated diarrhea, pseudomembranous colitis, and toxic megacolon (life-threatening inflammation of the colon that can lead to sepsis and death). CDI is the most well-known cause of bacterial diarrhea in health care settings, primarily affecting patients who have recently taken antibiotics (typically within the past two months). Pseudomembranous colitis is a more severe form of CDI, characterized by the visualization of pseudomembranous plaques on the colon surface via colonoscopy. C. difficile predominantly produces toxins A and B. A third toxin—binary toxin—has been identified in about 5% of C. difficile isolates. The ability of the organism to form spores enhances its virulence and allows C. difficile to survive for months in the hospital environment (CDC, 2024b; Lamont et al., 2025a, 2025b; Mada & Alam, 2024; Salen & Stankewicz, 2023).
The prevalence of CDIs in the United States has significantly increased since 2000, with only a small decline in recent years. CDC (2024b) data indicates that CDIs cause almost 500,000 illnesses and 29,300 deaths in the United States each year, and 1 in 6 patients diagnosed with a CDI will have a recurrence. According to the Infectious Disease Society of America (IDSA), surveillance for hospital-acquired CDIs should be conducted in all inpatient facilities to screen for elevated rates or outbreaks (CDC, 2024b; IDSA, 2023; Lamont et al., 2025a; Mada & Alam, 2024; Salen & Stankewicz, 2023).
Risk Factors and Protective Factors
The most commonly acknowledged and modifiable risk factor for CDI is antibiotic use within the past month. Other well-known risk factors include age over 65, hospitalization within the past month, and significant comorbid conditions. Additional risk factors encompass BMI over 30, enteral feeding, gastrointestinal surgery, cancer, chemotherapy, hematopoietic stem cell transplantation, inflammatory bowel disease, cirrhosis, NSAID use, and potentially gastric acid suppression. CDI is endemic in acute care facilities due to high antimicrobial use and significant contamination with C. difficile spores. Inappropriate antibiotic prescribing, such as using unnecessary or unsuitable antibiotics, increases the risk of patients developing CDI (CDC, 2024b; Lamont et al., 2025a; Mada & Alam, 2024; Monegro et al., 2023).
Signs and Symptoms
Given that CDI most commonly occurs following antibiotic use, clinical manifestations typically develop within 10 days of starting antibiotics; however symptoms may appear as early as the first day of antibiotic use or as late as 10 weeks later. CDI is characterized by at least three (but may be more than 20) watery or unformed stools per day. The stools typically exhibit a characteristic odor. Patients with CDI may also have abdominal cramping, anorexia, nausea, fever, and leukocytosis. Rarely, patients with CDI have occult fecal blood (CDC, 2024b; Lamont et al., 2025b; Mada & Alam, 2024).
Diagnosis
According to the IDSA guidelines, laboratory testing for CDI should not be performed on patients who have received a laxative within the previous 48 hours. Diagnostic testing should be done for symptomatic patients (Bishop & Tiruvoipati, 2022; Lamont et al., 2025b; McDonald et al., 2018). To help distinguish asymptomatic carriage from active infection, a two-tiered testing approach is preferred, in which a test with high sensitivity (e.g., glutamate dehydrogenase [GDH] or nucleic acid amplification testing [NAAT]) is combined with a test with high specificity (toxin A/B enzyme immunoassays [EIA]). The four types of laboratory tests for CDI include:
- Molecular tests:
- NAATs, which use a polymerase chain reaction and have a high sensitivity with a turnaround time of only a few hours; use alone is not encouraged due to the inability to determine asymptomatic carriage from active infection
- Antigen tests:
- EIA for GDH, a test with high sensitivity, but not recommended alone due to low specificity
- Toxin tests:
- Toxin A/B EIA, which uses monoclonal antibodies to detect toxin A and polyclonal antibodies to detect toxin B; these are rapid tests with high specificity and low sensitivity, so it is advised to use in combination with another high-sensitivity test
- Stool cultures:
- A stool culture, which has high specificity and low sensitivity for C. difficile; however, this test has a 48- to 96-hour turnaround time, so it is not as clinically useful
- In addition:
- Colon endoscopy to detect pseudomembranous colitis
- Radiographs of abdomen or pelvis (Bishop & Tiruvoipati, 2022; CDC, 2024b; Lamont et al., 2025b; McDonald et al., 2018)
The least expensive and preferred test combination is a high-sensitivity GDH, followed by high-specificity toxin A/B EIA if the GDH is positive. According to the IDSA, repeat testing should be done when there is a recurrence of symptoms of CDI infection following a successful course of treatment; however, there is no clinical value in testing if the patient remains asymptomatic after treatment. Hospitals are required to report CDI cases to the NHSN and, depending on state regulations, to certain local authorities (Bishop & Tiruvoipati, 2022; CDC, 2024b; IDSA, 2023; Lamont et al., 2025b; McDonald et al., 2018).
Treatment and Management
The first step in treating and managing CDI is the discontinuation of the provoking antibiotic therapy. Symptoms resolve in approximately 20% of patients by stopping the prescribed antibiotic. A 10-day course of fidaxomicin (Dificid) or oral vancomycin (Vancocin) is recommended for the treatment of an initial episode of CDI. Fidaxomicin (Dificid) is more expensive but recommended due to its superior recurrence prevention and safety profile. In situations with limited availability of vancomycin (Vancocin) or fidaxomicin (Dificid), a 10-day course of metronidazole (Flagyl) may be prescribed. For a repeat episode of CDI, a repeat course of oral vancomycin (Vancocin) or fidaxomicin (Dificid) is indicated. Bezlotoxumab (Zinplava), a promising human monoclonal antibody, was approved in 2016 by the US Food and Drug Administration for patients at high risk for CDI recurrence, but was discontinued in January of 2025 for unknown reasons (Bishop & Tiruvoipati, 2022; CDC, 2024b; Ernst, 2025; Kelly et al., 2025).
Fecal microbiota transplantation (FMT) is recommended for patients with multiple recurrences of CDI who have not responded to antibiotics. FMT is based on the concept that recurrent CDI persists because of the altered colonic microbiota in the bowel due to prior antibiotic therapy. FMT reestablishes the normal fecal microbiota by administering healthy donor feces through a nasogastric (NG) tube, a naso-jejunal tube, esophagogastroduodenoscopy, colonoscopy, or a retention enema (Bishop & Tiruvoipati, 2022; Kelly et al., 2025; MedlinePlus, 2022).
Prevention
HCPs must prevent transmission when caring for patients with a suspected or confirmed CDI. Hospitalized patients who have three or more watery or unformed stools in 24 hours, in the absence of another cause, should be placed in contact isolation and tested for CDI. Because patients diagnosed with CDI will continue to shed the organism, contact precautions should be maintained for several days after frequent, watery, or unformed stools have resolved. When a patient with CDI is transferred to a new facility, the nurse must notify the receiving facility that the patient has a history of CDI. The American Association of Family Physicians (AAFP) recommends the prophylactic use of probiotics in hospitalized patients who are immunocompromised and receiving antibiotics. Hand hygiene is another critical prevention strategy. In routine situations, HCPs should perform hand hygiene before donning and after removing gloves when entering a patient's room with a suspected or confirmed CDI. Handwashing with soap and water is necessary, as alcohol-based products do not kill C. difficile. For this reason, handwashing with soap and water is the preferred method of hand hygiene during CDI outbreaks. Disposable patient care equipment should be used whenever possible. Reusable equipment requires thorough cleaning with a sporicidal disinfectant (Bishop & Tiruvoipati, 2022; Cagel et al., 2022; CDC, 2024a, 2024b; Kelly et al., 2025).
Nursing Implications
Nursing care for patients with suspected or confirmed CDI begins with scrupulous hand hygiene, along with prompt initiation of contact precautions. An important consideration when placing patients with CDI in isolation is the requirement for a private room with a dedicated toilet. In situations where a private room is unavailable, patients may be cohorted (i.e., placed in a semi-private room with another patient with confirmed or suspected CDI; CDC, 2024b; Kelly et al., 2025).
In addition to initiating and maintaining appropriate isolation precautions for the duration of the CDI, nurses are responsible for monitoring and reporting the occurrence of three or more watery, unformed stools in 24 hours, monitoring the patient's lab work for C. difficile test results, and administering prescribed antibiotic therapy. Nurses must also ensure appropriate cleaning and disinfection of affected patients' rooms, including daily use of a sporicidal agent or a 1:10 bleach solution in areas with high CDI rates. In addition, some hospitals have added terminal disinfection with UV radiation or hydrogen peroxide vapor to their cleaning regimen. Data released in March 2022 suggests that utilizing aerosolized hydrogen peroxide can effectively reduce C. difficile spores and prevent infection. One study of a large acute care facility that implemented aerosolized hydrogen peroxide into their disinfection protocol showed a reduced rate of hospital-acquired CDIs by 41%, from 4.6 per 10,000 patient days to 2.7 per 10,000 patient days (Association for Professionals in Infection Control and Epidemiology [APIC], 2022; CDC, 2024b; Kelly et al., 2025).
Central Line-Associated Bloodstream Infection
Epidemiology and Pathophysiology
Central line–associated bloodstream infections (LABSIs) are severe and potentially fatal bloodstream infections that can occur from a breach in sterile technique during the insertion procedure of a central line, improper or inadequate care or management of the line, and medication administration through the line. Central lines are vascular access devices (VADs) that provide direct access to the major vessels in the venous circulatory system and remain in situ for long periods. Since the VAD provides a portal of entry and a direct pathway to the venous system, an infectious agent can quickly spread throughout the bloodstream, resulting in critical and systemic illness. Bloodstream infections can induce hemodynamic changes, leading to organ dysfunction, sepsis, shock, and death. According to the NHSN (2025), there was a 13% decrease in CLABSIs across US hospitals between 2022 and 2023; however, more than 28,000 CLABSIs still occur in acute care facilities (including ICUs) each year. The estimated cost of each CLABSI is around $46,000, with a mortality rate over 25% (CDC, 2024d, 2024h; Haddadin et al., 2022; Young & Yuo, 2025).
Risk Factors and Protective Factors
Risk factors for CLABSI include immunosuppression, the presence of multi-lumen catheters, a history of chemotherapy treatment, antibiotic resistance, extended central line duration, heavy microbial colonization at the insertion site or catheter hub, excessive catheter manipulation, and patients receiving total parenteral nutrition (TPN) or with nutritional deficiencies or hypoalbuminemia. Protective factors against CLABSI include the use of single-lumen devices, oral statin intake, and antibiotic administration (Jacob, 2024; Lafuente Cabrero et al., 2023).
Signs and Symptoms
Signs and symptoms of CLABSI depend on the severity of the illness. The most common symptoms include fever and chills. Patients at the extreme ends of the age spectrum (i.e., very advanced or very young age) may have atypical symptoms, including altered mental status, hypotension, and lethargy. Assessment of the catheter exit site may show inflammation, redness, swelling, or purulent drainage, and the patient may report tenderness with palpation of the area (Calderwood, 2023a; Haddadin et al., 2022).
Diagnosis
Blood cultures must be obtained to identify the causative organism and successfully treat the CLABSI. This should be done before initiating empiric antibiotics. Proper technique must be used, and the patient's skin must be thoroughly disinfected to prevent contamination when obtaining the blood cultures. Blood cultures are obtained from two sites when a CLABSI is suspected: one directly from the central line and the second from a peripheral vein. An adequate amount of blood (at least 20 to 30 mL per patient or 10 to 15 mL per site) must be obtained. A diagnosis of CLABSI requires the same pathogen to be found in both blood cultures, with the bacterial count at least three times higher in the culture taken directly from the central line (Calderwood, 2023a; Haddadin et al., 2022; Wilson, 2025).
Treatment and Management
Once blood cultures are obtained, all non-tunneled catheters should be removed. Tunneled catheters should be removed if the patient has persistent symptoms lasting over 36 hours, severe sepsis, shock, blood cultures that remain positive 72 hours after the initiation of appropriate treatment, infection with a difficult-to-treat organism (e.g., S. aureus, Pseudomonas, or fungi), or the recurrence of a CLABSI. Treatment of a CLABSI targets the causative organism based upon blood culture results, antimicrobial susceptibility results, host factors, and the overall clinical picture. Empiric antibiotic therapy should be initiated promptly while awaiting culture and sensitivity results. Once antimicrobial susceptibility is determined, treatment should be adjusted to a more specific and appropriate medication to target the organism (Allon & Sexton, 2024; Haddadin et al., 2022). In patients with an uncomplicated CLABSI (e.g., without endocarditis, hardware, or immunocompromise), the following IV antimicrobial therapy and duration are recommended:
- Staphylococci: vancomycin (Vancocin), daptomycin (Cubicin), penicillins or cephalosporins such as nafcillin (Unipen) or cefazolin (Ancef) for 5 to 7 days
- Enterococcus: ampicillin (Amplin) for 7 to 14 days
- Gram negative bacilli: ceftriaxone (Rocephin) or imipenem (Primaxin) for 10 to 14 days
- Candida: an echinocandin (e.g., micafungin [Mycamine] or caspofungin [Kabifungin]) or fluconazole (Diflucan) for 14 days (Calderwood, 2023b; Haddadin et al., 2022)
Prevention
In 2017, the CDC published the most updated revisions to the Intravascular Catheter-Related Infections Guidelines. In partnership with several other accredited organizations, these guidelines determined the evidence-based practice (EBP) standards for preventing CLABSI and other HAIs. In another collaborative effort, the Society for Healthcare Epidemiology of America (SHEA), the IDSA, the APIC, the Joint Commission (TJC), and the American Hospital Association (AHA) updated their Strategies to Prevent Central Line-Associated Bloodstream Infections in Acute-Care Hospitals in 2022. Table 1 offers an overview of these critical aspects of VAD care to prevent a CLABSI (Buetti et al., 2022; CDC, 2024h; CMS, 2024; Gorski et al., 2021).
Table 1
Summary of Recommendations to Prevent CLABSI
Essential Practices | |
Before insertion |
|
At insertion |
|
After insertion |
|
Additional Approaches |
|
Approaches that should NOT be considered routine part of CLABSI prevention |
|
(Buetti et al., 2022)
Nursing Implications
Medical asepsis for patients at risk for CLABSIs must include hand hygiene and appropriate infection control precautions, such as disinfecting the catheter hub or injection port before accessing a CVC. To prevent CLABSIs and increase compliance, TJC implemented the "Scrub the Hub!" campaign to remind HCPs to disinfect the catheter hub before accessing CVCs. Antiseptic barrier caps have also been shown to decrease the risk of CLABSIs. These devices are in constant contact with the catheter hub and optimize disinfection without active scrubbing of the hub. All IV tubing should be changed per policy, usually within 24 hours for intermittent infusions and 96 hours for continuous infusions (excluding lines used for lipids or blood products). All ordered antibiotics should be administered as prescribed to ensure the patient receives the proper treatment. Nurses should monitor for signs that the CLABSI is resolving or worsening (Ernstmeyer & Christman, 2023; Gillis et al., 2022; Haddadin et al., 2022; Opalka & Ricciuti, 2024; Ullman & Chopra, 2024). For more information on central lines, refer to the Vascular Access Devices NursingCE course.
Catheter-Associated Urinary Tract Infection
Epidemiology and Pathophysiology
A urinary tract infection (UTI) involves any part of the urinary system, including the urethra, bladder, ureters, and kidneys. UTIs account for over 9.5% of all infections reported by acute care hospitals. According to the NHSN (2025), 12% to 16% of hospitalized adults will have an indwelling urinary catheter at some point during their hospitalization. Indwelling urinary catheters contribute to 75% of health care-acquired UTIs. A CAUTI is diagnosed based on a positive urine culture if an indwelling urinary catheter has been in place for more than 48 hours. CAUTIs can occur from unsterile catheterizations, repeated catheterizations, and improper drainage system management. Gram-negative bacilli are the primary bacteria that cause UTIs (ANA, n.d.-a; Imam, 2024; NHSN, 2025; Trautner & Gupta, 2024).
Risk Factors and Protective Factors
Risk factors for developing a CAUTI include extended catheter use, patients assigned female at birth, advancing age, having DM, being immunocompromised, and opening a closed urinary drainage system. Another factor predisposing a patient to develop a CAUTI is a suboptimal aseptic technique when the indwelling catheter is inserted. A protective factor is using the proper sterile techniques when inserting and managing indwelling urinary catheters (Imam, 2024; Trautner & Gupta, 2024).
Signs and Symptoms
Signs of a CAUTI may resemble those of a UTI; however, dysuria and frequency are not typical symptoms of a CAUTI but may be present following catheter removal if an infection is already present. Common symptoms include a fever greater than 38.0° C (100.4° F), suprapubic tenderness, and costovertebral angle pain or tenderness without another cause. Patients may also have nonspecific symptoms such as malaise, flank pain, and altered mental status (Imam, 2024; NHSN, 2025; Trautner & Gupta, 2024).
Diagnosis
The initial diagnostic test for CAUTI is a urinalysis, followed by urine culture and sensitivity testing. If a CAUTI is suspected, the indwelling urinary catheter must be removed. A urine sample can be obtained from a newly inserted indwelling urinary catheter to avoid culture results containing colonized bacteria. Nurses should always use aseptic techniques and sterile supplies to obtain a urine specimen for culture and sensitivity testing. The urine sample should be obtained before initiating treatment with broad-spectrum antibiotics. Once available, targeted antibiotic treatment is based on the urine culture and sensitivity results (Imam, 2024; Trautner & Gupta, 2024).
For a patient to be diagnosed with a CAUTI at any age, all the following criteria must be met:
- The presence of an indwelling urinary catheter for at least two consecutive days while at the health care facility, corresponding with symptom presentation (the same day or the day before symptoms appeared)
- The presence of at least one of the symptoms listed above
- The presence of no more than two different organisms in the urine culture (NHSN, 2025; Trautner & Gupta, 2024)
Treatment and Management
The most common causes of CAUTIs include Escherichia coli (24%), Candida species (24%), Klebsiella pneumoniae (10%), and Enterococcus (14%). Empiric broad-spectrum antibiotic therapy can be initiated while waiting for culture and sensitivity results. Initial broad-spectrum antibiotic choices include penicillins, beta-lactams, cephalosporins, fluoroquinolones, or carbapenems. Patients should be treated with antibiotics for five to seven days; if systemic signs are present, the course of treatment should be extended to 10-14 days. The catheter should be removed or exchanged, and intermittent catheterization should be used if possible (Imam, 2024; Latthe & Latthe, 2024; Monegro et al., 2023; Sabih & Leslie, 2024; Trautner & Gupta, 2024; Werneburg, 2022).
Prevention
The most effective strategies to prevent CAUTIs are to avoid using an indwelling urinary catheter and to remove an existing catheter as soon as possible. For patients with an indwelling catheter inserted before surgery, removing the catheter postoperatively within 24 hours is critical for prevention. Intermittent catheterization is preferable to an indwelling catheter whenever possible due to lower bacteriuria rates. Nurses must also utilize appropriate aseptic techniques and sterile equipment for catheter insertion in the hospital environment. If breaks in aseptic technique, disconnection of the closed system, or leaks occur, the catheter and drainage bag should be replaced using aseptic technique and sterile supplies. A nurse-driven CAUTI prevention tool from the ANA features a decision-making tree based on the currently utilized 2009 CDC criteria for inserting an indwelling urinary catheter to determine whether insertion is indicated (ANA, n.d.-a; Imam, 2024; Trautner & Gupta, 2024).
Nursing Implications
According to evidence-based guidelines, the vital elements of nursing care that prevent infection are focused on the appropriate management of urinary catheters, including the following:
- Only insert an indwelling urinary catheter when indicated
- Remove the urinary catheter as soon as it is no longer indicated
- Properly secure indwelling catheters after insertion
- Maintain a sterile closed drainage system
- Replace the catheter and drainage bag using aseptic technique
- Obtain urine samples by aspirating urine from the sampling port using a sterile syringe
- Maintain unobstructed urine flow
- Always keep the urinary drainage bag below the level of the bladder; do not place the bag on the floor
- Perform routine hygiene and do not use antiseptics to clean the periurethral area (ANA, n.d.-a, n.d.-b; Trautner & Gupta, 2024)
Hospital-Acquired Pneumonia
Epidemiology and Pathophysiology
Pneumonia is defined as an excess of fluid in the lungs that results from an inflammatory process. The inflammation can be triggered by an invasion of an infectious organism or inspiration of an irritating agent, and it occurs in the alveoli, interstitial spaces, and bronchioles. Hospital-acquired pneumonia (HAP) refers to pneumonia that occurs 48 hours or more after admission to a hospital. The most common bacterial causes of HAP include S. aureus (including MRSA) and Pseudomonas aeruginosa (Klompas, 2024a; NHSN, 2025; Rogers & Brashers, 2023).
Risk Factors and Protective Factors
Risk factors for HAP include increased age, chronic lung disease, gram-negative colonization of the upper gastrointestinal tract, an altered level of consciousness, recent aspiration, the presence of a tracheostomy or NG tube, poor nutritional status, immunocompromise, the use of medications that increase gastric pH (e.g., histamine-2 antagonists), or alkaline tube feedings. Risk factors for developing HAP following surgery include being functionally debilitated, age over 70, and having abdominal or thoracic surgery (Klompas, 2025; Sethi, 2024a; Rogers & Brashers, 2023).
Signs and Symptoms
Signs and symptoms of HAP mimic those of community-acquired pneumonia: malaise, fever, chills, cough, dyspnea, hypoxia, and chest pain (Sethi, 2024a; Rogers & Brashers, 2023).
Diagnosis
Sputum is often obtained for Gram stain, culture, and sensitivity testing in an inpatient setting. The offending organism is not identified in many cases. A sputum sample can be obtained easily from patients who can cough into specimen containers, but these specimens are often contaminated with upper airway organisms. Patients who are extremely ill or unable to cough for collection may need to be suctioned in order to obtain a specimen using a sputum trap. There are several steps that the medical team can take to optimize the quality of a sputum sample. The specimen should be obtained before antibiotic administration. The mouth should be rinsed before expectoration. The patient should avoid eating or drinking for 1 to 2 hours before expectoration, and the specimen should be inoculated onto the culture media immediately after collection. Diagnosis can also be made based on chest x-ray results or chest CT combined with clinical data. Based on patient presentation, a bronchoscopy is sometimes performed, and blood cultures may be obtained (Boruchoff & Weinstein, 2023; Klompas, 2024a; Rogers & Brashers, 2023).
Treatment and Management
Patients with suspected HAP should be treated with antibiotics based on the results of noninvasive respiratory cultures (i.e., spontaneous expectoration, sputum, and nasotracheal suctioning). The current guidelines recommend using narrow-spectrum empiric antibiotics whenever possible to prevent resistance. Despite the risk for resistance, the adequacy of the initial antibiotic used plays a significant role in overall patient outcomes. Table 2 outlines the recommendations from the ATA/IDSA guidelines on empiric treatment for VAP in units where MRSA coverage and double antipseudomonal/gram-negative coverage are appropriate. Prescribers are advised to select one gram-positive option from Row A, one gram-negative option from Row B, and one gram-negative option from Row C (Kalil et al., 2016; Klompas, 2024b; Rogers & Brashers, 2023; Sethi, 2024a).
Table 2
ATA/IDSA Empiric Antibiotic Regimen Recommended for HAP/VAP
Row A Coverage for gram-positive organisms with MRSA activity | Vancomycin (Vancocin) 15 mg/kg IV every 8 to 12 hours, or linezolid (Zyvox) 600 mg IV every 12 hours |
Row B Coverage for gram-negative organisms with antipseudomonal activity (β-lactam-based agents) | Piperacillin-tazobactam (Zosyn) 4.5 g IV every 6 hours, cefepime (Maxipime) 2 g IV every 8 hours, ceftazidime (Fortaz) 2 g IV every 8 hours, imipenem (Primaxin) 500 mg IV every 6 hours, meropenem (Merrem) 1 g IV every 8 hours, or aztreonam (Azactam) 2 g IV every 8 hours |
Row C Coverage for gram-negative with antipseudomonal activity (non-β-lactam based agents) | Ciprofloxacin [Cipro] 400 mg IV every 8 hours, levofloxacin [Levaquin] 750 mg IV daily, amikacin [Amikin] 15 to 20 mg/kg IV daily, gentamicin [Gentak] or tobramycin [Nebcin] 5 to 7 mg/kg daily, colistin [Coly Mycin M] 5 mg/kg IV once followed by dosing per creatinine clearance every 12 hours, or polymyxin B [Poly Rx] 2.5 to 3 mg/kg/day every 12 hours |
(IDSA, 2023; Klompas et al., 2022)
Prevention and Nursing Implications
To prevent the development of HAP, nurses must encourage pulmonary hygiene and ambulation. Patients should consume adequate fluids to maintain hydration. Nurses should assess each patient for signs of aspiration using an evidence-based tool such as the Toronto Bedside Swallowing Screening Test or the Simple Standardized Bedside Swallowing Assessment. Nurses should also provide or promote thorough and frequent oral care. Performing proper hand hygiene and implementing standard precautions can also prevent the development of HAP (American Speech-Language-Hearing Association, n.d.; Klompas, 2025; Rogers & Brashers, 2023).
Ventilator-Associated Pneumonia
Epidemiology and Pathophysiology
A specific type of HAP is ventilator-associated pneumonia (VAP), which develops 48 to 72 hours after endotracheal intubation or within 48 hours of extubation. Ventilators directly connect the environment and the patient's lower respiratory passageways. Infectious organisms enter through the tube, invade the ordinarily sterile lower respiratory tract, colonize the lungs, and overwhelm the host's defense system. VAP can develop from poor technique while suctioning the airway, use of contaminated respiratory equipment, or ineffective hand hygiene. According to Papazian and colleagues (2020), bacterial invasion primarily occurs from the aspiration of oropharyngeal secretions contaminated by endogenous flora around the endotracheal tube (ET) cuff. VAP is one of the most frequent ICU-acquired infections, occurring in 5% to 40% of patients on mechanical ventilation for over two days. Incidence rates vary from 0.6% to 0.9% of hospitalized patients, depending on the setting and diagnostic criteria. Data from NHSN indicates that there were over 4,500 cases in the US in 2023. VAP carries an estimated mortality rate of approximately 15% to 30%, extends the length of stay by 11 to 13 days, and increases health care costs by about $40,000 (Klompas, 2024a; CDC, 2024d; Magill et al., 2018; Monegro et al., 2023; NHSN, 2025; Papazian et al., 2020; Shebl & Gulick, 2023).
Risk Factors and Protective Factors
Intubation is the most significant risk factor for developing VAP. Insertion of an ET tube bypasses the body's natural airway defenses, impairs the cough reflex and mucociliary clearance of irritants and pathogens, and facilitates the micro-aspiration of secretions containing bacteria. Bacteria may also form a biofilm within the ET tube that antibiotics or the body's defenses cannot reach. Additional risk factors for VAP include emergent intubation, nasotracheal intubation, not having the head of the bed elevated, and suboptimal oral care. Protective factors and strategies to prevent VAP include elevating the head of the bed, early exercise and mobilization, minimizing sedation, good oral care, and minimizing duration of intubation (Klompas, 2025; Sethi, 2024b).
Signs and Symptoms
Symptoms of VAP include fever, tachypnea, tachycardia, and increased respiratory or post-oral secretions. The patient may also have leukocytosis, purulent secretions, or worsening hypoxemia (Kollef, 2024; Sethi, 2024b).
Diagnosis and Treatment
VAP is diagnosed based on patient symptoms such as fever, increased secretions, hypoxemia, or the presence of leukocytosis and a chest x-ray with evidence of a new infiltrate; however, no symptom, sign, or x-ray finding is specific to VAP due to similarities to atelectasis, pulmonary embolism, and pulmonary edema. The most updated 2016 IDSA Guidelines for Management of Adults with Hospital-Acquired VAP recommend that sputum specimens be obtained by using noninvasive sampling such as endotracheal aspiration. The IDSA guidelines endorse using an antibiogram, which specifies local antibiotic resistance data, to guide empiric treatment decisions. Empiric antibiotic therapy is started as the initial treatment for VAP due to these patients being critically ill (Kalil et al., 2016). According to Sethi (2024b), "the mortality associated with hospital-acquired VAP is high, despite the availability of effective antibiotics" (para. 15). Once sputum culture results become available, they should guide the most effective pathogen-specific therapy. A seven-day course of antimicrobial therapy is recommended (Kalil et al., 2016; Klompas et al., 2022; Klompas, 2024a, 2024b; Sethi, 2024b).
Prevention
Reducing the exposure to risk factors for VAP is the most efficient way to prevent VAP, including avoiding intubation and using noninvasive ventilation whenever possible, minimizing sedation, and maintaining and improving physical conditioning. An important measure to prevent VAP includes keeping the patient in an upright or semi-upright position at 30 to 45 degrees. This reduces the risk of aspiration and is the simplest and most effective method of preventing VAPs. Other verified prevention practices include utilizing noninvasive ventilation options, including continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP), and high-flow oxygen instead of intubation. Using noninvasive oxygenation avoids bypassing innate protective factors that occur with ET intubation. Although this is not possible for all patients, in appropriate cases, using a CPAP, BiPAP, or high-flow oxygen eliminates the need for intubation. Interrupting sedation daily, assessing readiness to extubate daily, performing spontaneous breathing trials with sedatives turned off, implementing early mobilization and exercise, and changing ventilator circuits only when they are visibly soiled or malfunctioning can also decrease a patient's risk of developing VAP (Klompas, 2025; NHSN, 2025; Papazian et al., 2020; Sethi, 2024b; The TEAM Study Investigators and the ANZICS Clinical Trials Group, 2022).
Nursing Implications
Nursing care for patients with VAP includes administering antibiotics as prescribed, monitoring lab work, assessing the patient's response to treatment, and consistently implementing infection prevention and control strategies. A patient diagnosed with VAP requires consistent hand hygiene and standard precautions, including gown and gloves (CDC; 2024c; Klompas, 2024b; Seigel et al., 2024). For more information on HAP and VAP, refer to the Pneumonia NursingCE course.
Surgical Site Infection
Epidemiology and Pathophysiology
The CDC defines a surgical site infection (SSI) as an infection that occurs because of a surgical procedure, often near the incision or entry point, within 30 days of the procedure (90 days if an implant is used). SSIs occur when a pathogen enters the body through a surgical incision. SSIs can develop from a breach in sterile technique, improper skin preparation, contamination during dressing changes, or using a contaminated antiseptic solution (Evans, 2024). Approximately 0.5% to 3% of patients who undergo surgery develop an SSI (Zabaglo et al., 2024). According to the NHSN (2025), SSIs carry a 3% mortality rate and are the most expensive HAI, with an estimated annual cost of $3.3 billion US dollars and an additional 9.7 inpatient days per episode, each of which costs over $20,000.
Risk Factors and Protective Factors
Risk factors for developing an SSI include older age, medical comorbidities (e.g., DM, coexisting infections), BMI over 30, malnutrition, and surgical factors (e.g., length of the procedure, surgical technique, skin asepsis, and antimicrobial prophylaxis). SSIs are most common following colon surgery, coronary artery bypass graft (CABG), vascular surgery, cesarean delivery, joint arthroplasty involving prosthesis, and spinal fusions (Evans, 2024; Monegro et al., 2023; Zabaglo et al., 2024).
Signs and Symptoms
The scoring system ASEPSIS has been used to describe an SSI using objective data. ASEPSIS stands for:
- Additional treatment
- Serous discharge
- Erythema
- Purulent exudate
- Separation of the deep tissues
- Isolation of bacteria
- length of inpatient Stay (Evans, 2024)
Other symptoms include fever, pain or tenderness at the incision site, and localized edema (Evans, 2024; Kim et al., 2021; Zabaglo et al., 2024).
Diagnosis
Laboratory tests such as leukocyte count, neutrophils, erythrocyte sedimentation rate, and c-reactive protein are used, along with assessment data, to determine whether an SSI may be present. In the presence of an infection, these laboratory values increase compared to the patient's baseline. An MRI with contrast may be indicated for some SSI sites to determine whether an infection is present (Evans, 2024; Kim et al., 2021).
Treatment and Management
A diagnosis of SSI requires treatment with empirical antibiotics. Once the organism is identified, antibiotic therapy should be aligned with the organism's sensitivity. In cases of implant infection, it may be necessary to remove the device (Evans, 2024; Kim et al., 2021; Zabaglo et al., 2024).
Prevention
SSI prevention has gained more attention due to the extended length of stay and high costs associated. Preoperative antibiotic prophylaxis and skin decontamination before the patient arrives at the surgical area (i.e., showering with an antiseptic agent the night before surgery) and before any incision is made can prevent common pathogens attributed to SSIs. Surgical asepsis should be practiced during all surgical procedures. Smoking cessation should be encouraged, proper bowel preparation should be done prior to colon surgeries, and preexisting infections should be treated before the patient undergoes a non-emergent or elective surgery. To prevent an SSI postoperatively, proper hand hygiene and infection prevention strategies must be utilized when caring for the surgical site (Anderson & Sexton, 2024; Ernstmeyer & Christman, 2023; Long et al., 2024; Zabaglo et al., 2024).
Nursing Implications
Nurses need to implement interventions that prevent SSIs from developing and monitor patients for signs of an SSI. Prevention includes proper hand hygiene and standard precautions. It is essential to perform wound care and dressing changes as ordered, as well as when dressings are soiled. Any drains present postoperatively should also be emptied regularly. Monitor each patient and surgical site for signs of infection, including erythema, heat, pain, edema, vital sign changes, and worsening laboratory results (Anderson & Sexton, 2024; Evans, 2024).
Multidrug-Resistant Organisms
Multidrug-resistant organisms (MDROs) are defined as microorganisms (primarily bacteria) that are resistant to one or more classes of antimicrobial agents; common types include MRSA and VRE. The CDC reports that an estimated 2.8 million infections are caused by MRDOs, leading to over 35,000 deaths annually. MDROs have increased in prevalence over the last few decades. Although transmission most frequently occurs in acute care facilities, all health care settings are affected by the emergence and transmission of antibiotic-resistant bacteria. Therefore, MDROs have important implications for patient safety, infection control, and the proper selection of antibiotics. Options for managing MDRO infections are limited; these infections are more difficult to eradicate and are associated with increased length of stay, higher costs, and higher mortality rates. The severity and extent of illness caused by MDROs vary by population and setting; therefore, prevention and control strategies must be tailored to the specific needs of each population and facility. In response, the Healthcare Infection Control Practices Advisory Committee (HICPAC) developed guidelines for the control and management of MDROs in 2006. Updated in 2022, these guidelines outline the epidemiology of emerging MDROs and evidence-based prevention and treatment strategies. The prevention of antibiotic-resistant bacteria depends on appropriate clinical practices that should be incorporated into routine patient care. The core HICPAC prevention categories and brief overview are listed in Table 3 (CDC, 2024f, 2024i; Holubar & Deresinski, 2024).
Table 3
MDRO Prevention and Control
Administrative measures/adherence monitoring |
|
Education and training of HCPs |
|
Judicious use of antimicrobial agents |
|
Surveillance |
|
Infection control precautions to prevent transmission of MRDOs |
|
Environmental measures |
|
(CDC, 2024i)
Methicillin-Resistant Staphylococcus aureus
Epidemiology and Pathophysiology
Staphylococci are gram-positive aerobic organisms that are a part of the human bacterial flora or microbiota. S. aureus is the most pathogenic of these staphylococci and can commonly cause skin infections, pneumonia, endocarditis, and osteomyelitis. Methicillin (Staphcillin) was the treatment of choice for infections caused by beta-lactamase-producing penicillin-resistant S. aureus beginning in October 1960. However, strains resistant to methicillin (Staphcillin) emerged by 1968. MRSA is the term used to describe S. aureus strains resistant to beta-lactam antibiotics (e.g., penicillins, cephalosporins, and carbapenems), which have become endemic in health care settings. MRSA is a frequent pathogen in HAIs and has been associated with significant morbidity, mortality, and increased hospital length of stay. The higher morbidity and mortality rates related to MRSA are due primarily to other factors, such as the delayed initiation of appropriate antibiotic therapy, less effective antibiotic therapy, or the increased severity of underlying illness for hospitalized patients with infections caused by MRSA (Bush & Vazquez-Pertejo, 2024; CDC, 2024i, 2024j).
Risk Factors and Protective Factors
Risk factors for hospital-acquired MRSA infections include prolonged hospitalization, compromised immune system, invasive devices such as CVCs, antibiotic treatment, or proximity to individuals infected or colonized with MRSA (CDC, 2024i; Harris, 2024; Siddiqui & Koirala, 2023).
Protective factors for hospital-acquired MRSA infection include hand hygiene for patients and HCPs, meticulous environmental cleaning, and following contact precautions when caring for patients colonized with MRSA and those diagnosed with an active MRSA infection. The minimum required PPE includes a gown and gloves. If the patient has a respiratory infection or pneumonia caused by MRSA, HCPs must wear goggles and a face mask due to potential contact with respiratory secretions. HCPs hands can become a vehicle for MRSA transmission from contact with skin, wounds, dressings, secretions, equipment, or environmental surfaces contaminated with MRSA. Thorough hand hygiene and equipment cleaning (i.e., stethoscope) between patients is essential to decrease transmission (CDC, 2024g, 2024i, 2024j; Harris, 2024; Siegel et al., 2024).
Signs and Symptoms
MRSA is the leading cause of both HAP and VAP, and can also cause endocarditis, CAUTIs, bloodstream infections (including CLABSIs), soft tissue infections, and wound infections (including SSIs). MRSA symptoms vary depending on the site of the infection. Patients with infection of soft tissue or wounds or SSIs may have erythema, tenderness, edema, and drainage from the site. Patients with a bloodstream infection, CLABSI, pneumonia, VAP, or a CAUTI often have fatigue, fever, pain, or swelling at the site of the infection (Harris, 2024; Klompas, 2024a; Siddiqui & Koirala, 2023).
Diagnosis and Treatment
MRSA is diagnosed based on culture results; however, treatment should not be delayed while awaiting confirmation of infection. The selection of an appropriate empiric antibiotic is based on the location of the infection, potential risks, and clinical data. Culture and sensitivity results will guide the selection of the most appropriate antibiotic to treat the infection. Other diagnostic tests that may be ordered include a CBC and urinalysis with culture and sensitivity. For patients with an infection of the lung, bone, joint, or another internal structure, imaging studies are required (i.e., a chest x-ray, CT scan, or echocardiogram; Harris, 2024; Siddiqui & Koirala, 2023).
Vancomycin (Vancocin) IV is the antibiotic of choice to treat most hospital-acquired MRSA infections. It can be used as both empiric and definitive therapy, as most MRSA strains are susceptible to vancomycin (Vancocin). Vancomycin (Vancocin) dosing is adjusted based on the patient's serum trough level and renal function. Trough levels are obtained just before the administration of the fourth dose. IV daptomycin (Cubicin) can be used when vancomycin (Vancocin) is unavailable or is not well-tolerated by the patient. Depending on the severity of infection and treatment response, the duration of therapy may last from 5 to 14 days (Siddiqui & Koirala, 2023).
Prevention
The reservoir for MRSA in hospitals includes colonized or infected patients, or HCPs and contaminated objects or surfaces in the patient care environment. These factors highlight the importance of adhering to established guidelines to effectively control and prevent the spread of MRSA within health care settings. The 2022 collaborative practice recommendation for acute-care hospitals from SHEA, IDSA, and APIC on strategies to prevent MRSA transmission and infection are the gold standard. The following are the basic guidelines (Klompas et al., 2022):
- A MRSA monitoring program and risk assessment should be implemented
- Hand hygiene compliance following World Health Organization (WHO) or CDC recommendations must be promoted
- Contact precautions should be used for MRSA-colonized and -infected patients
- Appropriate cleaning and disinfection of the environment and equipment is necessary
- An alert system to notify HCPs of new MRSA colonization or infection in patients and readmission of colonized or infected patients through a laboratory-based system should be utilized
- MRSA data and outcome measures should be given to key stakeholders
- HCP, patient, and family should be educated regarding MRSA
- A stewardship program for antimicrobial use should be utilized
Additional recommendations include active surveillance testing, screening, decolonization practices, and the use of gowns and gloves in the ICU regardless of MRSA status. Screening is completed using a polymerase chain reaction MRSA test or cultures of the nares, oropharynx, or perineum. If the patient is MRSA positive, proper contact precautions can be initiated (CDC, 2024j; IDSA, 2023; Klompas et al., 2022; Siddiqui & Koirala, 2023).
Nursing Implications
Nursing care for patients with hospital-acquired MRSA infections includes performing hand hygiene, maintaining contact precautions, and administering antibiotics as prescribed. The administration of antibiotics on time is vital to attaining adequate serum levels of the medication to resolve the infection. Since vancomycin (Vancocin) dosing is based on the serum trough level, nurses must understand the appropriate timing of obtaining a blood sample. Additionally, it is crucial to closely monitor the patient's laboratory results, including antibiotic peak and trough levels as well as WBC, to ensure values return to normal as the infection resolves (CDC, 2024j; Siddiqui & Koirala, 2023).
Vancomycin-Resistant Enterococci
Epidemiology and Pathophysiology
Enterococci are Gram-positive, non-spore-forming cocci that colonize the gastrointestinal and biliary tracts and the female genital tract. These bacteria are also found in the environment. Enterococci can cause various infections, including UTIs, intraabdominal infections, bacteremia, and endocarditis. Enterococci are less virulent than S. aureus and mainly infect individuals with an underlying condition, impairing their ability to fight off infection. Vancomycin (Vancocin) is an antibiotic commonly used to treat drug-resistant enterococci infections; however, certain strains of enterococci have become resistant to vancomycin (Vancocin). The incidence and prevalence of vancomycin-resistant enterococci (VRE) have increased significantly in the past few decades, particularly in ICUs. VRE is shed through the stool, found on the skin, and spread when a person comes into contact with a contaminated object. Colonization with VRE precedes VRE infection, but not all patients colonized with VRE will develop an active infection. Infection with VRE increases treatment costs and mortality rates compared to vancomycin (Vancocin)-susceptible enterococci (Anderson, 2025; CDC, 2024j; Levitus et al., 2023; Said et al., 2024).
Risk Factors and Protective Factors
Risk factors for VRE colonization or infection include prolonged hospital or ICU stays, invasive procedures, immunocompromise, severe illness, abdominal surgery, enteral nutrition, and prior exposure to vancomycin (Vancocin) or third-generation cephalosporins. Since VRE can live on almost any surface for days or weeks and still be infectious, being in close contact with an infected individual is also a risk factor. Exposure to HCPs assigned to care for another patient with VRE colonization or infection also increases the risk. Prior exposure to antimicrobials is the most significant predictor of VRE colonization (Anderson, 2025; Levitus et al., 2023; Said et al., 2024).
Signs and Symptoms
VRE can cause various symptoms based on the location and type of infection. Some common sites for VRE infections include the urinary tract, the bloodstream, and wounds associated with catheters or surgical procedures. Common symptoms of VRE consist of fever and pain at the site, as well as swelling, redness, and purulent drainage for wounds (Levitus et al., 2023; Miller, 2024).
Diagnosis and Treatment
To make a diagnosis, a specimen from the potential source of infection should be sent for culture and sensitivity. Culture results will guide the selection of the most appropriate antibiotic therapy. Antibiotic therapy may also change depending on the site of the infection. The provider may need to consult with an infectious disease specialist to determine a plan of care due to the antibiotic resistance of VRE and its various strains (Anderson, 2025; Bush & Vazquez-Pertejo, 2023; Levitus et al., 2023; Said et al., 2024).
Prevention
Use of good infection prevention practices, such as contact precautions (i.e., wearing a gown and gloves when caring for patients with VRE) and frequent hand hygiene by HCPs, patients, and visitors can limit the spread of VRE in health care settings. Patients with VRE should follow all instructions the HCP gives and keep their hands clean, especially after touching the infected area or using the bathroom. Environmental factors, such as proper cleaning and disinfecting of equipment and utilizing dedicated equipment to single-patient use when possible, can prevent the spread of infection (Anderson, 2025; Siegel et al., 2024; Said et al., 2024).
Nursing Implications
Nursing care for patients with hospital-acquired VRE infections follows the same guidelines as care for those with MRSA. This involves practicing hand hygiene, using contact precautions, and antibiotic stewardship, including administering antibiotics on time, which is critical for attaining therapeutic blood levels and infection resolution. Nurses must also monitor the patient's laboratory values for signs that the infection is resolving, such as the WBC count returning to normal (Anderson, 2025; Levitus et al., 2023; Said et al., 2024).
Healthcare Professional Protection
Ensuring the safety of HCPs is crucial for maintaining a healthy and effective workforce, as well as for preventing the spread of infections within health care settings. According to the CDC (2024e), protecting HCPs from infectious disease exposures in the workplace involves eight primary components:
- Leadership support
- To be successful, infection prevention programs require visible and tangible support from all levels of the health care facility's leadership.
- Education and training of HCP on infection prevention
- Training should be adapted to reflect the diversity of the workforce and the type of facility and tailored to meet the needs of each category of HCP being trained.
- Patient, family, and caregiver education
- Include information about how infections are spread, how they can be prevented, and what signs or symptoms should prompt reevaluation and notification of the patient's HCP. Instructional materials and delivery should address varied levels of education, language comprehension, and cultural diversity.
- Performance monitoring and feedback
- Performance measures should be tailored to the care activities and the population served.
- Standard precautions
- Standard precautions are the basic practices that apply to all patient care, regardless of the patient's suspected or confirmed infectious state, and applied to all settings where care is delivered. These practices protect HCPs and prevent HCPs and the environment from transmitting infections to other patients.
- Transmission-based precautions
- Implementation of transmission-based precautions may differ depending on the patient care settings (e.g., inpatient, outpatient, LTCF), the facility design characteristics, and the type of patient interaction, and should be adapted to the specific healthcare setting.
- Temporary invasive medical devices for clinical management
- Early and prompt removal of invasive devices should be part of the plan of care and included in regular assessment.
- Occupational health
- It is the professional responsibility of all health care organizations and individual personnel to ensure adherence to federal, state, and local requirements concerning immunizations; work policies that support safety of HCPs; timely reporting of illness by employees to employers when that illness may represent a risk to patients and other HCPs; and notification to public health authorities when the illness has public health implications or is required to be reported (CDC, 2024e).
In response to the HAI epidemic, Project Firstline is a collaborative effort between the CDC, AHA, and the Health Research & Educational Trust whose aim is to "provide infection control training and education to frontline health care workers and public health personnel" (AHA, n.d., para. 3).
Prevention
In 2000, the Institute of Medicine (IOM; now called the National Academy of Medicine) released its landmark report, To Err Is Human. The report relayed the astronomical data surrounding medical errors in acute care hospitals, citing nearly 98,000 hospitalized patient deaths due to preventable medical errors annually. This momentous study ignited a focus on medical practices, spawning new policies and procedures and setting performance standards and expectations for patient safety and quality improvement. In addition, the report identified several factors that contribute to patient harm and drew attention from national agencies to examine strategies to deliver safe, effective, and high-quality health care. In response, TJC generated standards requiring health care organizations to create a culture of safety. In 2002, TJC published its first set of National Patient Safety Goals (NPSGs), requiring organizations to focus on priority safety practices pertaining to patient care. The NPSGs are updated annually, and the 2025 NPSG for infection control is outlined in Table 4. Infection control programs within health care organizations are coordinated and implemented by HCPs trained in infection control practices and are designed to reduce the risk of HAIs. Hospitals execute infection tracking and surveillance systems, alongside evidence-based infection control practices, to reduce the rates of HAIs, improve patient safety, and reduce morbidity and mortality (AHA, n.d.; IOM et al., 2000; TJC, 2025).
Table 4
2025 NPSG Goal 7
NPSG.07.01.01: Reduce the risk of health care-associated infections: Comply with either the current CDC or WHO hand hygiene guidelines Element(s) of Performance for NPSG.07.01.01: Implement a program that follows categories IA, IB, and IC of either the current CDC or WHO hand hygiene guidelines Set goals for improving compliance with hand hygiene guidelines Improve compliance with hand hygiene guidelines based on established goals |
(TJC, 2025; WHO, n.d.)
Hand Hygiene
Proper hand hygiene, a form of medical asepsis, is the most effective risk-reduction strategy to prevent HAIs. According to WHO, HCPs in ICUs wash their hands or perform hand hygiene in less than 60% of instances when it is indicated. Performing hand hygiene in the presence of the patient and family promotes trust and models good behavior for others. Figure 2 illustrates the five moments for hand hygiene during patient care. The term "hand hygiene" refers to handwashing with an antimicrobial or plain soap and water, and alcohol-based products such as gels, foams, and rinses. Alcohol-based products contain an emollient that does not require the use of water. According to the CDC, in the absence of visibly soiled hands or when contamination from spore-forming organisms (e.g., C. difficile) is unlikely, approved alcohol-based products for hand disinfection are preferred over antimicrobial or plain soap and water because of their superior microbicidal activity, reduced drying of the skin, and convenience in the absence of a sink (AHA, n.d.; CDC, 2024a, 2024c; Ernstmeyer & Christman, 2023; WHO, n.d., 2021).
Figure 2
Five Moments for Hand Hygiene
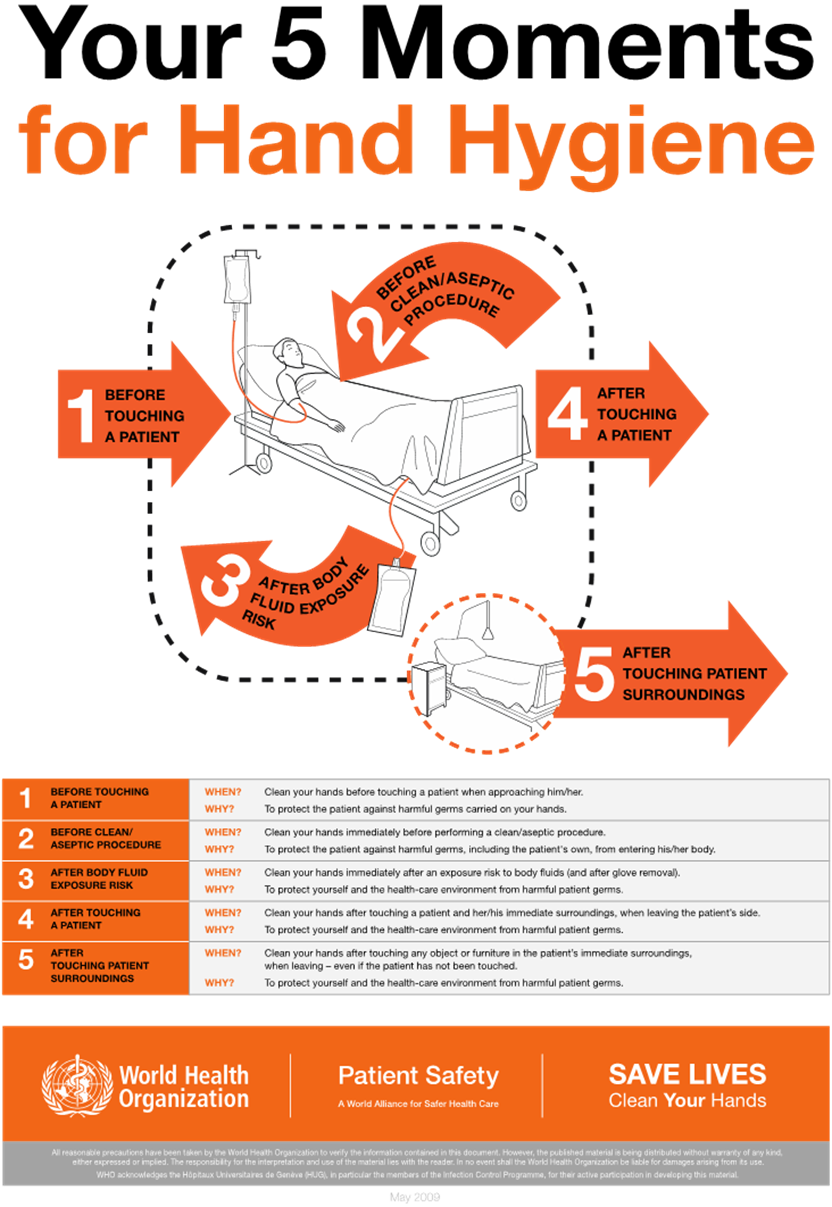
(WHO, 2021)
As outlined in the CDC hand hygiene guidelines, HCPs should use an alcohol-based hand rub in the following clinical situations:
Immediately before direct contact with a patient (e.g., touching)
Before performing an aseptic task (e.g., placing an indwelling device) or handling invasive medical devices (e.g., IV site, urinary catheter)
Before transitioning care from a soiled body site to a clean body site on the same patient
After touching a patient or the patient's immediate environment (e.g., bed linens, surfaces, IV pole)
After contact with blood, body fluids, or contaminated surfaces
Immediately after removing gloves (CDC, 2024a, 2024c)
To use an alcohol-based hand sanitizer properly, the product should be applied to the hands, covering all surfaces, and rubbed together until the hands feel dry. This process should take approximately 20 seconds. HCPs should wash hands with soap and water instead of using an alcohol-based hand rub in the following clinical scenarios:
- When hands are visibly soiled
- Prior to eating and after using restroom facilities
- After caring for a patient with known or suspected infectious diarrhea or known or suspected exposure to spores (e.g., norovirus, CDI; CDC, 2024a, 2024c)
To clean the hands using soap and water, wet them with water, apply the amount of soap product recommended by the manufacturer, and rub the hands together vigorously for at least 15 to 20 seconds, covering all surfaces of the hands and fingers. Next, the hands should be rinsed with water and dried with a disposable towel. Finally, the CDC recommends using a disposable towel to turn off the faucet to prevent recontamination of the hands (CDC, 2024a, 2024c).
HCPs are advised to inspect their hands for breaks, cuts, or lacerations in the skin or cuticles before the start of each workday. These open areas provide portals of entry and exit for organisms. If any breaks in skin integrity are identified, a dressing should be applied before caring for patients. Artificial nails are discouraged since they harbor microorganisms. Fingernails should be trimmed to one-quarter of an inch, and rings should be avoided if possible. If the areas beneath the fingernails are soiled, they should be cleaned with a nail cleaner when available (CDC, 2024a, 2024c).
Personal Protective Equipment
The Occupational Safety and Health Administration (OSHA) recommends PPE be used by HCPs for protection. In health care settings, PPE such as gloves, masks, eyewear, and gowns are necessary for specific clinical situations to prevent the transmission of infectious materials through contact with patients and their blood, body fluids, secretions, or excretions. Choosing which PPE items are appropriate for each task should be based on how the organism is transmitted and on facility policies and procedures (CDC, n.d.-b; OSHA, n.d.).
Standard Precautions
Standard precautions are the basis of infection control practices and are of utmost importance to prevent the transmission of infectious agents and communicable diseases in health care settings. Standard precautions apply to the care of all patients in health care settings (regardless of the suspected or confirmed presence of an infectious agent). They are the first line of defense to break the chain of infection and protect HCPs and patients. Standard precautions are premised on the concept that every patient's blood or body fluids are potentially contaminated with infectious agents. They are employed with blood, blood products, body fluids, secretions, excretions (except sweat), non-intact skin, and mucous membranes. In addition to hand hygiene practices, standard precautions include selecting and using proper PPE based on the level of anticipated contact with a patient and the potential for exposure to infectious material, including blood, body fluid, or splash exposure. Providing care using standard precautions includes hand hygiene, gloves, a gown, a facemask, and a face shield; respiratory hygiene/cough etiquette; and safe injection practices. The use of face masks during spinal/epidural access procedures also falls within the definition of standard precautions. The efficacy of standard precautions is related directly to how well individuals and institutions adhere to the recommended guidelines (CDC, n.d.-b, 2024e; Siegel et al., 2024).
In 2013, based on a robust evidence base, the CDC recommended that respiratory hygiene/cough etiquette be incorporated into infection control as a component of standard precautions. These should be instituted in the health care setting at the first point of contact with a potentially infected person to prevent the transmission of all respiratory infections. Respiratory hygiene or cough etiquette applies to anyone entering a health care setting (patients, visitors, and staff) with signs or symptoms of illness (coughing, congestion, rhinorrhea, or increased respiratory secretions; Siegel et al., 2024). The components of respiratory hygiene/cough etiquette include the following:
- Covering the mouth and nose when coughing and sneezing
- Using disposable facial tissues to contain respiratory secretions, with prompt disposal into a hands-free receptacle
- Wearing a surgical mask when coughing to minimize contamination of the surrounding environment
- Turning the head when coughing and staying at least three feet away from others, especially in common waiting areas
- Washing hands with soap and water or alcohol-based hand rub after contact with respiratory secretions (AHA, n.d.; CDC, n.d.-b, 2024e; Siegel et al., 2024).
Transmission-Based Precautions
Transmission-based precautions are the second tier of infection control and are intended for use alongside standard precautions. Transmission-based precautions are reserved for patients suspected of being infected or colonized with specific infectious agents. Also referred to as isolation precautions, transmission-based precautions are based on the infectious organism's mode of transmission. The major categories of transmission-based protection include contact, droplet, and airborne precautions (CDC, 2024e; Siegel et al., 2024). These are used for patients with highly transmissible pathogens when the route of transmission is not entirely interrupted by standard precautions. Regardless of the specific type of transmission-based precautions required, the following principles should be routinely adhered to:
- Thorough performance of hand hygiene before entering and leaving the room of a patient in isolation
- Proper disposal of contaminated supplies and equipment according to agency policy
- Application of knowledge of the mode of infection transmission when using PPE
- Protection of all persons from exposure during the transport of an infected patient outside of the isolation room
- Prioritization of single private rooms when available, but cohort rooming may be implemented during outbreaks of infections (i.e., the placement of patients infected with the same organism in the same room, based on organizational needs; AHA, n.d.; Siegel et al., 2024).
For more information on PPE, refer to the Personal Protective Equipment NursingCE course.
Antibiotic Stewardship
According to the CDC, antibiotic stewardship in acute care facilities is crucial for reducing HAIs. The implementation of a hospital antimicrobial stewardship program has significantly reduced HAIs. The essential elements of a hospital antimicrobial stewardship program consist of the following:
- Leadership commitment: dedicating necessary human, financial, and information technology resources
- Accountability: appointing a single leader responsible for program outcomes; experience with successful programs shows that a physician leader is effective
- Drug expertise: appointing a single pharmacist leader responsible for working to improve antibiotic use
- Action: implementing at least one recommended action, such as systemic evaluation of ongoing treatment needs after a period of initial treatment (i.e., "antibiotic time out" after 48 hours)
- Tracking: monitoring antibiotic prescribing and resistance patterns
- Reporting: regularly reporting information on antibiotic use and resistance to prescribers, nurses, and relevant staff
- Education: educating clinicians about resistance and optimal prescribing (AHA, n.d.; Holubar & Deresinski, 2024)
In Summary
Prevention is key when approaching HAIs. Recognizing the importance of proactive measures, the AAFP has outlined specific preventative EBPs for HAIs:
- Institutions should establish local infection prevention processes, including ongoing educational programs, checklists and treatment "bundles" and local reporting and tracking programs
- Urinary catheters should be used for the shortest duration possible and removed as soon as they are no longer required
- Central lines should be used for the shortest duration possible and removed as soon as they are no longer required
- Noninvasive positive pressure ventilation should be attempted before intubation when clinically appropriate to prevent VAP
- Probiotics should be considered to prevent CDI in hospitalized, immunocompromised patients who are receiving antibiotics
- Antibiotics should be used for the shortest possible duration, discontinued when appropriate, and targeted to specific organisms to reduce the risk of CDI and the development of MDROs (Cagle et al., 2022)
References
Allon, M., & Sexton, D. J. (2024). Tunneled hemodialysis catheter-related bloodstream infection (CRBSI): Management and prevention. UpToDate. Retrieved April 7, 2025, from https://www.uptodate.com/contents/tunneled-hemodialysis-catheter-related-bloodstream-infection-crbsi-management-and-prevention
American Hospital Association. (n.d.). Infection control and prevention. Retrieved April 9, 2025, from https://www.aha.org/infection-control-and-prevention
American Nurses Association. (n.d.-a). ANA CAUTI prevention tool. Retrieved April 7, 2025, from https://www.nursingworld.org/practice-policy/work-environment/health-safety/infection-prevention/ana-cauti-prevention-tool
American Nurses Association. (n.d.-b). Infection prevention & control. Retrieved April 7, 2025, from https://www.nursingworld.org/practice-policy/work-environment/health-safety/infection-prevention/
American Speech-Language-Hearing Association. (n.d.). Swallowing screening. Retrieved April 7, 2025, from https://www.asha.org/practice-portal/clinical-topics/adult-dysphagia/swallowing-screening/
Anderson, D. J. (2025). Vancomycin-resistant enterococci: Epidemiology, prevention, and control. UpToDate. Retrieved April 7, 2025, from https://www.uptodate.com/contents/vancomycin-resistant-enterococci-epidemiology-prevention-and-control
Anderson, D. J., & Sexton, D. J. (2024). Overview of control measures for prevention of surgical site infection in adults. UpToDate. Retrieved April 7, 2025, from https://www.uptodate.com/contents/overview-of-control-measures-for-prevention-of-surgical-site-infection-in-adults
Association for Professionals in Infection Control and Epidemiology. (2022). New study finds aerosolized hydrogen peroxide can significantly reduce C. difficile infections in hospital settings. https://apic.org/news/new-study-finds-aerosolized-hydrogen-peroxide-can-significantly-reduce-c-difficile-infections-in-hospital-settings/
Bishop, E. J., & Tiruvoipati, R. (2022). Management of Clostridioides difficile infection in adults and challenges in clinical practice: Review and comparison of current IDSA/SHEA, ESCMID and ASID guidelines. The Journal of Antimicrobial Chemotherapy, 78(1), 21–30. https://doi.org/10.1093/jac/dkac404
Blot, S., Ruppé, E., Harbarth, S., Asehnoune, K., Poulakou, G., Luyt, C. E., Rello, J., Klompas, M., Depuydt, P., Eckmann, C., Martin-Loeches, I., Povoa, P., Bouadma, L., Timsit, J. F., & Zahar, J. R. (2022). Healthcare-associated infections in adult intensive care unit patients: Changes in epidemiology, diagnosis, prevention and contributions of new technologies. Intensive & Critical Care Nursing, 70, 103227. https://doi.org/10.1016/j.iccn.2022.103227
Boruchoff, S. E., & Weinstein, M. P. (2023). Sputum cultures for the evaluation of bacterial pneumonia. UpToDate. Retrieved April 7, 2025, from www.uptodate.com/contents/sputum-cultures-for-the-evaluation-of-bacterial-pneumonia
Buetti, N., Marschall, J., Drees, M., Fakih, M. G., Hadaway, L., Maragakis, L. L., Monsees, E., Novosad, S., O'Grady, N. P., Rupp, M. E., Wolf, J., Yokoe, D., & Mermel, L. A. (2022). Strategies to prevent central line-associated bloodstream infections in acute-care hospitals: 2022 Update. Infection Control and Hospital Epidemiology, 43(5), 553–569. https://doi.org/10.1017/ice.2022.87
Bush, L. M., & Vazquez-Pertejo, M. T. (2023). Enterococcal infections. Merck Manual Professional Version. https://www.merckmanuals.com/professional/infectious-diseases/gram-positive-cocci/enterococcal-infections?query=VRE
Bush, L. M., & Vazquez-Pertejo, M. T. (2024). Staphylococcal infections. Merck Manual Professional Version. https://www.merckmanuals.com/professional/infectious-diseases/gram-positive-cocci/staphylococcal-infections
Cagle, S. D., Hutcherson, B. L., & Wiley, A. T. (2022). Health care-associated infections: Best practices for prevention. American Family Physician, 105(3), 262–270. https://www.aafp.org/pubs/afp/issues/2022/0300/p262.html
Calderwood, M. S. (2023a). Intravascular non-hemodialysis catheter-related infection: Clinical manifestations and diagnosis. UpToDate. Retrieved April 8, 2025, from https://www.uptodate.com/contents/intravascular-non-hemodialysis-catheter-related-infection-clinical-manifestations-and-diagnosis
Calderwood, M. S. (2023b). Intravascular non-hemodialysis catheter-related infection: Treatment. UpToDate. Retrieved April 8, 2025, from https://www.uptodate.com/contents/intravascular-non-hemodialysis-catheter-related-infection-treatment
Centers for Disease Control and Prevention. (n.d.-a). Healthcare- and community-associated infections. Antimicrobial Resistance & Patient Safety Portal, Centers for Disease Control and Prevention. Retrieved April 7, 2025, from https://arpsp.cdc.gov/profile/infections?tab=nhsn
Centers for Disease Control and Prevention. (n.d.-b). Healthcare-associated infections (HAIs). Retrieved April 7, 2025, from https://www.cdc.gov/healthcare-associated-infections/
Centers for Disease Control and Prevention. (2024a). About hand hygiene for patients in healthcare settings. https://www.cdc.gov/clean-hands/about/hand-hygiene-for-healthcare.html
Centers for Disease Control and Prevention. (2024b). C. diff: Facts for clinicians. https://www.cdc.gov/c-diff/hcp/clinical-overview/
Centers for Disease Control and Prevention. (2024c). Clinical safety: Hand hygiene for healthcare workers. https://www.cdc.gov/clean-hands/hcp/clinical-safety/
Centers for Disease Control and Prevention. (2024d). Current HAI progress report. https://www.cdc.gov/healthcare-associated-infections/php/data/progress-report.html
Centers for Disease Control and Prevention. (2024e). Guidelines and guidance library. https://www.cdc.gov/infection-control/hcp/guidance/index.html
Centers for Disease Control and Prevention. (2024f). HAI and antimicrobial use prevalence surveys. https://www.cdc.gov/healthcare-associated-infections/php/haic-eip/antibiotic-use.html
Centers for Disease Control and Prevention. (2024g). Infection control guidance: Preventing Methicillin-resistant Staphylococcus aureus (MRSA) in healthcare facilities. https://www.cdc.gov/mrsa/hcp/infection-control/?CDC_AAref_Val=https://www.cdc.gov/mrsa/healthcare/inpatient.html
Centers for Disease Control and Prevention. (2024h). Intravascular catheter-related infection (BSI) prevention guidelines. https://www.cdc.gov/infection-control/hcp/intravascular-catheter-related-infection/index.html
Centers for Disease Control and Prevention. (2024i). Multidrug-resistant organisms (MDRO) management guidelines. https://www.cdc.gov/infection-control/hcp/mdro-management/index.html
Centers for Disease Control and Prevention. (2024j). Preventing MRDOs. https://www.cdc.gov/healthcare-associated-infections/php/preventing-mdros/index.html
Centers for Medicare & Medicaid Services. (2024). Hospital-acquired condition reduction program. https://www.cms.gov/medicare/quality/value-based-programs/hospital-acquired-conditions
Evans, H. L. (2024). Overview of the evaluation and management of surgical site infection. UpToDate. Retrieved April 7, 2025, from https://www.uptodate.com/contents/overview-of-the-evaluation-and-management-of-surgical-site-infection
Ernst, D. (2025). C. difficile prevention therapy Zinplava discontinued. Medical Professionals Reference. https://www.empr.com/news/c-difficile-prevention-therapy-zinplava-discontinued/
Ernstmeyer, K., & Christman, E. (Eds.). (2023). Nursing skills [Internet], Chapter 4 aseptic technique. (9th Ed.). Open Resources for Nursing (Open RN). https://www.ncbi.nlm.nih.gov/books/NBK596727
Gillis, V. E. L. M., van Es, M. J., Wouters, Y., & Wanten, G. J. A. (2022). Antiseptic barrier caps to prevent central line-associated bloodstream infections: A systematic review and meta-analysis. American Journal of Infection Control, 51(7), 827–835. https://doi.org/10.1016/j.ajic.2022.09.005
Gorski, L. A., Hadaway, L., Hagle, M. E., Broadhurst, D., Clare, S., Kleidon, T., Meyer, B. M., Nickel, B., Rowley, S., Sharpe, E., & Alexander, M. (2021). Infusion therapy standards of practice, 8th edition. Journal of Infusion Nursing, 44(1S), S1–S224. https://doi.org/10.1097/NAN.0000000000000396
Haddadin, Y., Annamaraju, P., & Regunath, H. (2022). Central line-associated bloodstream infections. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK430891/
Harris, A. (2024). Methicillin-resistant Staphylococcus aureus: Beyond the basics. UpToDate. Retrieved June 13, 2022, from https://www.uptodate.com/contents/methicillin-resistant-staphylococcus-aureus-mrsa-beyond-the-basics
Holubar, M., & Deresinski, S. (2024). Antimicrobial stewardship in hospital settings. UpToDate. Retrieved April 8, 2025, from https://www.uptodate.com/contents/antimicrobial-stewardship-in-hospital-settings
Imam, T. H. (2024). Catheter-associated urinary tract infections. Merck Manual Professional Version. https://www.merckmanuals.com/professional/genitourinary-disorders/urinary-tract-infections-utis/catheter-associated-urinary-tract-infections
Infectious Disease Society of America. (2023). Compendium of strategies update. https://www.idsociety.org/practice-guideline/compendium-of-strategies-to-prevent-hais/
Institute of Medicine, Committee on Quality of Health Care in America, Kohn, L. T., Corrigan, J. T., & Donaldson, M. S. (2000). To err is human: Building a safer health system. The National Academies Press. https://doi.org/10.17226/9728
Iwu, C. D. (2024). Global research landscape of health care-associated infections among immunocompromised people before and after the start of the COVID-19 pandemic. Infectious Medicine, 3(3). https://doi.org/10.1016/j.Imj.2024.100127
Jacob, J. T. (2024). Intravascular catheter-related infection: Epidemiology, pathogenesis, and microbiology. UpToDate. Retrieved April 8, 2025, from https://www.uptodate.com/contents/intravascular-catheter-related-infection-epidemiology-pathogenesis-and-microbiology
The Joint Commission. (2025). Hospital: 2025 national patient safety goals. https://www.jointcommission.org/standards/national-patient-safety-goals/hospital-national-patient-safety-goals/
Kalil, A. C., Metersky, M. L., Klompas, M., Muscedere, J., Sweeney, D., A., Palmer, L. B., Napolitano, L. M., O’Grady, N. P., Bartlett, J. G., Carratala, J., El Solh, A. A., Ewig, S., Frey, P. D., File, T. M., Jr., Restrepa, M. I., Roberts, J. A., Waterer, G. W., Cruse, P., Knight, S. L., & Brozek, J. L. (2016). Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clinical Infectious Diseases, 63(5), e61–e111. https://doi.org/10.1093/cid/ciw353
Kelly, C. P., Lamont, J. T., & Bakken, J. S. (2025). Clostridioides difficile infection in adults: Treatment and prevention. UpToDate. Retrieved April 7, 2025, from https://www.uptodate.com/contents/clostridioides-difficile-infection-in-adults-treatment-and-prevention
Kim, M. H., Park, J-H., & Kim, J. T. (2021). A reliable diagnostic method of surgical site infection after posterior lumbar surgery based on serial C-reactive protein. International Journal of Surgery Global Health, 4(5), e61. https://doi.org/10.1097/GH9.0000000000000061
Klompas, M., Branson, R., Cawcutt, K., Crist, M., Eichenwald, E. C., Greene, L. R., Lee, G., Maragakis, L. L., Powell, K., & Priebe, G. P., Speck, K., Yokoe, D. S., & Berenholtz, S. M. (2022). Strategies to prevent ventilator-associated pneumonia, ventilator-associated events, and nonventilator hospital-acquired pneumonia in acute-care hospitals: 2022 Update. Infection Control & Hospital Epidemiology. 43(6):687–713. https://doi.org/10.1017/ice.2022.88
Klompas, M. (2024a). Epidemiology, pathogenesis, microbiology, and diagnosis of hospital-acquired and ventilator-associated pneumonia in adults. UpToDate. Retrieved April 7, 2025, from https://www.uptodate.com/contents/epidemiology-pathogenesis-microbiology-and-diagnosis-of-hospital-acquired-and-ventilator-associated-pneumonia-in-adults
Klompas, M. (2024b). Treatment of hospital-acquired and ventilator-associated pneumonia in adults. UpToDate. Retrieved April 7, 2025, from https://www.uptodate.com/contents/treatment-of-hospital-acquired-and-ventilator-associated-pneumonia-in-adults
Klompas, M. (2025). Risk factors and prevention of hospital-acquired and ventilator-associated pneumonia in adults. UpToDate. Retrieved April 7, 2025, from https://www.uptodate.com/contents/risk-factors-and-prevention-of-hospital-acquired-and-ventilator-associated-pneumonia-in-adults
Kollef, M. H. (2024). Clinical presentation and diagnostic evaluation of ventilator-associated pneumonia. UpToDate. Retrieved April 7, 2025, from https://www.uptodate.com/contents/clinical-presentation-and-diagnostic-evaluation-of-ventilator-associated-pneumonia
Lafuente Cabrero, E., Terradas Robledo, R., Civit Cuñado, A., García Sardelli, D., Hidalgo López, C., Giro Formatger, D., Lacueva Perez, L., Esquinas López, C., & Tortosa Moreno, A. (2023). Risk factors of catheter- associated bloodstream infection: Systematic review and meta-analysis. PloS One, 18(3), e0282290. https://doi.org/10.1371/journal.pone.0282290
Lamont, J. T., Bakken, J. S., & Kelly, C. P. (2025a). Clostridioides difficile infection in adults: Epidemiology, microbiology, and pathophysiology. UpToDate. Retrieved April 7, 2025, from https://www.uptodate.com/contents/clostridioides-difficile-infection-in-adults-epidemiology-microbiology-and-pathophysiology
Lamont, J. T., Kelly, C. P., & Bakken, J. S. (2025b). Clostridioides difficile infection in adults: Clinical manifestations and diagnosis. UpToDate. Retrieved April 7, 2025, from https://www.uptodate.com/contents/clostridioides-difficile-infection-in-adults-clinical-manifestations-and-diagnosis
Latthe, P. M., & Latthe, A. M. (2024). Catheter-associated urinary tract infections - A review. Wadia Journal of Women and Child Health, 3, 79–82. https://doi.org/10.25259/WJWCH_39_2024
Levitus, M., Rewane, A., & Perera, T. B. (2023). Vancomycin-resistant Enterococci. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK513233/
Long, D. R., Cifu, A., Salipante, S. J., Sawyer, R. G., Machutta, K., & Alverdy, J. C. (2024). Preventing surgical site infections in the era of escalating antibiotic resistance and antibiotic stewardship. JAMA Surgery, 159(8), 949–956. https://doi.org/10.1001/jamasurg.2024.0429
Mada, P. K., & Alam, M. U. (2024). Clostridioides difficile infection. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK431054/
Marchaim, D., & Kaye, K. (2023). Nosocomial infections in the intensive care unit: Epidemiology and prevention. UpToDate. Retrieved April 7, 2025, from https://www.uptodate.com/contents/nosocomial-infections-in-the-intensive-care-unit-epidemiology-and-prevention
McDonald, L. C., Gerding, D. N., Johnson, S., Bakken, J. S., Carroll, K. C., Coffin, S. E., Dubberke, E. R., Garey, K. W., Gould, C. V., Kelly, C., Loo, V., Shaklee Sammons, J., Sandora, T. J., & Wilcox, M. H. (2018). Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clinical Infectious Diseases, 66(7), e1–e48. https://doi.org/10.1093/cid/cix1085
MedlinePlus. (2022). Fecal microbiota transplant. https://medlineplus.gov/ency/article/007703.htm
Miller, W. R. (2024). Treatment of enterococcal infections. UpToDate. Retrieved April 8, 2025, from https://www.uptodate.com/contents/treatment-of-enterococcal-infections
Monegro, A. F., Muppidi, V., & Regunath, H. (2023). Hospital-acquired infections. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK441857/
National Healthcare Safety Network. (2025). Patient safety component manual. Centers for Disease Control and Prevention, National Healthcare Safety Network. https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf
The National Institute for Occupational Safety and Health. (2022). Chain of infection components. Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. https://www.cdc.gov/niosh/learning/safetyculturehc/module-2/3.html
Occupational Safety and Health Administration. (n.d.). Personal protective equipment. Retrieved April 7, 2025, from https://www.osha.gov/personal-protective-equipment
Opalka, K., & Ricciuti, K. (2024). QAPI 153—scrub away the CLABSI: A visual aide for infection reduction. American Journal of Infection Control, 52(6), S73. https://doi.org/10.1016/j.ajic.2024.04.140
Papazian, L., Klompas, M., & Luyt, C. (2020). Ventilator-associated pneumonia in adults: A narrative review. Intensive Care Medicine, 46, 888-906. https://doi.org/10.1007/s00134-020-05980-0
Rajesh, E., Katragadda, R., & Ramani, C. P. (2021). Bacteriological profile and antimicrobial resistance pattern of ventilator associated pneumonia in tertiary care hospital. Indian Journal of Microbiology Research, 8(3), 191-195. https://doi.org/10.18231/j.Ijmr.2021.039
Rogers, J. L. and Brashers, V. L. (Eds.). (2023). McCance and Huether's pathophysiology: The biologic basis for disease in adults and children (9th ed.). Elsevier.
Sabih, A., & Leslie, S. W. (2024). Complicated urinary tract infections. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK436013/
Said, M. S., Tirthani, E., & Lesho, E. (2024). Enterococcus infections. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK567759/
Salen, P., & Stankewicz, H. A. (2023). Pseudomembranous colitis. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK470319/
Sethi, S. (2024a). Hospital-acquired pneumonia. Merck Manual Professional Version. https://www.merckmanuals.com/professional/pulmonary-disorders/pneumonia/hospital-acquired-pneumonia
Sethi, S. (2024b). Ventilator-associated pneumonia. Merck Manual Professional Version. https://www.merckmanuals.com/professional/pulmonary-disorders/pneumonia/ventilator-associated-pneumonia
Shebl, E., & Gulick, P. G. (2023). Nosocomial pneumonia. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK535441/
Siddiqui, A. J., & Koirala, J. (2023). Methicillin-resistant Staphylococcus aureus. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK482221/
Siegel, J. D., Rhinehart, E., Jackson, M., Chiarello, L., & the Healthcare Infection Control Practices Advisory Committee. (2024). 2007 guideline for isolation precautions: Preventing transmission of infectious agents in healthcare settings. https://www.cdc.gov/infection-control/media/pdfs/guideline-isolation-h.pdf
The TEAM Study Investigators and the ANZICS Clinical Trials Group. (2022). Early active mobilization during mechanical ventilation in the ICU. The New England Journal of Medicine, 387(19), 1747–1758. https://doi.org/10.1056/NEJMoa2209083
Trautner, B. W., & Gupta, K. (2024). Catheter-associated urinary tract infection in adults. UpToDate. Retrieved April 7, 2025, from https://www.uptodate.com/contents/catheter-associated-urinary-tract-infection-in-adults
Ullman, A. J., & Chopra, V. (2024). Routine care and maintenance of intravenous devices. UpToDate. Retrieved April 8, 2025, from https://www.uptodate.com/contents/routine-care-and-maintenance-of-intravenous-devices
US Department of Health and Human Services. (n.d.). Health care-associated infections. Healthy People 2030, Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. Retrieved April 7, 2025, from https://odphp.health.gov/healthypeople/objectives-and-data/browse-objectives/health-care-associated-infections
US Department of Health and Human Services. (2024). National HAI targets & metrics. https://www.hhs.gov/oidp/topics/health-care-associated-infections/targets-metrics/index.html
Werneburg G. T. (2022). Catheter-associated urinary tract infections: Current challenges and future prospects. Research and Reports in Urology, 14, 109–133. https://doi.org/10.2147/RRU.S273663
Wilson, M. L. (2025). Detection of bacteremia: Blood cultures and other diagnostic tests. UpToDate. Retrieved April 8, 2025, from https://www.uptodate.com/contents/detection-of-bacteremia-blood-cultures-and-other-diagnostic-tests
World Health Organization. (n.d.). Infection prevention and control, hand hygiene. Retrieved April 9, 2025, from https://www.who.int/teams/integrated-health-services/infection-prevention-control/hand-hygiene
World Health Organization. (2021). Five moments for hand hygiene [Image]. https://www.who.int/publications/m/item/five-moments-for-hand-hygiene
Young, M. P., & Yuo, T. H. (2025). Central venous catheters: Overview of complications and prevention in adults. UpToDate. Retrieved April 7, 2025, from https://www.uptodate.com/contents/central-venous-catheters-overview-of-complications-and-prevention-in-adults
Zabaglo, M., Leslie, S. W., & Sharman, T. (2024). Postoperative wound infections. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK560533/
Powered by Froala Editor