This module explores the prevalence and incidence of human papillomavirus (HPV), current guidelines, and recommendations for screenings and treatment. Healthcare professionals (HCPs) should understand the importance of HPV vaccination and the potential barriers for adolescents and young adults.
Course preview
Human Papillomavirus (HPV)
Disclosure Statement
This module explores the prevalence and incidence of human papillomavirus (HPV), current guidelines, and recommendations for screenings and treatment. Healthcare professionals (HCPs) should understand the importance of HPV vaccination and the potential barriers for adolescents and young adults.
At the completion of this activity, learners will be able to:
- describe the pathophysiological changes that occur with HPV
- identify risk factors for HPV
- describe clinical manifestations associated with HPV
- discuss nursing care aimed at preventing and treating HPV and HPV-related cancers
- discuss future HPV research
Human papillomavirus is a sexually transmitted infection (STI). Currently, it is the most common STI in the United States and was identified in 43 million Americans in 2018 (Centers for Disease Control and Prevention [CDC], 2022b; Hinkle & Cheever, 2018; Norris, 2020). Most sexually active women and men will contract a genital HPV infection at some point in their lives, but most of these infections will remain clinically silent and resolve without treatment or complications (Hahn & Spach, 2019). Individuals with HPV may not experience symptoms. According to the CDC (2019), by age 50, at least 4 out of 5 females will have experienced an HPV infection, with the highest number of HPV infections among those ages 20 to 24. If the infection initially occurs after age 30, the person’s risk for cervical cancer increases. Globally, cervical cancer is the fourth leading cause of cancer in women and the second most common cause in young adult women (ages 15-44 years; Hoffman & Sullivan, 2020). Furthermore, each year over 33,000 cases of cancer across all genders are linked to HPV in the US. The direct annual cost of genital HPV infection is estimated at just over 1.7 billion dollars (Hahn & Spach, 2019).
Anatomy and Physiology
Female Reproductive System
The female reproductive system consists of multiple organs and the release of several hormones. These organs have various functions related to sexual stimulation and fetal growth. The female reproductive system includes the ovaries, fallopian tubes, uterus, vagina, and external genitals (see Figure 1).
Sperm can enter the body during sexual intercourse. During penetration, semen enters the vagina and passes through the cervix and uterus to get to the fallopian tubes. The vagina contains an acidic environment to protect it from pathogens and a stratified squamous epithelium, known as the vaginal microbiome, which seeks to protect the body from disease. The external genitals are also called the vulva, consisting of the clitoris, mons pubis, labia majora, labia minora, and Bartholin’s glands (which promote lubrication of the vagina during sexual intercourse; Scanlon & Sanders, 2019).
Figure 1
Female Reproductive Organs
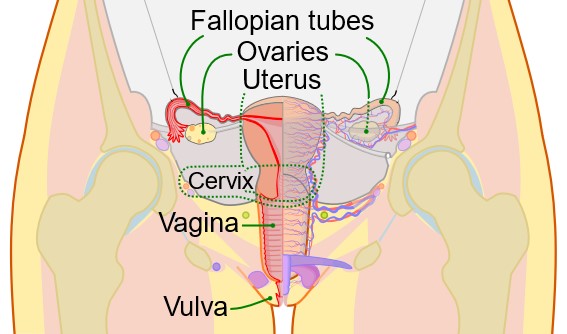 (CDC & Mysi, 2007) Male Reproductive SystemThe male reproductive system includes several organs related to sperm development and ejaculation (see Figure 2). The scrotum contains the testes. The prostate gland secretes fluid and contracts to expel the sperm into the urethra during ejaculation. The bulbourethral glands, just below the prostate, assist with neutralizing acidic urine. Allowing the semen to become alkaline assists with overcoming the vagina’s naturally acidic environment. The urethra is the longest duct and is in the penis. The penis is an external genital organ; the most distal portion is the glans penis. The glans penis might be covered by prepuce unless it was removed during circumcision (Scanlon & Sanders, 2019). Figure 2Male Reproductive Organs
(CDC & Mysi, 2007) Male Reproductive SystemThe male reproductive system includes several organs related to sperm development and ejaculation (see Figure 2). The scrotum contains the testes. The prostate gland secretes fluid and contracts to expel the sperm into the urethra during ejaculation. The bulbourethral glands, just below the prostate, assist with neutralizing acidic urine. Allowing the semen to become alkaline assists with overcoming the vagina’s naturally acidic environment. The urethra is the longest duct and is in the penis. The penis is an external genital organ; the most distal portion is the glans penis. The glans penis might be covered by prepuce unless it was removed during circumcision (Scanlon & Sanders, 2019). Figure 2Male Reproductive Organs 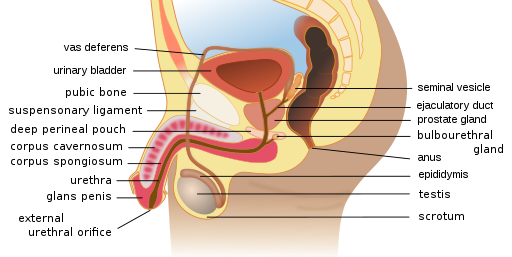 (Wumingbai, 2021) Pathophysiology HPV can be transmitted via skin contact during vaginal, anal, and oral sexual intercourse (Hoffman & Sullivan, 2020). The virus comprises a double-stranded deoxyribonucleic acid (DNA) that promotes lesions on the squamous epithelium. These lesions are visible condyloma acuminata (i.e., genital warts). The DNA replicates, causing the basal membrane to become occupied by the virus. In the early stages, low-grade intraepithelial lesions develop. These lesions are known and graded according to their neoplasia; thus, in its early stage, a low-grade intraepithelial lesion is known as cervical intraepithelial neoplasia (CINI), which envelopes one-third of the epithelium. CIN II covers two-thirds of the epithelium, and CIN III covers the entire epithelium (see Figure 3; Hoffman & Sullivan, 2020). CIN I is low risk, whereas CIN II and CIN III demonstrate higher risk.
(Wumingbai, 2021) Pathophysiology HPV can be transmitted via skin contact during vaginal, anal, and oral sexual intercourse (Hoffman & Sullivan, 2020). The virus comprises a double-stranded deoxyribonucleic acid (DNA) that promotes lesions on the squamous epithelium. These lesions are visible condyloma acuminata (i.e., genital warts). The DNA replicates, causing the basal membrane to become occupied by the virus. In the early stages, low-grade intraepithelial lesions develop. These lesions are known and graded according to their neoplasia; thus, in its early stage, a low-grade intraepithelial lesion is known as cervical intraepithelial neoplasia (CINI), which envelopes one-third of the epithelium. CIN II covers two-thirds of the epithelium, and CIN III covers the entire epithelium (see Figure 3; Hoffman & Sullivan, 2020). CIN I is low risk, whereas CIN II and CIN III demonstrate higher risk.
Figure 3
Progression of CIN to Cancer

(Balasubramaniam et al., 2019)
HPV has over 40 types and 100 subtypes, affecting the genitals, mouth, and throat. The types are differentiated according to the risk of cervical cancer development. CIN-specific subtypes, such as 16 and 18, place individuals at a greater risk of developing uncontrolled cervical dysplasia and cervical cancer. These types are evident in 70% of cervical cancer patients. However, 90% of HPV cases are deemed low-risk and present asymptomatically or with condyloma acuminata (genital warts). The incubation period for HPV ranges from 3 weeks to 8 months to decades; the longer the incubation period is, the more likely it will correlate with a cervical cancer diagnosis (Hoffman & Sullivan, 2020; Hinkle & Cheever, 2018; Norris, 2020).
If a woman contracts HPV, her risk for cervical cancer increases if she smokes, is immunosuppressed, or is exposed to hormone alterations (including pregnancy and medications; Norris, 2020). Preventing HPV-related cervical cancer can also occur through annual pap smears for women. However, not all types of HPV will cause cervical cancer; low-risk types include 6, 11, 42, 43, 44, 54, 61, 70, and 72. Genital warts tend to be visible with types 6 and 11; these genital warts are visible and palpable on the vulva, vagina, cervix, and anus. HPV types more frequently associated with cervical malignancy include 16, 18, 31, 33, 45, and 52 (American College of Obstetricians and Gynecologists [ACOG], 2022; Hinkle & Cheever, 2018). In nearly all cases of cervical cancer, patients will test positive for infectious HPV (Hoffman & Sullivan, 2020). Cervical cancer tends to occur within 3 to 7 years following changes in cervical cellular structure (ACOG, 2022).
Risk Factors and Protective Measures
Annually, over 6 million new cases of HPV are diagnosed. Individuals at greatest risk for developing HPV are young adults under age 25, individuals who are sexually active at a young age with a first partner at age 16 or younger, and those with multiple sex partners or a partner with multiple sex partners (Norris, 2020). In addition, lower socioeconomic status, smoking, poor nutrition, oral contraceptive use, douching, and herpes simplex virus type 2 are other risk factors for HPV (Hoffman & Sullivan, 2020).
...purchase below to continue the course
Male patients are at risk for contracting HPV as well. HPV can contribute to the development of penile, anal, and oropharyngeal cancers in men. Immunocompromised men, including those with HIV, are at risk of developing cancer. Men who receive anal sex are more likely to develop anal cancer (CDC, 2022b). More specifically, they are at risk of developing squamous cell carcinoma of the anus due to HPV-16 (Donà et al., 2022). As a result, patient education in disease prevention is a critical nursing intervention.
HPV Immunization
Pediatric nurses in outpatient clinics can educate patients and their families on HPV prevention through safe sex practices and vaccination in the teenage years. More specifically, vaccination is recommended by the Advisory Committee on Immunization Practices (ACIP) of the CDC (2019) and ACOG (2022) in males and females before becoming sexually active; vaccination is also encouraged for both males and females into their third decade of life (see Table 1; Frandsen & Smith Pennington, 2018; Hinkle & Cheever, 2018). Gardasil was initially a quadrivalent vaccine offering protection against four different types of HPV; it was approved for use in 2006 for females aged 9 through 26. This series of three injections was expanded for males of the same age in 2009. Originally approved in late 2014, Gardasil-9 (see Table 1) is a nine-valent vaccine with increased coverage of additional high-risk HPV types. Since late 2016, only the nine-valent formulation has been distributed in the US. The FDA expanded the use of Gardasil-9 in 2018 to ages 9-45. The CDC recommends dosage based on the patient’s age at initial vaccination. In patients between 9 and 14, the 2-dose series should be given with 6-12 months between doses; in those 15 and older, the 3-dose series is recommended at 0, 2, and 6 months. If children are sexually abused, have HIV or are immunocompromised, HPV vaccination is encouraged at age 9. A three-dose series is also recommended in those who did not wait the recommended 6 months between their first two doses or are immunocompromised. The HPV vaccine does not interfere with other common adolescent vaccines (meningitis, Tdap) when given concurrently at a different site. Nurses should encourage the completion of the entire HPV vaccination series by discussing its effectiveness in preventing cervical cancer. Vaccination should only be held during an acute illness or pregnancy. If the vaccination series is interrupted, vaccination should be provided as soon as possible; however, the series does not need to be restarted, nor is an additional dosage recommended. In those unvaccinated individuals over 26, a conversation regarding the potential risks and benefits of vaccination should inform shared decision-making regarding the potential for vaccination. If risk factors for exposure persist and the risks of vaccination are low, this may be beneficial. Vaccination in this older cohort is less effective, as most individuals over 26 have already been sexually exposed to the HPV virus (ACOG, 2022; Frandsen & Smith Pennington, 2018; CDC, 2021, 2022a; Hoffman & Sullivan, 2020; Meites et al., 2019; NCI, 2019; Siegel et al., 2020).
Table 1
HPV Immunization Details
Medication | HPV 9-valent recombinant (Gardasil 9) |
Age of Immunization
| All unvaccinated adults or adolescents aged 9–26 years Unvaccinated adults aged 26-45 based on individual shared decision-making |
Dosage | 2 doses: 0.5 mL IM at 0 and 6-12 months in the deltoid or anterolateral thigh 3 doses: 0.5 mL IM at 0, 2, and 6 months in the deltoid or anterolateral thigh |
Targeted HPV Subtypes | 6, 11, 16, 18, 31, 33. 45, 58, 52 |
Side Effects | Pain, swelling, erythema, pruritus, ecchymosis, bleeding, headaches, fever, nausea, dizziness, tiredness, diarrhea, abdominal pain, sore throat, and fainting |
Contraindications | A prior history of a yeast allergy A prior history of anaphylaxis related to any vaccine component Deferred in pregnant patients |
(CDC, 2021; Frandsen & Smith Pennington, 2018; Karch, 2020; Merck & Co, 2022; Norris, 2020)
Benefits of Vaccination
The CDC (2019) supports the HPV vaccination, declaring the benefits outweigh the potential side effects or risks. Since the introduction of the HPV vaccine, HPV infections and cervical precancers have dropped significantly. Over the past 10 years, females aged 12-19 have experienced a 71% decrease in high-risk HPV infections associated with cancer and anogenital warts. During the same timeframe, females aged 30-45 had a 61% decrease in high-risk HPV infections. Precancerous cervical lesions caused by HPV types 16 and 18 have dropped 40% among vaccinated women. This protection remains high after 10 years, with no decrease in protection when measuring antibodies from the vaccine in the blood. Approximately 31,200 of the 33,700 HPV-related cancers diagnosed yearly can be prevented through vaccination. Routine cervical cancer screenings provide early recognition of cellular change and risk, yet there are no recommended screening tests for other cancers associated with HPV. Vaccination can prevent the development of oropharyngeal, penile, and anal cancers in addition to cervical and vaginal (CDC, 2019).
Barriers to Vaccination
Since the HPV vaccination is administered to minors, barriers often stem from parental or legal guardian beliefs, concerns, costs, opposition to the vaccine, or perceived risk versus benefits (Zheng et al., 2021). In a study by Johns Hopkins Medicine (2018), parents chose to avoid the HPV vaccination series for their children due to concerns regarding the safety of the vaccine, a lack of necessity, a lack of knowledge about HPV, and the failure of healthcare providers to advocate for the clinical benefits of vaccination. Historically, providers have cited that parents chose to avoid vaccination for HPV over concerns that sexual activity would be encouraged. Still, this study identified many other barriers to overcome with parents. The top reason for boys and girls was parents' perception of a lack of necessity. Healthcare providers are encouraged to educate parents on vaccination safety and increased protection from HPV infection and associated cancers (Johns Hopkins Medicine, 2018).
Missed clinical opportunities are also cited by the CDC (2019) as a primary reason for low HPV vaccination rates. Other factors identified include healthcare providers’ knowledge gap of the vaccine and HPV, discomfort discussing sexual behaviors, cost, and safety concerns. Escoffery and colleagues (2019) identified that barriers to HPV vaccination included a lack of training among healthcare providers, limited staff resources, complications with accessing electronic health records, limited staff buy-in, cultural and language barriers, competing priorities, a lack of funding, insufficient staff training, difficulties with state immunization registries, misinformation, vaccine stigma, and limited health literacy.
Patients with an allergy to any ingredients should not receive the vaccination. For instance, if a patient has an allergy to polysorbate 80, amorphous aluminum hydroxyphosphate sulfate, or yeast, they should not receive HPV recombinant, quadrivalent (Gardasil), or 9-valent recombinant (Gardasil 9). An allergy to yeast may provoke anaphylaxis (Merck & Co, 2022). As a result, assessing for allergies before administering the HPV vaccination is necessary.
Benefits of Condom Usage
Condoms can prevent contracting HPV and other STIs (CDC, 2022c). When applied correctly, the risk of developing HPV-related neoplasia and cancer is decreased (see Table 2; Skorstengaard et al., 2019). Valasoulis and colleagues (2022) found that proper utilization of condoms during sexual intercourse reduces the reoccurrence of biomarkers and disease among patients treated for CIN.
Table 2
Correct Usage of Male Condoms
Do | Don’t |
Apply a condom before penetration | Reuse condoms or apply more than one at a time |
Read the package and check the expiration date | Use an expired condom |
Check the condom before placement to assess for tears or defects | Use a condom with tears or defects |
Place condoms in a cool, dry setting | Place the condom in a wallet, as heat and friction will alter the integrity of the condom |
Use latex or polyurethane condom | Use a condom made from material to which either partner has an allergy |
Pinch air out of the condom before placement | Use oil-based products as lubricants |
Unroll the condom to the base of the penis | Use nonoxynol-9 due to potential skin irritation |
Hold the condom in place after sexual intercourse and dispose |
|
Perform hand hygiene |
|
(CDC, 2022c)
Barriers to Condom Usage
If a condom is incorrectly placed, the risk of contracting HPV increases (CDC, 2022b). Areas exposed and not covered by a condom may also result in HPV transmission (New Zealand HPV Project, 2022). Individuals at risk for not using condoms may include adolescents and those experiencing substance abuse, lower socioeconomic status, lower educational attainment, and a lack of access (Goldenberg et al., 2020). Nurses have a unique opportunity to assess and address all these factors through education and coordination of resources.
Signs and Symptoms
Generally, HPV is asymptomatic and resolves without treatment in 2 years for 90% of patients. However, if an individual is immunocompromised or has specific subtypes of HPV, they are at risk for developing cervical dysplasia, genital warts, and cervical cancer. Genital warts on external surfaces tend to be small, flat, and flesh-appearing, with a rough surface and pedunculated features for all genders. Internal genital warts (i.e., on the vagina, urethra, anus, or mouth) may have a cauliflower-like appearance. Symptomatic patients may experience itching, burning, or tenderness in affected areas (Hoffman & Sullivan, 2020; Norris, 2020).
Diagnosis
Since HPV tends to be asymptomatic, screening for the virus is essential. HPV testing can be performed during STI screening at pap smears. A cervical swab sample is placed under a microscope to look for changes in cellular structure that demonstrate malignancy. At this time, HPV DNA can be identified using a hybridization solution. The testing confirms whether high-risk HPV DNA is present, as it can contribute to cervical cellular malignancy. This testing can be done alone or with cytology (referred to as co-testing; Hoffman & Sullivan, 2020; Norris, 2020). According to the ACS, all women over 25 should receive HPV testing with their pap smears. Testing for HPV is based on an assessment of age and risk (ACS, 2020a, b). According to ACOG (2021) and the US Preventive Services Task Force, cervical cancer screening should begin earlier (age 21). Please see Table 3 for recommendations from both groups.
Table 3
ACS 2020 Cervical Cancer Screening Guidelines versus 2021 ACOG/USPSTF Guidelines
Age Range | 2020 ACS Guidelines | 2021 ACOG/USPSTF Guidelines |
0-20 | No screening recommended |
21-24 | No screening recommended | Cytology alone every 3 years |
25-29 | Three options: primary HPV testing every 5 years, OR co-test every 5 years OR cytology alone every 3 years | Cytology alone every 3 years, although primary HPV testing every 5 years may be considered in average-risk patients |
30-65 | Three options: primary HPV testing every 5 years, OR co-test every 5 years OR cytology alone every 3 years |
65+ | the decision to continue screening beyond 65 years depends on individual risk factors and past medical history women who have undergone regular screening in the past 10 years with normal results and no history of CIN-2 or higher can stop screening once screening is stopped, it should not be started again |
Women who had a total hysterectomy with removal of the cervix | No screening recommended unless it was performed to treat cervical precancer or cancer |
(ACS, 2020a; 2020b)
In addition, if a patient expresses concern over genital warts or vulvar pruritus or if an abnormal pap smear result occurs, further examination for HPV is warranted. Pap results may demonstrate atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesions. HCPs should rule out other conditions, including vaginitis, by examining the vulva, lesion assessment, and biopsy collection. If further testing is warranted, a colposcopy may be ordered to examine the cervix and vagina (Norris, 2020).
A colposcope—a low-power binocular microscope—allows visualization of the mucosal layers of the cervix and vagina. Acetic acid aids in the visualization of the columnar epithelium by removing mucus. Lugol’s solution is applied to examine the cervix and vagina during the procedure. The solution uses iodine, so nurses must assess patients for an iodine allergy. Biopsies may be required if the tissue stains by the Lugol’s solution appear lighter in color than the typical brown staining (Hoffman & Sullivan, 2020).
For male patients, HPV biopsy results may demonstrate intraepithelial neoplasia of the penis and anus (Norris, 2020). Neoplasia can result in penile or anal cancer. If HPV affects the throat, tongue, or tonsils, patients are also at risk for developing oropharyngeal cancer, so a visualization scope and biopsy may be required (CDC, 2022b).
Treatment and Management
Treating HPV involves avoiding transmission to other individuals, preventing and eliminating genital warts, monitoring for changes in cervical cellular structure, and limiting psychological distress for patients (Norris, 2020).
HPV Non-Pharmacological Management
Treatment should include preventing HPV through robust patient education on safe sexual practices. Nurses can educate individuals on condom usage in outpatient settings, including schools, pediatric offices, and obstetrics/gynecology clinics (Norris, 2020). Besides condoms, nurses can educate patients on using dental dams to prevent HPV infection in the mouth (ACOG, 2022). Patients should be encouraged to maintain a healthy lifestyle to promote greater well-being in areas such as diet, smoking cessation, and limited alcohol intake (Hoffman & Sullivan, 2020).
Patients may require psychosocial care for anger and frustration related to developing warts or not knowing who infected them. Nurses can provide support by acknowledging the patient’s distress, educating on preventing disease transmission, and coordinating care with social workers to tend to the patient’s psychosocial needs. Nurses should assess for pain and encourage the patient to report worsening pain or vaginal discharge (Hinkle & Cheever, 2018; Hoffman & Sullivan, 2020).
HPV Pharmacological Management
If a patient has external genital warts, they may be prescribed a topical ointment (applied by the healthcare provider) of trichloroacetic acid or podophyllin (Podofin, Podocon). They may also require cryotherapy (thermal-induced cytolysis) or surgical removal of genital warts. Electrocautery or laser treatment may be warranted for abundant genital warts. Patients may be prescribed topical products that can be self-administered at home, such as podofilox (Condylox) 0.5% gel or solution, imiquimod (Aldara) 5% cream, or sinecatechin (Veregen) 15% ointment. These agents work by collapsing the area surrounding the wart and encouraging an immune response. While treatment for HPV is usually successful, the infection is viral, and treatments are not curative. They are designed to elicit an immune response. Given this, patients with weakened immune systems, those with diabetes, smokers, and pregnant people have an increased risk of pharmacological resistance. Nurses should educate patients on the risk of local skin irritation and pain with topical applications. If a patient is experiencing pain, nurses should provide analgesia and reassess the treatment’s efficacy (Hinkle & Cheever, 2018; Hoffman & Sullivan, 2020). Nurses should educate patients not to use over-the-counter wart removers for genital warts (ACOG, 2022).
HPV Tissue Removal
If the tissue is high-grade neoplasia (CIN II or III), genital warts and the surrounding tissue should be extracted to assess for cervical cancer. Tissue can be removed by surgical ablation, excision, laser conization, or loop electrosurgical excision. Ablation utilizes temperature changes to remove the affected area via cryosurgery (freezing) or a laser. The impacted area may be excised using cold knife colonization (CKC). Using a laser, cervical tissue can be removed via laser conization. An electrosurgical excision uses a painless current to remove the tissue (Hoffman & Sullivan, 2020).
Future Research
Screening for HPV
New research is being conducted to identify whether menstrual blood can assist in diagnosing HPV. Naseri and colleagues (2022) examined modified menstrual pads (i.e., Q-Pad) from 107 menstruating women for HPV. The pads demonstrated HPV in HPV-positive women. Among participants, 94% preferred using the Q-Pad over traditional clinician testing to screen for HPV. Nurses can uniquely advocate for patients in terms of ease of screening.
Effects of the COVID-19 Pandemic
HPV vaccination, which has been available since 2006, has been widely successful in decreasing HPV rates and creating herd immunity. However, throughout the COVID-19 pandemic, HPV vaccinations have declined due to missed appointments and redirected healthcare priorities, such as caring for patients with COVID-19, efforts to prevent possible exposure by limiting clinic visits, and reduced staffing; as a result, traditional clinic visits were replaced with telehealth appointments (Kaltwasser, 2022; Rosenblum et al., 2022; Ryan et al., 2022).
Damgacioglu and colleagues (2022) examined the impact of decreased HPV vaccination rates due to the COVID-19 pandemic on oropharyngeal cancers in men, predicting an overwhelming increase in HPV-related oropharyngeal cancers. However, the intensity of this increase will be based on the speed at which vaccination coverage can be prioritized and the rate of vaccination uptake (Damgacioglu et al., 2022). Nurses can educate patients and their families on the necessity of catching up on their vaccination schedule at medical appointments (Kaltwasser, 2022; Rosenblum et al., 2022).
References
American College of Obstetricians and Gynecologists. (2021). Updated cervical cancer screening guidelines. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/04/updated-cervical-cancer-screening-guidelines
American College of Obstetricians and Gynecologists. (2022). Human papillomavirus (HPV) vaccination. https://www.acog.org/womens-health/faqs/hpv-vaccination
American Cancer Society. (2020a). The American Cancer Society guidelines for the prevention and early detection of cervical cancer. https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/cervical-cancer-screening-guidelines.html
American Cancer Society. (2020b). The HPV test. https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/screening-tests/hpv-test.htm
Balasubramaniam, S. D., Balakrishnan, V., Oon, C. E., & Kaur, G. (2019). Progression of CIN to cancer [image]. https://commons.wikimedia.org/wiki/File:HpvInfectedSquamousEpithelialCell.png
CDC, & Mysi. (2007). Scheme female reproductive system-en.svg [Image]. https://commons.wikimedia.org/wiki/File:Scheme_female_reproductive_system-en.svg
Centers for Disease Control and Prevention. (2019). Human papillomavirus (HPV). https://www.cdc.gov/hpv/index.html
Centers for Disease Control and Prevention. (2021). HPV vaccination recommendations. https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html
Centers for Disease Control and Prevention. (2022a). Child and adolescent immunization schedule. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html#note-hpv
Centers for Disease Control and Prevention. (2022b). HPV and men: Fact sheet. https://www.cdc.gov/std/hpv/stdfact-hpv-and-men.htm
Centers for Disease Control and Prevention. (2022c). Male (external) condom use. https://www.cdc.gov/condomeffectiveness/external-condom-use.html
Damgacioglu, H., Sonawane, K., Chhatwal, J., Lairson, D. R., Clifford, G. M., Giuliano, A. R., & Deshmukh, A. A. (2022). Long-term impact of HPV vaccination and COVID-19 pandemic on oropharyngeal cancer incidence and burden among men in the USA: A modeling study. The Lancet Regional Health – Americas, 8, 100143. https://doi.org/10.1016/j.lana.2021.100143
Donà, M. G., Giuliani, M., Rollo, F., Fenicia Vescio, M., Benevolo, M., Giglio, A., Giuliani, E., Morrone, A., & Latini, A. (2022). Incidence and clearance of anal high-risk Human Papillomavirus infection and their risk factors in men who have sex with men living with HIV. Scientific Reports, 12, 184. https://doi.org/10.1038/s41598-021-03913-5
Escoffery, C., Riehman, K., Watson, L., Priess, A. S., Fischer Borne, M., Halpin, S. N., Rhiness, C., Wiggins, E., & Kegler, M. C. (2019). Facilitators and barriers to the implementation of the HPV VACs (Vaccinate Adolescents Against Cancers) program: A consolidated framework for implementation research analysis. Preventing Chronic Disease, 16, 180406. http://dx.doi.org/10.5888/pcd16.180406
Frandsen, G., & Smith Pennington, S. (2018). Abrams’ clinical drug therapy: Rationales for nursing practice. Wolters Kluwer.
Goldenberg, S. M., Liyanage, R., Braschel, M., & Shannon, K. (2020). Structural barriers to condom access in a community-based cohort of sex workers in Vancouver, Canada: Influence of policing, violence, and end-demand criminalization. British Medical Journal Sexual & Reproductive Health, 46, 301-207. http://dx.doi.org/10.1136/bmjsrh-2019-200408
Hahn, A.W., & Spach, D. H. (2019). National STD curriculum: Human papillomavirus infection. https://cdn.std.uw.edu/pdf/pathogen-based/hpv/core-concept/all
Hinkle, J. L., & Cheever, K. H. (2018). Brunner & Suddarth’s textbook of medical-surgical nursing (14th ed.). Wolters Kluwer.
Hoffman, J. J., & Sullivan, N. J. (2020). Davis advantage for medical-surgical nursing: Making connections to practice (2nd ed.). F. A. Davis.
Johns Hopkins Medicine. (2018). HPV vaccine: Why parents really choose to refuse. ScienceDaily. www.sciencedaily.com/releases/2018/10/181024112236.htm
Kaltwasser, J. (2022). HPV Rates Continue to Decline, New Data Suggest Herd Immunity Growing. American Journal of Managed Care. https://www.ajmc.com/view/hpv-rates-continue-to-decline-new-data-suggest-herd-immunity-growing
Karch, A. (2020). Focus on nursing pharmacology (8th ed.). Wolters Kluwer.
Merck and Co. (2022). Gardasil 9 safety & side effects. https://www.gardasil9.com/adolescent/vaccine-side-effects
Meites, E., Szilagyi, P. G., Chesson, H. W., Unger, E. R., Romero, J. R., & Markowitz, L. E. (2019). Human papillomavirus vaccination for adults: Updated recommendations of the advisory committee on immunization practices. Morbidity and Mortality Weekly Report (MMWR), 68(32), 698-702. http://dx.doi.org/10.15585/mmwr.mm6832a3
Naseri, S., Cruz, G., Young, S., & Blumenthal, P. (2022). Screening for high-risk Human Papillomavirus using self-collected menstrual blood. Obstetrics & Gynecology, 139, 27S. https://doi.org/10.1097/01.AOG.0000826692.81266.e9
National Cancer Institute. (2019). Human papillomavirus (HPV) vaccines. Retrieved from https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-vaccine-fact-sheet
New Zealand HPV Project (2022). Will safe sex prevent HPV? https://www.hpv.org.nz/hpv-prevention/will-safe-sex-prevent-hpv
Norris, T. (2020). Porth's essentials of pathophysiology (5th ed.). Wolters Kluwer.
Rosenblum, H. G., Lewis, R. M., Gargano, J. W., Querec, T. D., Unger, E. R., & Markowitz, L. E. (2022). Human Papillomavirus vaccine impact and effectiveness through 12 years after vaccine introduction in the United States, 2003 to 2018. Annals of Internal Medicine, 175(7), 918-926. https://doi.org/10.7326/M21-3798
Ryan, G., Gilbert, P. A., Ashida, S., Charlton, M. E., Scherer, A., & Akelson, N. M. (2022). Challenges to adolescent HPV vaccination and implementation of evidence-based interventions to promote vaccine uptake during the COVID-19 pandemic: “HPV Is probably not at the top of our list.” Preventing Chronic Disease, 19, 210378. http://dx.doi.org/10.5888/pcd19.210378
Scanlon, V. C., & Sanders, T. (2019). Essentials of anatomy and physiology (8th ed.). F. A. Davis.
Siegel, R. L., Miller, K. D., & Jemal, A. (2020). Cancer statistics, 2020. CA Cancer Journal for Clinicians, 70, 7-30. https://doi.org/10.3322/caac.21590
Skorstengaard, M., Suhr, J., & Lynge, E. (2019). Condom use to enhance regression of cervical intraepithelial neoplasia: study protocol for a randomized controlled trial. Trials, 20, 473. https://doi.org/10.1186%2Fs13063-019-3564-4
Valasoulis, G., Michail, G., Pouliakis, A., Androutsopoulos, G., Panayiotides, I. G., Kyrgiou, M. Daponte, A., & Paraskevaidis, E. (2022). Effect of condom use after CIN treatment on cervical HPV biomarkers positivity: Prolonged follow up study. Cancers, 14(14), 3530. https://doi.org/10.3390/cancers14143530
Wumingbai. (2021). Human male reproductive system en.svg [Image]. https://commons.wikimedia.org/wiki/File:Human_male_reproductive_system_en.svg
Zheng, L., Wu, J. P., & Zheng, M. (2021). Barriers to and facilitators of human papillomavirus vaccination among people aged 9 to 26 Years: A systematic review. Sexually Transmitted Diseases. 48(12), e255-e262. https://doi.org/10.1097/OLQ.0000000000001407
 (CDC & Mysi, 2007) Male Reproductive SystemThe male reproductive system includes several organs related to sperm development and ejaculation (see Figure 2). The scrotum contains the testes. The prostate gland secretes fluid and contracts to expel the sperm into the urethra during ejaculation. The bulbourethral glands, just below the prostate, assist with neutralizing acidic urine. Allowing the semen to become alkaline assists with overcoming the vagina’s naturally acidic environment. The urethra is the longest duct and is in the penis. The penis is an external genital organ; the most distal portion is the glans penis. The glans penis might be covered by prepuce unless it was removed during circumcision (Scanlon & Sanders, 2019). Figure 2Male Reproductive Organs
(CDC & Mysi, 2007) Male Reproductive SystemThe male reproductive system includes several organs related to sperm development and ejaculation (see Figure 2). The scrotum contains the testes. The prostate gland secretes fluid and contracts to expel the sperm into the urethra during ejaculation. The bulbourethral glands, just below the prostate, assist with neutralizing acidic urine. Allowing the semen to become alkaline assists with overcoming the vagina’s naturally acidic environment. The urethra is the longest duct and is in the penis. The penis is an external genital organ; the most distal portion is the glans penis. The glans penis might be covered by prepuce unless it was removed during circumcision (Scanlon & Sanders, 2019). Figure 2Male Reproductive Organs  (Wumingbai, 2021) Pathophysiology HPV can be transmitted via skin contact during vaginal, anal, and oral sexual intercourse (Hoffman & Sullivan, 2020). The virus comprises a double-stranded deoxyribonucleic acid (DNA) that promotes lesions on the squamous epithelium. These lesions are visible condyloma acuminata (i.e., genital warts). The DNA replicates, causing the basal membrane to become occupied by the virus. In the early stages, low-grade intraepithelial lesions develop. These lesions are known and graded according to their neoplasia; thus, in its early stage, a low-grade intraepithelial lesion is known as cervical intraepithelial neoplasia (CINI), which envelopes one-third of the epithelium. CIN II covers two-thirds of the epithelium, and CIN III covers the entire epithelium (see Figure 3; Hoffman & Sullivan, 2020). CIN I is low risk, whereas CIN II and CIN III demonstrate higher risk.
(Wumingbai, 2021) Pathophysiology HPV can be transmitted via skin contact during vaginal, anal, and oral sexual intercourse (Hoffman & Sullivan, 2020). The virus comprises a double-stranded deoxyribonucleic acid (DNA) that promotes lesions on the squamous epithelium. These lesions are visible condyloma acuminata (i.e., genital warts). The DNA replicates, causing the basal membrane to become occupied by the virus. In the early stages, low-grade intraepithelial lesions develop. These lesions are known and graded according to their neoplasia; thus, in its early stage, a low-grade intraepithelial lesion is known as cervical intraepithelial neoplasia (CINI), which envelopes one-third of the epithelium. CIN II covers two-thirds of the epithelium, and CIN III covers the entire epithelium (see Figure 3; Hoffman & Sullivan, 2020). CIN I is low risk, whereas CIN II and CIN III demonstrate higher risk. 