About this course:
The purpose of this two-part learning series is to provide an overview of oncology nursing, outlining the core aspects of cancer diagnosis, staging, and treatment. It describes oncology nurses’ roles, responsibilities, and professional performance. Part one discusses the pathophysiology of cancer, early detection, prevention, and unique care considerations related to surgical and radiation oncology. Part two builds on these concepts, focusing on systemic therapies, side effect management, patient education, and oncologic emergencies.
Course preview
Oncology Nursing
The purpose of this two-part learning series is to provide an overview of oncology nursing, outlining the core aspects of cancer diagnosis, staging, and treatment. It describes oncology nurses’ roles, responsibilities, and professional performance. Part one discusses the pathophysiology of cancer, early detection, prevention, and unique care considerations related to surgical and radiation oncology. Part two builds on these concepts, focusing on systemic therapies, side effect management, patient education, and oncologic emergencies.
At the end of this module, learners will be able to:
- explain the pathophysiology of cancer and the basis for cancer diagnosis and staging
- describe the nurse's role and scope of practice in the care of oncology patients
- identify the nurse's role in the early detection and prevention of cancer
- discuss the types of surgical interventions for cancer and the nurse's role in caring for surgical oncology patients
- review the basic principles of radiation therapy, radiation safety, and nursing interventions for skin care associated with radiation therapy
- discuss the role of antineoplastic therapy in cancer treatment, common side effects, the basic principles of safe drug handling and administration, and nursing implications
- review the classes of targeted therapies and immunotherapies, side effects, and the nursing implications of each treatment
- identify the signs of hypersensitivity reactions and cytokine release syndrome, as well as appropriate nursing interventions
- recognize the signs and symptoms of the most common oncologic emergencies and discuss evidence-based interventions
- describe a nurse’s role in patient advocacy and end-of-life care
Part One: Surgical and Radiation Oncology
Cancer is a group of malignant diseases defined by uncontrolled cell growth, the ability to invade nearby tissues and lymph nodes, and the potential to spread throughout the body. Over the last several decades, scientific research has deepened our understanding of how cancer develops, grows, and spreads, shaping the way we define and treat it. Breakthroughs in treatment and drug development have successfully contributed to improved quality of life and better outcomes. However, cancer care has become more complex, placing oncology nurses at the heart of patient support and treatment. Nurses must stay up to date with the latest advancements in cancer biology, treatment strategies, symptom management, and the long-term needs of cancer survivors. Oncology nurses fill diverse and specialized roles throughout the cancer journey—from prevention and early detection to treatment and survivorship. As scientific discoveries continue to drive progress, their mission remains the same: delivering safe, timely, effective, and evidence-based care to improve patients’ lives (Maloney-Newton et al., 2023; Nettina & Nelson-Tuttle, 2024; Olsen et al., 2023; Siegel et al., 2024).
Incidence, Prevalence, and Survival Statistics
According to the American Cancer Society (ACS, 2025b), more than two million new cancers will be diagnosed in the United States in 2025, with approximately 618,120 cancer-related deaths. These numbers translate to 5,590 new cases and 1,690 deaths each day. In 2025, the cancer incidence rate for those assigned female at birth aged 50 to 64 will be approximately 832.5 cases per 100,000 and 830.6 per 100,000 for those assigned male at birth in the same age category. Female patients younger than age 50 have an 82% greater incidence rate than their male counterparts (141.1 vs 77.4 per 100,000). The most common cancers in those assigned female at birth include breast, lung, colorectal, and uterine. For those assigned male at birth, the most common cancers include prostate, lung, colorectal, bladder, and melanoma. Cancer is the second most common cause of death in the United States, exceeded only by heart disease. The age-standardized mortality rate is higher among those assigned male (180 per 100,000) than female (160 per 100,000) (ACS, 2022b, 2025b; National Cancer Institute [NCI], 2024b).
Regarding race and ethnicity, cancer mortality rates are highest among Indigenous Americans, with death rates from kidney, liver, stomach, and cervical cancers being two to three times greater than those in White individuals. Similarly, Black individuals experience twice the mortality rate of White individuals for prostate and stomach cancers and have the highest death rates from breast and uterine cancers, with uterine cancer mortality being two to three times higher than that of other racial groups. The overall risk of dying from cancer has declined by about one-third since 1991, dropping by 2% per year since 2015 compared to 1% per year in the 1990s. These improvements are attributed to reduced tobacco use and increased cancer prevention, detection, and screening, leading to earlier diagnoses and treatment (ACS, 2022b, 2025b; NCI, 2024b).
Cancer Disparities
Although cancer mortality is declining in the United States, certain groups continue to be at a heightened risk of developing and dying from the disease. While cancer affects all populations, racial/ethnic, socioeconomic, and geographic disparities lead to a disproportionately increased burden in some groups. These disparities are primarily attributed to structural racism and inequalities in wealth that serve as barriers to accessing high-quality health care. Regardless of race or ethnicity, cancer risk and mortality rise as socioeconomic status declines. Survival rates are lower for those identifying as African American or Black than those who identify as White across nearly all cancer types. For example, Black patients have a 41% higher risk of death from breast cancer than White patients, even though the incidence is about the same. In rectal cancer, only 41% of Black patients with stage I disease undergo proctectomy or proctocolectomy (cancer-curing surgery), compared to 66% of White patients (ACS, 2022a, 2025b; Miller et al., 2022; NCI, 2025a).
Similar gaps occur in the surgical treatment of non-small cell lung cancer, with 49% of Black patients with stages I and II and 16% with stage III receiving surgery, compared to 55% and 22% of White patients, respectively. These treatment disparities are further compounded by the fact that Black patients are less likely to be diagnosed with stage I disease than White patients for most cancers, with some of the largest disparities for breast (53% vs. 68%) and uterine (59% vs. 73%) cancers. Indigenous Americans have the highest rate of liver cancer, with a risk that is more than twice that of the White population. Black patients have the highest prostate cancer mortality among all ethnic groups and are twice as likely to die from the disease as White patients. Hispanic/Latino and Black patients have the highest cervical cancer rates and mortality. Research suggests differences in genetics, tumor biology, and immune environment of certain cancers (e.g., triple-negative breast, colorectal, and prostate cancer) in African Americans compared to individuals from other racial/ethnic groups. These biological differences may contribute to disparities in cancer incidence, aggressiveness, and response to treatment, but disparities are exacerbated by a lack of diversity in clinical trial participation, as the findings may not be applicable across populations. Furthermore, the COVID-19 pandemic limited access to cancer prevention, early detection, and treatment services. These delays in care have exacerbated existing cancer disparities due to the unequal burden the pandemic exerted on all communities of color (ACS, 2022a, 2025b; Miller et al., 2022; NCI, 2025a).
...purchase below to continue the course
Healthy cells follow an orderly process of growth, division, and programmed cell death (apoptosis) to maintain balance in the body. In contrast, cancer cells exhibit uncontrolled proliferation due to genetic mutations that disrupt normal regulatory mechanisms. Unlike healthy cells, which stop dividing when space or nutrients are limited, cancer cells continue to grow unregulated. As cancerous cells collect in an area, they form a malignant (cancerous) tumor. Key characteristics of cancer cells include the following (Maloney-Newton et al., 2023).
- Dysplasia and hyperplasia: Disorganized growth (dysplasia) and increased cell size and number (hyperplasia) lead to abnormal tissue structures.
- Evasion of apoptosis: Cancer cells bypass programmed cell death, allowing them to persist and accumulate.
- Angiogenesis: The ability to stimulate the formation of new blood vessels to supply the tumor with oxygen and nutrients.
- Immune system evasion: Cancer cells can disguise themselves to avoid detection and destruction by the immune system.
- Metastatic potential: Cancer cells can migrate through the bloodstream or lymphatic system to establish tumors in distant organs.
The Genetic Basis of Cancer
Cancer is fundamentally a genetic disease driven by mutations in specific genes that regulate cell growth and division. Healthy genes comprise deoxyribonucleic acid (DNA) sequences that contain information necessary for proper functioning. Three primary gene types are implicated in cancer development (Maloney-Newton et al., 2023; NCI, 2021).
- Proto-oncogenes: These genes normally promote cell growth and division. When mutated, they become oncogenes, driving uncontrolled proliferation.
- Tumor-suppressor genes: These genes regulate cell division and prevent tumor formation. Mutations in these genes remove growth restraints, leading to unrestricted cellular division.
- DNA repair genes: Responsible for correcting DNA damage, mutations in these genes lead to the accumulation and replication of genetic errors that contribute to cancer development.
Hereditary Versus Sporadic Cancer
While all cancers involve genetic mutations, not all cancers are hereditary. Hereditary cancer arises when individuals inherit a mutation in a cancer-related gene, increasing their risk of developing cancer over their lifetime. Inherited germline mutations are present in all cells of the body and are passed from parent to child. Breast cancer genes 1 (BRCA1) and 2 (BRCA2) are among the most common hereditary cancer mutations, significantly increasing the risk of breast, ovarian, prostate, and pancreatic cancers. Everyone is born with BRCA1 and BRCA2 genes. In their physiologic form, these genes prevent cancer by regulating the growth and division of specific cell types. However, harmful changes in the BRCA1/BRCA2 genes lead to a loss of function. BRCA1/BRCA2 mutations account for up to 10% of cases of breast cancer. Patients with BRCA mutations face up to a 50% lifetime risk of breast cancer, compared to 7% in the general population. Similarly, the risk of ovarian cancer is nearly 30% compared to less than 1% in the general population. Patients assigned male at birth are also affected by BRCA1/BRCA2 mutations, as they are eight times more likely to be diagnosed with breast cancer than those without a BRCA mutation. Mutated BRCA1/BRCA2 genes are inherited in an autosomal-dominant pattern and can be passed from either parent. Only one copy of the mutated gene in each cell is sufficient to increase the risk of developing cancer. A person with a BRCA1 or BRCA2 mutation has a 50% chance of passing it on to each child (Centers for Disease Control and Prevention [CDC], 2024c; NCI, 2024a).
Understanding Metastasis
Metastasis is the process by which cancer spreads beyond its original site to distant organs, significantly impacting treatment options and patient prognosis. Metastases occur when cancer cells detach from the primary tumor, invade local tissues, and travel through the bloodstream or lymphatic system. The most common sites of metastatic spread include the liver, lungs, bone, and central nervous system (CNS); however, each cancer type has its own unique patterns of spread. Since metastatic cancer retains the characteristics of the primary tumor, oncology nurses should educate patients that metastasis does not mean they have developed a new cancer type. Clear patient education can help alleviate misconceptions and improve adherence to treatment plans (Maloney-Newton et al., 2023; Nettina & Nelson-Tuttle, 2024; Olsen et al., 2023).
The Evolving Role of Oncology Nurses
As cancer treatment continues to advance, the role of oncology nurses has expanded to meet the growing complexity of patient needs. Oncology nurses are essential in direct patient care, symptom management, palliative and hospice care, and survivorship support. Beyond bedside care, they also serve as coordinators, patient navigators, educators, and leaders in cancer survivorship. The broad scope of oncology nursing includes specialized roles in critical care settings such as intensive care units (ICUs) and transplant care, as well as outpatient infusion centers, radiation therapy departments, and community-based cancer prevention and early detection programs. Oncology nurses practice in diverse environments, including academic and community hospitals, private clinics, radiation treatment facilities, and home health care settings. Over the past decade, there has been a notable shift in cancer treatment delivery from inpatient to outpatient settings. Inpatient care is now primarily reserved for surgical interventions, higher acuity cases, oncologic emergencies, and patients requiring advanced palliative care support. This transition has reinforced the importance of collaboration between nurses and physician/provider teams, ensuring comprehensive, efficient, and safe patient care. Oncology nurses are integral members of the interdisciplinary team, facilitating seamless communication and coordination to optimize treatment outcomes (Olsen et al., 2023; Siegel et al., 2024).
Key Responsibilities of Oncology Nurses
Oncology nurses manage complex patient needs, including symptoms of disease and anticipated side effects of various therapies. A deep understanding of cancer biology, symptomatology, and treatment modalities is essential for effectively educating patients and their families. Many patients struggle to process and retain medical information following a cancer diagnosis, often experiencing confusion, anxiety, and emotional distress. Oncology nurses play a crucial role in answering questions, addressing misconceptions, and advocating for their patients by communicating their needs and concerns to other healthcare providers (Greer et al., 2020; Nettina & Nelson-Tuttle, 2024; Olsen et al., 2023).
Cancer diagnoses can have profound physical, psychological, and social effects on both patients and their families. Oncology nurses assess patients' mental health histories, as preexisting conditions such as anxiety and depression can increase their risk of emotional distress throughout their cancer journey. Ongoing psychosocial assessments help nurses identify signs of emotional strain, maladaptive coping, or psychological challenges, prompting timely referrals to specialists, including dietitians, social workers, physical therapists, mental health counselors, and support groups. Ensuring a patient’s emotional well-being is just as critical as understanding their disease and treatment plan. As frontline providers in cancer care, oncology nurses must develop strong triage skills to identify and address oncologic emergencies promptly. Given the increasing specialization of cancer treatment, nurses have unique opportunities to cultivate expertise in specific disease areas such as breast cancer, gastrointestinal (GI) cancers, hematologic malignancies, or clinical trials. Due to the variability in practice settings and patient populations, defining the full scope of oncology nursing roles is challenging. Many oncology nurses describe their work as deeply meaningful and rewarding, influenced by their clinical environments, cultural contexts, and patient experiences (Greer et al., 2020; Nettina & Nelson-Tuttle, 2024; Olsen et al., 2023; Siegel et al., 2024).
Oncology Nursing Competencies
The Oncology Nursing Society (ONS) has established generalist oncology nurse competencies to outline the foundational knowledge and skills required for safe and effective practice. These competencies serve as a framework for ensuring high-quality patient care, supporting the professional growth of oncology nurses, and guiding career development. The ONS Generalist Oncology Nurse Competencies (2024) specifically aid novice oncology nurses in transitioning to entry-level practice, focusing on teamwork, professional development, evidence-based care, financial toxicity management, and quality metrics. In collaboration with the American Society of Clinical Oncology (ASCO), ONS has also developed safety standards for administering antineoplastic agents. The ASCO-ONS Antineoplastic Therapy Administration Safety Standards (Siegel et al., 2024) address critical aspects of oncology practice, including nursing benchmarks for ensuring patient safety during antineoplastic and immunotherapy administration. These guidelines apply across all oncology care settings and are considered the gold standard for safe practice. Additionally, state laws and the Nursing Practice Act define and regulate the scope of oncology nursing practice (Olsen et al., 2023; ONS, 2024; Siegel et al., 2024).
A Nurse's Role in the Early Detection and Prevention of Cancer
Risk and Protective Factors
While the definitive cause of cancer is not entirely understood, and many people develop the disease without any identifiable causes, numerous factors are known to increase the risk. Some theories postulate that cancer may occasionally occur from the spontaneous transformation of cellular processes and DNA alterations, but most theories blame carcinogens. Carcinogens are substances, radiation, or exposures that damage the cellular genetic material throughout a person's lifetime, resulting in cancer formation. Examples of carcinogens include tobacco, tanning beds, diesel exhaust, and ultraviolet (UV) radiation. Risk factors are categorized as modifiable and or non-modifiable based on the individual's ability to alter the risk. Age is the clearest risk factor for cancer, as cancer incidence rises alongside age. Other non-modifiable risk factors include family history, genetics, and most chemical and radiation exposures. Modifiable risk factors include diets high in fat, a body mass index (BMI) over 30 kg/m2, sedentary lifestyles, tobacco use, excessive alcohol intake, and sunlight exposure. Altering modifiable factors can serve as a protective factor to lower a person's risk of cancer, such as maintaining a healthy weight, smoking cessation, engaging in regular physical activity, and practicing sun safety to limit harmful UV exposure. Research demonstrates that additional cancer risk factors include chronic inflammation and hormones. In addition, some infections and viruses are associated with an increased risk of cancer. Hepatitis B and Hepatitis C are linked to hepatocellular cancer, human papillomavirus (HPV) can cause cervical cancer, and Epstein-Barr virus is associated with Hodgkin lymphoma. People in low- and middle-income countries are at increased risk for developing cancer through chronic infections. Primary and secondary prevention strategies decrease the morbidity and mortality of cancer (ACS, 2023c; Maloney-Newton et al., 2023; Olsen et al., 2023).
Primary Prevention
Primary prevention focuses on actions to inhibit cancer from developing. Many cancer deaths are preventable, so primary prevention involves minimizing harmful exposures and reducing or omitting unhealthy lifestyle behaviors. Cancer prevention offers the most cost-effective long-term strategy for cancer control worldwide. According to the ACS (2025a), at least 40% of newly diagnosed cancers in the United States are potentially avoidable, including 19% caused by cigarettes, 8% caused by excess body weight, and 5% caused by alcohol. Tobacco is the leading cause of preventable disease and death in the United States. Cigarette smoking kills more than 480,000 Americans annually (CDC, 2024a). Cigarette smoking causes about one of every five deaths in the United States each year, and the life expectancy for smokers is at least 10 years shorter than for nonsmokers. Tobacco use causes cancers throughout the body, including lung, bladder, head and neck, kidney, cervix, liver, and pancreas cancers. Skin cancers are primarily due to excessive sun exposure without proper protection and indoor tanning beds. Prevention strategies include sunscreen, lightweight clothing or hats to shield from direct exposure, reducing sunlight exposure during peak hours of the day when the UV rays are the strongest, and avoiding tanning beds altogether (ACS, 2025a; CDC, 2024a; Nettina & Nelson-Tuttle, 2024; Olsen et al., 2023).
Cancers related to hepatitis and HPV can be prevented through behavioral and lifestyle changes, such as practicing safe sex and getting vaccinated. According to the CDC (2024b), 85% of people will get an HPV infection in their lifetime, and approximately 13 million people become infected each year. HPV can cause cancers of the cervix, vagina, vulva, oropharynx (throat, tonsils, tongue), penis, and anus. Infections with high-risk HPV subtypes that cause most HPV-related cancers have dropped by nearly 90% due to the HPV vaccination. Cervical cancers linked to HPV have declined by 40% among those vaccinated. The HPV vaccine is highly effective and is recommended for all adolescents aged 11 to 12 years. Thus far, more than 135 million doses have been distributed across the United States (ACS, 2023c; CDC, 2024b).
Secondary Cancer Prevention
Cancer burden can also be reduced through early detection and treatment. Secondary cancer prevention involves screening to identify cancer before symptoms develop while the disease is treatable or potentially curable. The goal of screening is to reduce morbidity and increase survival. Screening helps detect patients in high-risk groups who require increased surveillance compared to the general population. Alternatively, patients in high-risk groups may undergo invasive interventions such as prophylactic mastectomy. Preventive surgeries can diminish the lifetime risk of breast or ovarian cancer in certain individuals belonging to high-risk categories. Screening colonoscopies promote the early detection and removal of precancerous polyps before they become invasive cancer. Other examples of standard screening tests include sigmoidoscopy, fecal occult blood testing (FOBT), digital rectal examination (DRE), mammography, Papanicolaou testing (Pap smear), and prostate-specific antigen (PSA) testing. In addition, institutions increasingly offer low-dose spiral computed tomography (CT) scans to detect curable stage I lung cancer in patients who meet the designated criteria (e.g., age and tobacco use history). The decision to perform routine screening tests is based on national guidelines, which determine whether the tests are cost effective and adequate to detect a potentially curable cancer in an otherwise asymptomatic person. In the formation of screening guidelines, several factors are considered, such as age, sex, family history, ethnicity/race, and iatrogenic factors (e.g., prior radiation therapy or diethylstilbestrol [DES] exposure). While screening is an effective secondary cancer prevention strategy, not all cancers can be detected early. For example, there are no effective screening tests for ovarian and pancreatic cancer, so most are diagnosed at advanced stages when symptoms present. Screening tests are imperfect and may not identify cancer in all patients. Each test has a risk-benefit ratio that must be considered and discussed with the patient. Oncology nurses play a vital role in assessing individual risk factors, monitoring for symptoms, and educating patients on the importance of lifestyle modifications and the benefits and limitations of screening. Fundamental training and teaching points are outlined in Table 1, as adapted from the ACS Guideline for Diet and Physical Activity for Cancer Prevention and the Guidelines for the Early Detection of Cancer (ACS, 2020a, 2023a; Maloney-Newton et al., 2023).
Table 1
Key Teaching Points: Cancer Prevention and Early Detection
|
|
|
|
|
|
|
|
|
|
|
*Average risk refers to guidelines for the general population; those at higher risk (e.g., with a strong family history, concerning symptoms, or other risk factors) should consult their physician for individualized screening guidelines.
(ACS, 2020a, 2023a, 2023b)
Refer to the Cancer Prevention and Early Detection NursingCE course for more detailed information on cancer screening.
The Relationship Between BMI and Cancer
Obesity is a risk factor for cancer development, progression, and recurrence. According to the CDC (2025), obesity is linked to at least 13 types of cancer, which comprise 40% of all cancers diagnosed in the United States each year (refer to Table 2). Measured as a BMI of 30 kg/m2 or higher, obesity is defined as excess fat accumulation in relation to height. Despite its negative impact on health, obesity rates are still expected to rise substantially over the next several decades. Nearly 20% of all cancers diagnosed in the United States are related to excess body weight, physical inactivity, and poor nutrition; aside from tobacco use, these are the three most crucial cancer risk factors that can be modified (ACS, 2020b).
Table 2
Thirteen Cancers Linked to Elevated BMI
1. Uterine cancer |
2. Adenocarcinoma of the esophagus |
3. Breast cancer (in postmenopausal patients) |
4. Colon and rectal cancers |
5. Gallbladder cancer |
6. Gastric cancer (primarily stomach cancer) |
7. Kidney cancer |
8. Pancreatic cancer |
9. Ovarian cancer |
10. Thyroid cancer |
11. Multiple myeloma (MM) |
12. Malignant meningioma |
13. Liver cancer |
(ACS, 2020b)
A primary example of the relationship between cancer and BMI is endometrial (uterine) cancer. Endometrial cancer is the most common gynecologic malignancy, with an estimated 69,120 new cases and 13,860 deaths in the United States in 2025 (ACS, 2025c). Historically recognized as a disease of postmenopausal patients, endometrial cancer has become more prevalent in younger, premenopausal patients. This increased incidence is attributed to the global obesity epidemic and associated metabolic disorders. Elevated BMI is cited as a causative factor for approximately 80% of endometrial cancers worldwide. Patients with a BMI over 25 are up to four times as likely to develop endometrial cancer than patients with a BMI under 25; patients with a BMI of 40 or more are nearly seven times more likely to develop cancer. The relationship is primarily driven by an overproduction of estrogen carried in excess adipose tissue. Additional factors such as insulin resistance, increased bioavailability of steroid hormones, and inflammation also contribute to cancer development. Obesity leads to poorer long-term health outcomes and is also found to impact the treatment course negatively. Despite a clear understanding of the relationship between BMI and endometrial cancer, mortality continues to rise. Furthermore, patients with endometrial cancer and a BMI over 30 are more likely to die from other obesity-related diseases than endometrial cancer. These conditions are some of the leading causes of preventable death, including heart disease, stroke, and type 2 diabetes (Kokts-Porietis et al., 2021; Sagnic, 2021; Smrz et al., 2021).
Cancer Signs and Symptoms
The presenting signs and symptoms of cancer can vary widely depending on the type of cancer. A patient may be asymptomatic at diagnosis, or signs may be vague and nonspecific, prolonging the time from symptom presentation until diagnosis. Some cancers, such as leukemia and lymphoma, often exhibit a cluster of manifestations that suggest the diagnosis, such as weight loss, night sweats, lymphadenopathy (enlarged lymph nodes), and excessive fatigue. The differential diagnosis for cancer is extensive, with many symptoms overlapping with those of common benign illnesses, such as viruses or tick-borne diseases. However, certain warning signs may indicate an underlying malignant process and should be evaluated. Ominous symptoms include unintentional weight loss, rectal bleeding, vaginal bleeding in a postmenopausal patient, a lump in the breast, abdominal bloating or distension that does not resolve, or unexplained pain. Other signs may include hoarseness, productive coughing with hemoptysis (blood-streaked sputum or coughing up blood clots), changes in bladder or bowel habits, and unusual bleeding or bruising. Cancer may present in various ways, so a comprehensive medical history and physical assessment are critical (ACS, 2020d; Maloney-Newton et al., 2023).
Overview of Cancer Diagnosis and Staging
Patients undergo a series of tests to establish the origin and extent of a cancer diagnosis. A tissue sample, bone marrow aspiration, or cytology specimen is necessary to determine the cell type, grade, and unique features. A tissue sample (or biopsy) is most commonly performed when evaluating solid tumors such as breast or pancreatic cancers. Patients may undergo a fine-needle aspiration (FNA), a procedure in which cancer cells are aspirated from the tumor using a needle and syringe. However, an FNA cannot always distinguish invasive from noninvasive cancer, and negative results do not entirely rule out malignancy; thus, a core-needle biopsy is commonly recommended. This technique uses a large-bore needle placed directly into the tumor, usually with ultrasound or CT guidance, to retrieve a small piece of cancer. A core biopsy typically provides enough tissue to diagnose most cancer types and can be performed in outpatient settings. At other times, tumor samples must be retrieved through surgical intervention such as lymph node excision or open biopsies performed under general anesthesia (Longo, 2019; Maloney-Newton et al., 2023).
A bone marrow biopsy is more commonly performed for hematologic malignancies such as leukemia or multiple myeloma. A small sample of the bone marrow is extracted for testing, most commonly from the hip bone. A wide needle is inserted into the hip bone and rotated to remove a sample of the bone and the marrow (i.e., fluid inside the bone). Cytology examines small clusters of cells in body fluids. It differs from tissue sampling in that cytology samples consist of a suspension of cells instead of tissue. Cytology examinations can be performed on various fluid collections such as cerebrospinal fluid (CSF), pleural fluid, or ascites. The fluid is extracted using a long, thin needle. Cytology can also be performed by scraping or brushing cells from the tissue or organ being tested—like the cervix, esophagus, or bronchi—using a small brushing instrument (e.g., Pap smear). The collection procedure varies depending on the location of the specimen. For example, a paracentesis is performed to collect fluid from the abdominal cavity, whereas a thoracentesis extracts cells from the pleura surrounding the lung (Longo, 2019; Maloney-Newton et al., 2023)
Tumor grading, also called histologic grading, measures cancer cells’ appearance compared to healthy cells under a microscope. It also describes how quickly the cells may grow and spread. This information is vital to determine the patient’s prognosis and treatment plan. Tumor grading systems differ for each cancer type but are primarily based on cellular differentiation and ranked from low-grade (Grade 1) to high-grade (Grade 3). Low-grade cancers look similar to healthy tissue, are the least aggressive, and grow and spread slower. High-grade cancers are poorly differentiated or undifferentiated (i.e., the cells do not resemble healthy tissue) and more aggressive. Poorly differentiated cancers typically have a poorer prognosis. Specialized staining is performed to look for specific markers or proteins in a tumor. Certain types of cancer have unique proteins (antibodies) on their cell’s surface, and immunohistochemical (IHC) staining helps identify whether these antibodies are present; this information helps determine the most effective treatment regimen. For instance, IHC testing for breast cancer cells evaluates whether the tumor overexpresses two hormone receptors, estrogen receptor (ER) and progesterone receptor (PR). For tumors that are positive for ER and/or PR, growth is fueled by hormones, and treatment includes hormone-blocking therapies. If these markers are negative, the patient will not benefit from hormonal treatments (Longo, 2019; Maloney-Newton et al., 2023).
Flow cytometry evaluates cells extracted from the bone marrow, lymph nodes, or bloodstream for antibody receptors. It measures the amount of DNA in cancer cells and looks for other biomarkers to guide treatment. Genetic testing is increasingly used in cancer care to identify targetable gene mutations and abnormal chromosomes as components of personalized medicine. Scientific advancements have led to the routine use of gene profiling and cytogenetic testing as foundational components of cancer diagnosis and treatment planning. Fluorescent in situ hybridization (FISH) testing uses fluorescent dyes linked to pieces of DNA that attach only to specific parts of particular chromosomes. Identifying these chromosomal alterations is vital to determine whether targeted drugs might be effective. For example, FISH testing can reveal if there are too many copies (amplification) of the human epidermal growth factor receptor 2 (HER2) gene, which creates a protein found on the surface of all breast calls. Overexpression of HER2 suggests that a tumor is likely to respond to anti–HER2-based targeted agents, such as trastuzumab (Herceptin; Longo, 2019; Maloney-Newton et al., 2023; Olsen et al., 2023).
Radiology imaging such as CT scans, magnetic resonance imaging (MRI), and positron emission tomography (PET) scans are other vital components of the cancer staging and diagnostic workup. Additional radiographic testing may be necessary depending on the type of cancer suspected. Accurate staging provides a collection of data that informs decision-making about treatment and guides treatment outcomes for each type of cancer. The American Joint Committee on Cancer (AJCC) has developed a simple classification system that can be applied to nearly all tumor types, which is called the tumor-node-metastasis (TNM) staging system. The TNM is preferred for most solid tumors and is a numeric assessment of the tumor size (T), presence or absence of regional lymph node involvement (N), and presence or absence of distant metastasis (M). However, there is no single standard evaluation tool for all cancers. Hematologic malignancies are staged differently than solid tumors. For example, Hodgkin and non-Hodgkin lymphomas are staged with the Ann Arbor Classification, whereas MM staging relies on the International Staging System (ISS) and Revised ISS (R-ISS). In general, the components of the staging workup depend on the tumor type, symptoms, pathophysiology, and classic spread pattern of each cancer (Maloney-Newton et al., 2023; Olsen et al., 2023).
Tumor Markers
Tumor markers are substances, or proteins, made in higher quantities by cancer cells than normal cells. In some patients, tumor markers are detected and measured in the bloodstream or urine. They can provide information about the cancer, such as how aggressive it is, whether it is responding to treatment, or if it is growing. However, they are nonspecific metrics and can sometimes increase due to benign causes, such as an acute infection or inflammation. Furthermore, not all cancers produce measurable tumor markers. Some common tumor markers include PSA, carcinoembryonic antigen (CEA), cancer antigen 125 (CA-125), and cancer antigen 27-29 (CA 27-29). Each tumor marker is specific to a type of disease process. For instance, PSA relates to prostate cancer; CEA is related to cancers of the rectum, colon, pancreas, and breast; CA-125 is linked to ovarian cancer; and CA 27-29 is associated with breast cancer (Maloney-Newton et al., 2023; NCI, 2023c).
Treatment Modalities
There are four main goals of cancer therapy: prevention, cure, control, and palliation. While prevention focuses on inhibiting cancer development, cure denotes treatment to eradicate the disease. Control refers to extending the patient's life when a cure is unlikely or impossible by preventing the growth of new cancer cells and reducing the size and impact of an existing disease. Finally, palliation centers on comfort when cure and control of the disease cannot be achieved. Oncology nurses should engage in ongoing discussions with patients regarding their care goals and whether their goals are being achieved. The primary categories of cancer treatment include surgery, radiation, chemotherapy, and biological therapies, as shown in Figure 1 (Maloney-Newton et al., 2023; Nettina & Nelson-Tuttle, 2024).
Figure 1
Cancer Treatment Modalities
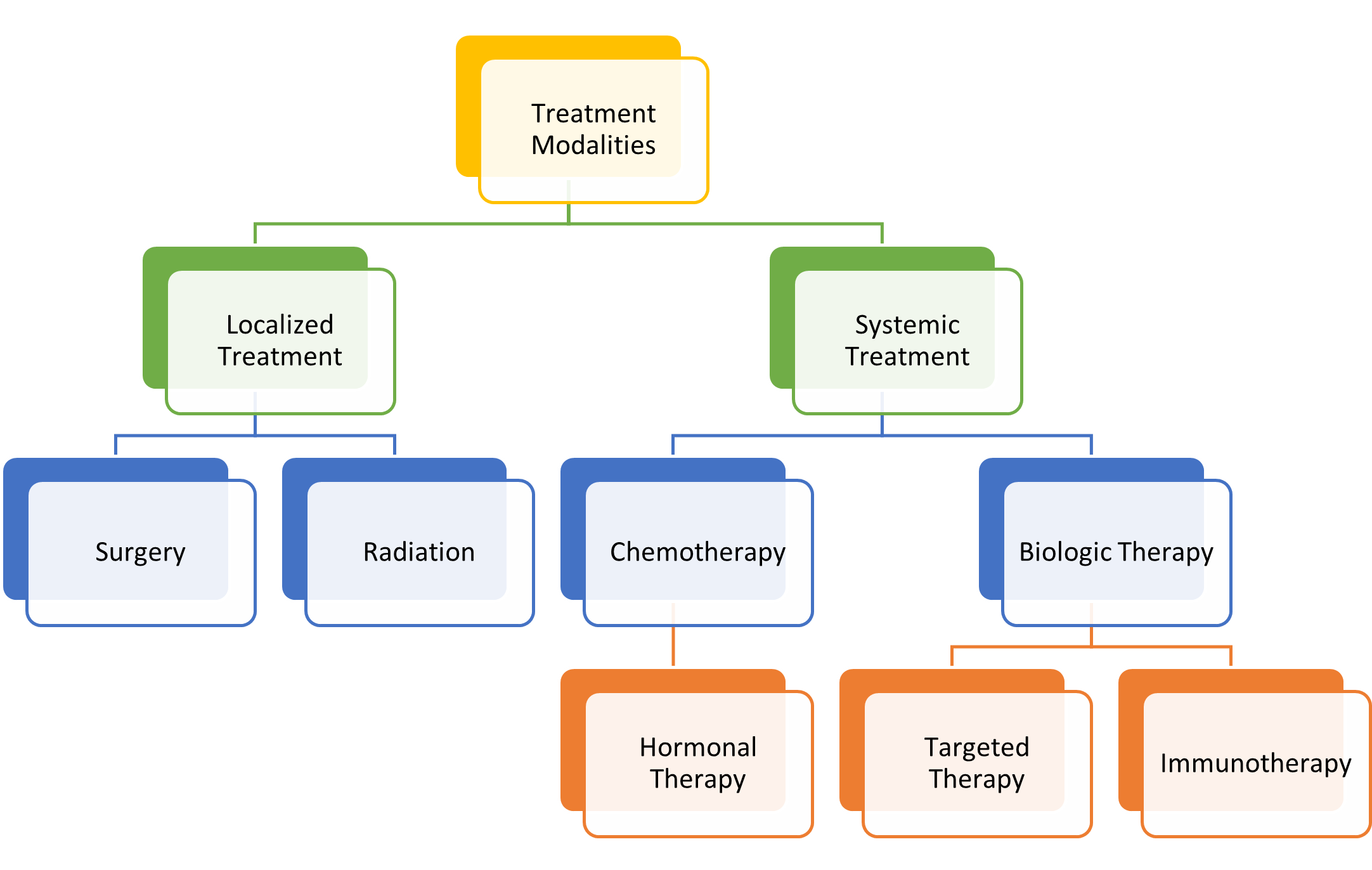
(Olsen et al., 2023)
Patients with cancer often receive a combination of treatment approaches throughout the disease trajectory. Scientific advancements have revolutionized cancer care, leading to innovative fields such as precision medicine, targeted therapies, and immunotherapy. Precision medicine tailors treatment based on the genomic profiling of a patient's tumor, identifying unique genetic mutations. This approach helps determine the most effective therapies with the least toxicity. Targeted therapies block the growth and spread of cancer by inhibiting specific genes, proteins, or blood vessels that are responsible for tumor progression. These treatments have successfully improved patient outcomes by selectively attacking cancer cells while sparing normal tissues. Immunotherapy leverages the body’s immune system to recognize and destroy cancer cells, much like it would respond to infections or foreign invaders. This therapeutic approach includes immune checkpoint inhibitors, Chimeric antigen therapy (CAR) T-cell therapy, and cancer vaccines. Radiation therapy is a localized treatment that has become increasingly precise, reducing damage to surrounding tissues. However, it still presents challenges, including side effects such as fatigue, skin reactions, and tissue fibrosis. Surgery is not indicated for all cancers, but clinical research has helped to better define the parameters and indications for surgical intervention and the ideal time for patients to undergo surgery (Maloney-Newton et al., 2023; Siegel et al., 2024).
The National Comprehensive Cancer Network (NCCN, 2025) is an alliance of leading cancer centers and world-renowned experts devoted to cancer care, research, and education. The NCCN provides evidence-based treatment guidelines according to cancer type, pathology, genetics, staging, inheritance patterns, and several other specific features through rigorous clinical trial research, data compiled across institutions, and annual expert panel reviews. The guidelines are widely utilized in cancer care and guide medical decision-making throughout the disease trajectory (NCCN, 2025).
Surgical Treatment
Surgical treatment remains a cornerstone of cancer management, especially for localized and regionally confined tumors. Depending on the cancer type and stage, surgery may serve a curative, reconstructive, or palliative purpose. Surgery is performed with curative intent when the goal is to remove all or a significant portion of the primary tumor. It may be the only treatment necessary or performed before or after other treatment modalities. There are other times when surgery is used in a palliative setting to reduce and alleviate distressing cancer symptoms. Table 3 outlines the various types of surgical intervention for cancer treatment (Maloney-Newton et al., 2023).
Table 3
Surgical Treatment of Cancer
Preventative/Prophylactic Surgery |
|
Primary Surgery |
|
Cytoreductive (debulking) Surgery |
|
Salvage Surgery |
|
Palliative Surgery |
|
Reconstructive Surgery |
|
(Maloney-Newton et al., 2023)
Surgery may be combined with other treatment modalities, such as preoperative chemotherapy (neoadjuvant therapy), intraoperative chemotherapy, radiation therapy, or postoperative chemotherapy (adjuvant treatment). The type and severity of side effects or surgical complications depend on the specific treatment modality and comorbid conditions. For example, some chemotherapy agents can delay wound healing, whereas others may impact cardiac function, imposing surgical risks and complications. In addition, patients with diabetes or underlying cardiac disease are at increased risk for infection or fluid overload. The nurse's role extends throughout the surgical continuum, from the preoperative to the postoperative period. Nursing responsibilities are broad and may include obtaining pertinent medical history and allergies and verifying surgical consent. In addition, nurses are responsible for presurgical counseling and teaching, such as preparing for surgery, infection control measures, expectations during surgery, and proper skin care (Maloney-Newton et al., 2023; Nettina & Nelson-Tuttle, 2024).
Nurses manage postoperative pain, nausea, and adverse reactions to anesthesia. They perform interventions to decrease the incidence and severity of surgical complications by promoting airway clearance, monitoring surgical sites, and performing aseptic wound care. Postoperative nursing care focuses on pulmonary rehabilitation, promoting coughing, deep breathing, and incentive spirometry to prevent pneumonia and lung infections. Nurses teach splinting of the incision with coughing, sneezing, or other movements to reduce pain and avoid wound dehiscence. They are responsible for ensuring and advocating for adequate pain control and administering pain medications. Nurses reposition patients in bed every two hours to prevent pressure injuries and skin breakdown. They monitor closely for signs and symptoms of infection, such as fevers, redness, drainage, swelling, or warmth at the incision site, and promptly report suspicious changes or concerns to the rest of the care team (Maloney-Newton et al., 2023; Nettina & Nelson-Tuttle, 2024).
Patients with advanced cancer are hypercoagulable or at increased risk for venous thromboembolism (VTE). Hypercoagulability associated with cancer is multifactorial. However, research suggests tissue damage triggers the coagulation cascade, causing abnormalities within the fibrinolytic pathways, platelet function, and increased inflammatory markers in blood circulation. Surgery compounds this preexisting risk, increasing the propensity to develop a blood clot in the postoperative period (Longo, 2019). Nurses must ensure patients receive appropriate VTE prophylaxis after surgery to mitigate their risk, such as sequential compression devices (SCDs), compression stockings, and anticoagulation therapy. Early mobility and adequate hydration are encouraged to reduce VTE risk postoperatively. Nurses are responsible for monitoring for signs of a blood clot, such as unilateral leg swelling, calf or knee discomfort, redness, warmth, shortness of breath, hypoxemia, or tachycardia. These symptoms must be promptly reported to the provider, as they can quickly progress and lead to fatal outcomes. Surgical nurses often manage many invasive tubes, drains, and catheters in the postoperative period. Cautious assessment and monitoring of each site are crucial to identify acute signs of infection or other complications. Surgical complications may include infection, bleeding, thrombosis, bowel obstruction or ileus, acute respiratory distress syndrome, aspiration pneumonia, and cardiac dysfunction (Longo, 2019; Maloney-Newton et al., 2023).
Successful recovery extends beyond the hospital stay, requiring thorough discharge planning and patient education. Surgical oncology nurses prepare patients for discharge, ensure all needs are addressed, teach patients and caregivers how to care for surgical wounds, and coordinate follow-up visits. Patients and their caregivers must understand how to administer prescribed medications, such as analgesics and antibiotics, dosing schedules, and expected side effects. They must be educated on wound care techniques and how to monitor for signs of infection. Nutrition is critical postoperatively, as it has implications for wound healing, infection control, and overall prognosis. Protein and caloric malnutrition are common complications in patients who have undergone prior cancer therapy or are immunocompromised. Inadequate nutrition can increase the risk of wound dehiscence, sepsis, and prolonged hospitalizations. In addition, the management of any temporary or permanent lines, catheters, or body alterations, such as ostomy care, must be reviewed. Communication with home health agencies is imperative to convey postoperative instructions and ensure a seamless transition without disruption in care. Patients may experience a significant emotional disruption when undergoing surgery, and some patients first learn of their cancer diagnosis during their postoperative recovery. Oncology nurses are uniquely positioned to defuse distressing events, provide emotional support, and connect patients with available support resources (Maloney-Newton et al., 2023).
Radiation Therapy
Radiation therapy, also known as radiotherapy, has significantly advanced since its inception with Cobalt therapy in the 1950s. Modern radiation treatment planning incorporates sophisticated computer algorithms to optimize dose delivery. Radiation therapy plays a pivotal role in cancer treatment, with more than half of cancer patients receiving it at some point during their care. It delivers high doses of targeted radiation to tumors while minimizing exposure to surrounding healthy tissue. This approach induces biological changes in cellular DNA, leading to cell death over time. While both healthy and cancerous cells are affected, healthy cells have a greater ability to repair themselves. Rapidly dividing cancers, such as lymphomas and squamous cell carcinomas of the head and neck, tend to be more sensitive to the effects of radiation than cancers that divide more slowly, such as sarcoma. The primary goals of radiation therapy include tumor eradication, symptom palliation, quality of life improvement, and survival prolongation while minimizing adverse effects (Maani & Maani, 2022; Maloney-Newton et al., 2023; McQuestion et al., 2021).
Radiation therapy may serve as a standalone treatment, or it may be combined with other modalities such as surgery and chemotherapy. Chemotherapy can act as a radiosensitizer, enhancing cancer cell susceptibility to radiation effects. Patients who undergo surgery may need adjuvant radiation therapy (i.e., radiation administered after surgery) to eliminate residual cancer cells. Palliative radiation is frequently used to alleviate pain and other distressing symptoms. For example, in patients with metastatic disease to the spine, palliative radiation can reduce tumor-induced nerve compression, which can relieve neuropathy, pain, and neurological symptoms. Similarly, in brain tumors, radiation therapy can help manage symptoms such as headaches, nausea, vomiting, or visual disturbances. Radiation therapy is typically fractionated, meaning it is administered in multiple small doses over weeks rather than a single large dose. Dose fractionation allows healthy cells time to recover between treatments while maintaining tumor control. The total number of radiation sessions depends on tumor characteristics, location, the patient’s health status, and treatment goals. With conventional fractionation, patients usually receive daily weekday treatments over several weeks, taking weekend breaks. Some cancers are treated with hyper-fractionation dosing, which divides the same total dose into more fractions by giving smaller doses per fraction via twice-a-day treatments (usually at least 6 hours apart). Radiation can be delivered externally or internally, and some patients may receive both modalities. Table 4 compares external and internal radiation therapies (Maani & Maani, 2022; Maloney-Newton et al., 2023; McQuestion et al., 2021; Nettina & Nelson-Tuttle, 2024).
Prior to initiating radiation therapy, patients undergo a simulation procedure using CT or MRI imaging to map the tumor and surrounding structures precisely. Small, pinhead-sized tattoos or markers are often applied to the skin to ensure accurate targeting of radiation beams. When the simulation plan is completed, the radiation oncologist meticulously reviews all the data, and multiple validations of the treatment plan are performed to optimize therapeutic efficacy while minimizing toxicity. While some forms of radiation therapy are administered in an inpatient setting, most are delivered on an outpatient basis in hospital-based radiation departments or free-standing radiation centers. Individual treatment sessions typically last less than 15 minutes, allowing most patients to maintain their daily routines (Maani & Maani, 2022; McQuestion et al., 2021; Nettina & Nelson-Tuttle, 2024).
Table 4
Comparison of External and Internal Radiation Therapy
External Radiation | Description | Key Features |
External beam radiation therapy (EBRT) | Radiation is delivered from an external source to the tumor site. |
|
Intensity-modulated radiation therapy (IMRT) | Varying doses of radiation are delivered to different areas of the tumor. |
|
Image-guided radiation therapy (IGRT) | Imaging scans (CT, MRI, PET) are used during treatment to track tumor changes. |
|
Stereotactic radiosurgery (SRS) & stereotactic body radiation therapy (SBRT) | Highly focused radiation is delivered in fewer sessions, while preserving surrounding healthy tissue. |
|
Tomotherapy | CT imaging is combined with IMRT for highly targeted therapy. |
|
Intraoperative radiation therapy (IORT) | A concentrated radiation dose is delivered directly to the tumor bed during surgery. |
|
Internal Radiation | Description | Key Features |
Low-dose rate (LDR) brachytherapy | Radioactive implants stay in place for hours to days. |
|
High-dose rate (HDR) brachytherapy | Higher doses of radiation are delivered internally over short periods. |
|
Intracavitary brachytherapy | Radioactive material is placed inside a body cavity. |
|
Interstitial brachytherapy | Radioactive sources are implanted directly into the tumor. |
|
Systemic radiation therapy | Radioactive substances are administered orally or via injection. |
|
(Maani & Maani, 2022; Maloney-Newton et al., 2023; McQuestion et al., 2021)
LDR brachytherapy typically requires hospitalization and isolation to prevent exposure to others. Some LDR treatments require the patient to be confined to a bed to avoid the dislodgment of the radioactive applicator for 1 to 3 days. LDR brachytherapy is used for prostate, oral, and cervical cancer. By contrast, HDR brachytherapy is generally performed on an outpatient basis. Each treatment lasts a few minutes, and hospitalization and bed rest are not indicated. Staff, visitors, and family members are not exposed to radiation. The treatment is delivered in a radiation-shielded room to protect others from exposure, and patients are not considered "radioactive" after treatment. They can safely go about their regular routines and lifestyles without potentially exposing others. HDR brachytherapy is used to treat many cancers, such as lung, breast, and esophageal cancers (Maani & Maani, 2022; Maloney-Newton et al., 2023).
Radiation Safety
Federal and state agencies strictly regulate the use of radioactive substances in health care to ensure safety and minimize radiation exposure. Radiation oncology nurses must undergo extensive training in the critical components of radiation safety. They are required to wear dosimeter badges to monitor their cumulative radiation exposure over time. To protect themselves from exposure, nurses must follow three fundamental principles of radiation safety: time, distance, and shielding. Time refers to minimizing the duration of exposure near the source by efficiently clustering patient care activities. Distance emphasizes maintaining as much physical space as possible between the nurse and the radiation source. Shielding involves the use of protective barriers between the healthcare provider and the radiation source, such as wearing lead aprons or portable shields when proximity to the radiation source is unavoidable. Beyond self-protection, nurses play a critical role in educating patients and their families on radiation safety. Patients receiving internal radiation, such as implanted radioactive seeds or devices, often require specific precautions to prevent unintentional exposure to others. Before discharge, patients and caregivers must be educated on safety guidelines, including limiting close contact with young children and pregnant individuals until radiation levels decline to a safe threshold. Additionally, health care facilities implement policies to protect radiation personnel who are pregnant or planning pregnancy. Pregnant nurses are generally advised to avoid direct patient care involving radiation exposure and should notify their supervisor as soon as pregnancy is confirmed or suspected. By adhering to these safety measures, nurses not only safeguard themselves but also contribute to a safe environment for patients, families, colleagues, and the community (Maani & Maani, 2022; Maloney-Newton et al., 2023; McQuestion et al., 2021; Nettina & Nelson-Tuttle, 2024).
Nursing Implications in Radiation Therapy
The ONS offers an international web-based continuing education course, the ONS/ONCC Radiation Therapy Certificate Course, which oncology nurses practicing in radiation specialties are strongly encouraged to complete. While teaching is a primary responsibility, as safety is of utmost concern for this patient population, nurses also play a vital role in symptom management (ONS, 2025b).
Acute Effects of Radiation
Radiation therapy can cause acute and latent side effects, which vary based on the treatment site and radiation dose. Because radiation is a localized treatment, side effects are primarily site-specific, such as local inflammation and skin reactions (e.g., irritation, skin breakdown, blistering, and burning). However, common generalized side effects include fatigue and anorexia. The acute effects of radiation are usually transient, begin about 2 weeks after starting treatment, and subside within 2 weeks of completing treatment. Radiation affects cells with rapid renewal characteristics that quickly turn over, such as the mucous membranes, GI tract, and bone marrow. Other factors such as age, nutritional status, and prior or concurrent chemotherapy also impact the severity of symptoms. Additional acute and long-term effects may include alopecia, lymphedema, and sexual dysfunction (McQuestion et al., 2021; Nettina & Nelson-Tuttle, 2024; ONS, 2025b; Palmer et al., 2021).
Radiation targeting the GI tract, such as the stomach, colon, or rectum, can cause nausea, vomiting, diarrhea, painful defecation, dehydration, weight loss, and skin breakdown. If the anus is affected, patients may experience fecal incontinence, rectal bleeding, or hemorrhoids. Head and neck radiation can cause numerous complications, such as oral ulceration (mucositis), esophageal ulceration (stomatitis), painful swallowing (dysphagia), and dry mouth (xerostomia). These patients often require a feeding tube to ensure adequate nutrition and prevent cachexia. Nutrition is a core component of treatment and influences patients’ ability to tolerate therapy. Therefore, many physicians recommend percutaneous endoscopic gastrostomy (PEG) tube placement before treatment begins. Radiation to the breast or chest wall can impact the heart and lungs, leading to late effects such as cardiotoxicity (damage to the heart function or muscle) or pulmonary fibrosis (scarring of the lungs). Radiation to the cervix and vagina can lead to vaginal atrophy, inducing symptoms of vaginal dryness, irritation, scarring, and sexual dysfunction. Radiation fields that affect the bladder can cause cystitis (inflammation of the bladder), leading to dysuria (painful urination), hematuria (blood in the urine), urinary incontinence, and loss of pelvic floor muscular strength. Radiation near the spine can cause bone marrow suppression, such as neutropenia, anemia, and thrombocytopenia (Maloney-Newton et al., 2023; McQuestion et al., 2021; ONS, 2025b; Palmer et al., 2021).
Skin Care
Up to 95% of patients receiving radiation therapy will experience some degree of skin reaction. Radiation oncology nurses play a critical role in educating, assessing, and managing radiation dermatitis or radiodermatitis. This condition occurs in response to radiation exposure and is caused by changes to the basal layer of the epidermis and the dermis. Cumulative doses of radiation weaken the skin integrity, depleting stem cells from the basal layer of the epidermis and leading to varying degrees of radiodermatitis. Acute skin reactions generally begin 7 to 14 days after starting treatment, and the first signs include dryness and slight erythema. As treatment continues, symptoms can progress to bright red erythema, a rash, and desquamation. Patients often describe the skin as feeling similar to a sunburn. Several factors heighten the risk of skin reactions, such as poor nutrition, excess fat tissue causing skin folds to be in the radiation field, sun exposure, and the use of topical irritants. Desquamation is the sloughing of the top layer of the skin and involves two stages—dry and moist. The first stage, dry desquamation, is the peeling of the top layer of skin, which becomes increasingly uncomfortable as the underlying nerve endings are exposed to the air. Dry desquamation is most common in intertriginous regions where the skin rubs together, such as beneath the breast or axillae. Moist desquamation refers to the peeling of the skin with serous fluid leakage and is very uncomfortable (Maloney-Newton et al., 2023; McQuestion et al., 2021; Palmer et al., 2021).
Radiodermatitis can impact a patient's quality of life, cause pain, limit activities, and delay treatment. In severe cases, radiodermatitis can cause treatment cessation. Preventing radiation skin reactions is difficult, particularly for patients with conditions such as inflammatory breast cancer, where intense skin reactions are expected. Radiation oncology nurses must understand how to meticulously monitor for, assess, document, grade, and manage radiation dermatitis promptly. Several grading tools are available for evaluating radiodermatitis, and selection can vary across treatment facilities and physician preferences. Some commonly used tools include the Radiation Therapy Oncology Group (RTOG) Acute Radiation Morbidity Scoring Criteria, ONS Radiation Therapy Patient Care Record, and the Radiation-Induced Skin Reaction Assessment Scale. The same scale should be used consistently to ensure accurate and reliable assessment and documentation. The management of radiation dermatitis is complex and varied; currently, no gold standard exists for the prevention or control of radiodermatitis. However, expert opinion and consensus have formulated general guidelines for radiation oncology nurses as outlined in Table 5 (Maloney-Newton et al., 2023; McQuestion et al., 2021; Palmer et al., 2021).
Table 5
Radiation Skin Care Management
Healthcare Provider Precautions |
|
Key Teaching Points |
|
Skin Irritation, Rash, Pruritus |
|
Dry Desquamation |
|
Moist Desquamation |
|
Patient Personal Hygiene |
|
Patient Safety |
|
(Maloney-Newton et al., 2023; McQuestion et al., 2021; ONS, 2025b; Palmer et al., 2021)
Radiation Recall
Radiation recall is an uncommon but severe inflammatory response triggered by certain chemotherapy agents administered during or after radiation therapy. This reaction manifests as an extreme sunburn-like redness, swelling, tenderness, blistering, and peeling at previously irradiated sites. Although the response mechanism is poorly understood, it usually affects the part of the body that receives radiation. It can develop weeks, months, or even years after radiation therapy has ended. Treatment generally consists of corticosteroids to reduce inflammation; rarely, chemotherapy is delayed until the skin heals (Maloney-Newton et al., 2023; McQuestion et al., 2021).
Late Effects of Radiation Therapy
Delayed effects appear more than two months (in many cases, years) after the radiation exposure. Advancements in radiation therapy have helped to minimize damage to healthy tissue; however, treatment is directed to the same area each time, and radiation rays sometimes scatter. Tissues and organs near the treated cancer site can receive smaller doses of radiation despite efforts to prevent this. Late effects of radiation are based on the location of the radiation treatment site and the dose/duration of treatment. Some late effects include cataracts, permanent hair loss, and neuropsychology problems such as impaired memory or cognition. Others may experience impaired thyroid or adrenal function. Hypothyroidism is a common late effect of radiation therapy to the neck, head, and chest due to the impact of radiation on the thyroid gland (McQuestion et al., 2021; ONS, 2025; Palmer et al., 2021).
Radiation-induced heart disease is a complication of radiation to the chest wall, such as in breast cancer or lymphomas, due to the heart’s positioning within the radiation field. These patients can develop cardiomyopathy, congestive heart failure, and damage to the heart muscle, inducing an overall decline in cardiac function. Newer techniques, such as the deep inspiration breath hold (DIBH) during breast cancer radiation treatment, are used to minimize this risk. During DIBH, the patient holds their breath deeply for about 20 seconds while the radiation is being delivered to the breast, temporarily displacing the heart outside the radiation field (Degrande et al., 2023). Radiation to the posterior chest wall may cause lung complications such as pulmonary fibrosis and interstitial lung disease. Infertility, sterility, and sexual dysfunction are also common delayed and ongoing effects of radiation. Some patients may sustain a decreased range of motion in the treated area due to scarring and loss of tissue elasticity. Vaginal dilators are recommended for patients who have undergone radiation therapy to the vagina. Pelvic floor training exercises (e.g., Kegel exercises) are advised for patients experiencing urinary dysfunction, such as incontinence or loss of sphincter tone, as a means of strengthening the muscles of the pelvic floor. Edema and lymphedema are potential side effects due to the disruption of the lymphatic system. Skin sensitivity with sun exposure to the affected area may persist for life, so patients must be counseled on sun safety practices. Young children who have undergone radiation treatment may exhibit slowed or halted bone growth (McQuestion et al., 2021; ONS, 2025b; Palmer et al., 2021).
Secondary malignancies are the most severe and dreaded late complication of cancer therapy and comprise about 15% to 20% of all cancer diagnoses. Although most secondary cancers are linked to chemotherapy exposure, some are caused by radiation. Research demonstrates that radiation-induced secondary malignancies are biologically aggressive cancers and histologically different from the primary tumor. The exact mechanism is unknown, but these diagnoses typically present 5 to 10 years after radiation therapy for hematologic malignancies and about 10 to 60 years later for solid tumors (Khanna et al., 2021). A study on non-Hodgkin lymphoma survivors found a 13% higher risk of secondary malignancies compared to the general population. Chemotherapy and radiation therapy increase this risk, particularly for hematological cancers. Younger age at diagnosis, being assigned male at birth, and certain racial backgrounds were also linked to higher secondary malignancy risk (Li et al., 2022).
According to Palmer and colleagues (2021), secondary malignancies are the leading cause of non-relapse late effects among childhood cancer survivors. Adolescents and young adult cancer survivors are prone to developing second cancers, especially lung cancer. A study of 26,894 cancer survivors found that a BMI over 25 (42.8%) or over 30 (17.2%) at diagnosis significantly increased the risk of a second primary cancer. Over a median 7.9-year follow-up, 13.9% developed a second cancer. Survivors with a BMI over 25 had a 15% higher risk, while survivors with a BMI over 30 faced a 34% higher risk, especially for obesity-related cancers. These findings emphasize the need for weight management in cancer survivorship care (Bodelon et al., 2024).
Part Two: Systemic Therapies and Oncologic Emergencies
Oncology nurses are responsible for all aspects of care related to safe handling, administering, and monitoring of anti-cancer therapies. Thus, they must remain knowledgeable of treatment regimens, drug actions, and side effect profiles. Nurses safeguard patient care by evaluating and interpreting laboratory data, calculating drug dosages to certify accuracy, and responding promptly to hypersensitivity reactions (HSRs) and infusion reactions. HSRs are oncologic emergencies because they can rapidly progress to life-threatening situations. Oncology nurses manage intravenous (IV) lines, including central venous access devices (CVAD), which require meticulous care and ongoing monitoring. Nurses educate patients and caregivers about anti-cancer drugs, their side effects, and the signs and symptoms to monitor and report. Proficient assessment and triage skills are necessary, as the signs of infection in immunocompromised patients are vague. Finally, nurses support patients emotionally, frequently reassessing their physical and mental health, advocating for their needs, and coordinating care (Maloney-Newton et al., 2023; Siegel et al., 2024).
The ASCO and the ONS collaborated in 2008 to create safety standards for administering anti-cancer agents. The ASCO/ONS Antineoplastic Therapy Administration Safety Standards were revised in 2024 and address oncology practice–related issues and standards for oncology nurses to promote safe care for patients receiving chemotherapy, targeted therapy, and immunotherapy. These standards apply across settings and patient populations and serve as the gold standard for oncology nursing practice. In alignment with these practice standards, this course will discuss safe administration procedures, including the appropriate use and disposal of personal protective equipment (PPE) and assessing, monitoring, and managing patients receiving antineoplastic therapy. Furthermore, patient and family education on treatment-related side effects and the management of post-treatment care will be explored (ONS, 2024; Siegel et al., 2024;).
Antineoplastic Therapy Overview
Antineoplastic therapy, also called chemotherapy or cytotoxic therapy, refers to a group of high-risk, hazardous drugs intended to destroy cancer cells. Chemotherapy drugs are distributed throughout the body via the bloodstream and can cause significant morbidity and mortality. Therefore, specialized educational requirements and training are required for oncology nurses who administer antineoplastic therapy and other hazardous cancer medications. While each antineoplastic class has a unique mechanism of action, chemotherapy generally works by interfering with the normal cell cycle to impair DNA synthesis and prevent cell replication. Chemotherapy is given with varying intents, and oncology nurses must understand the rationale for each. Neoadjuvant chemotherapy is administered to shrink a tumor, so the primary treatment (e.g., surgery) is less extensive. Adjuvant chemotherapy is given after the primary treatment to prevent recurrence and reduce micro-metastases (i.e., tiny cancer cells too small to appear on diagnostic tests). For potentially curative treatment regimens, maximum tolerated doses of drugs are delivered on a specific schedule to achieve the greatest efficacy. Palliative chemotherapy is administered for symptom control and to improve quality of life. Chemoprevention is the use of medications to prevent cancer in individuals from high-risk groups. Myeloablation obliterates bone marrow in preparation for stem cell or bone marrow transplantation with high-dose, intensive chemotherapy (ACS, 2019; Maloney-Newton et al., 2023; Nettina & Nelson-Tuttle, 2024; Siegel et al., 2024).
Classification of Chemotherapy Agents
A basic understanding of the cell cycle is required to comprehend how chemotherapy works against cancer. The cell cycle is a five-stage process of reproduction that occurs in healthy and cancerous cells. Gap 0 (G0) is also called quiescence and is the resting stage in which cells are temporarily out of the cell cycle. During this stage, cellular activity continues to occur except for reproduction. During Gap 1 (G1), RNA and protein synthesis occur. This stage is considered the gap between resting and DNA synthesis. Synthesis (S) is when cellular DNA is duplicated in preparation for division. Protein and RNA syntheses continue during Gap 2 (G2) as the cell constructs the mitotic apparatus. Finally, Mitosis (M) is when cellular division occurs (Maloney-Newton et al., 2023; NCI, 2023b; Rodgers, 2024).
Chemotherapy agents are typically classified according to their mechanism and phase of action during the cell cycle. Cell cycle-specific drugs exert cytotoxic effects at particular stages within the cycle. These agents are not active against cancer cells during the G0 phase and are schedule-dependent, meaning they are most effective when administered in divided doses or by continuous infusion. Continuous infusion may occur for up to 7 days at a slow rate, allowing the drug to reach as many cells as possible when they are actively dividing and amenable to dying. Cell cycle-nonspecific drugs exert a broader impact, as they are active during all cell-cycle phases. Nonspecific agents are dose-dependent and are most effective as bolus doses; the cell kill is directly proportional to the amount of drug given. Table 6 displays the classification of the most common chemotherapy agents according to their mechanism of action and effect on the cell cycle (ACS, 2019; Amjad et al., 2023; NCI, 2023b).
Table 6
Classification of Antineoplastic Agents
Category | Examples | Mechanism | Effect on Cell Cycle |
Antimetabolites |
|
|
|
Alkylating agents |
|
|
|
Antitumor antibiotics |
|
|
|
Vinca alkaloids |
|
|
|
Taxanes (Microtubules) |
|
|
|
Podophyllotoxins |
|
|
|
Camptothecins |
|
|
|
(ACS, 2019; Amjad et al., 2023; NCI, 2023b)
Drug Resistance
Cancer drug resistance is a major challenge in oncology, limiting the effectiveness of treatments and leading to disease progression. Drug resistance occurs when cancer stops responding to the prescribed treatment and is a significant barrier to curing cancer. While a patient may have an initial robust response to treatment, drug resistance eventually emerges. Resistance develops from multiple mechanisms but is broadly characterized into intrinsic resistance (existing before treatment) and acquired resistance (developing during therapy following exposure to a drug). In some cases, cancers can develop multidrug resistance (resistance to multiple drugs), resulting in minimal cell death and the growth of drug-resistant tumors. Cancer can adapt and mutate, particularly in response to treatment. While there are several proposed rationales for cancer drug resistance, it is often multifactorial and a combination of mechanisms. Some of the most well-cited resistance mechanisms are described in Table 7 (ACS, 2019; Khan et al., 2024; Maloney-Newton et al., 2023; Nussinov et al., 2021).
Table 7
Types of Drug Resistance
Mechanism | Description |
Genetic mutation and amplification |
|
Pathway alterations and bypass mechanisms |
|
Increased drug efflux |
|
Enhanced DNA repair |
|
Tumor microenvironment influence |
|
Phenotypic changes and cell plasticity |
|
- (Khan et al., 2024; Nussinov et al., 2021)
Drug resistance is the primary reason cancer drugs are given in combination. Since cancer cells are destructively efficient in their division, replication, and spread patterns, combining therapies with diverging mechanisms of action can overcome resistance. Further, as cancer progresses, it develops new mutations. If cancer becomes resistant to a drug or a group of drugs, it is more likely to become resistant to others. Thus, adhering to evidence-based treatment guidelines is vital, as the most effective treatment protocol should always be administered first (Khan et al., 2024; Nussinov et al., 2021).
The Safe Administration of Antineoplastic Therapy
Antineoplastic therapy administration should be viewed as a process rather than an isolated act of medication administration. Oncology nurses must attain in-depth knowledge and understanding of the mechanism of action, expected side effects of each drug, and safe infusion practices to be deemed competent in chemotherapy administration. The 2024 ASCO-ONS Antineoplastic Therapy Administration Safety Standards provide comprehensive guidelines to ensure the safe administration of antineoplastic therapies. These standards are organized into several key domains, which aim to enhance patient safety, improve the quality of care, and reduce the risk of errors when administering antineoplastic therapies. Health care organizations are encouraged to integrate these guidelines into their clinical practices to uphold the highest standards of oncological care. A high-level overview of the standards is provided in Table 8. Additionally, ONS (2025) offers several online courses for initial didactic preparation and continuing education for nurses who administer antineoplastic treatments. The ONS/ONCC Chemotherapy/Immunotherapy Certificate Course is the most widely used advanced-level credentialing program, which teaches nurses how to administer antineoplastic therapy safely. Nurses who complete this course earn the ONS Chemotherapy Immunotherapy Provider Card, which expires every 2 years. While each institution or practice can determine how to assess competence in performing antineoplastic-related skills, most accredited cancer centers and hospitals require nurses to have an active ONS Chemotherapy Immunotherapy Provider Card to administer antineoplastic therapy (ONS, 2024, 2025a).
Table 8
ASCO-ONS Antineoplastic Therapy Administration Safety Standards
Standard | Recommendations |
Safe environment |
|
Treatment planning and informed consent |
|
Ordering, preparing, and dispensing antineoplastic agents |
|
Administration of antineoplastic agents |
|
Monitoring for treatment-related toxicities |
|
Documentation and communication |
|
(Siegel et al., 2024)
Antineoplastic therapy is most commonly administered by IV or mouth, but certain drugs may be administered via other routes, such as:
- Subcutaneous injection (under the skin)
- Intramuscular injection (deep into the muscle)
- Intrathecal (into the central nervous system [CNS])
- Intravesicular (into the bladder)
- Intraperitoneal (into the intraabdominal cavity) (Maloney-Newton et al., 2023)
Because chemotherapy can cause severe irritation, damage, and injury to the veins and subcutaneous tissue, many patients have an implantable CVAD (e.g., port). A port is a small device surgically implanted under the skin, usually in the chest wall, allowing direct access to the bloodstream. The port can be used to draw blood, infuse chemotherapy, and administer other medications (Nettina & Nelson-Tuttle, 2024; Siegel et al., 2024).
Refer to the NursingCE course on Vascular Access Devices to learn more about this topic.
Most chemotherapy agents are vesicants that should be given through a CVAD as they have a high potential for causing severe tissue damage if extravasated (i.e., leak outside the vein). The severity of extravasation depends on the drug's type and concentration. Initial symptoms can present as acute burning, pain, and swelling at the infusion site. These symptoms become increasingly severe in the hours, days, and weeks following the initial injury. Patients may develop blisters, which usually begin within 3 to 5 days and may be followed by peeling or sloughing of the skin with invasion and destruction of deeper structures. Tissue necrosis usually occurs within 2 to 3 weeks. In the most severe cases, damage can reach tendons, nerves, and joints, leading to functional and sensory impairment of the area, disfigurement, or loss of the limb entirely. Examples of vesicants include doxorubicin (Adriamycin), dactinomycin (Cosmegen), and vincristine (Oncovin). Peripheral IV sites must be assessed before, during, and after infusion for signs of erythema, swelling, or loss of blood return. Patients must be counseled to report any pain, burning, or other abnormal sensations during infusions. There are specific guidelines governing the management of peripheral IV sites for chemotherapy, such as location, placement, monitoring parameters, and frequency of checking for blood return. In general, all chemotherapy agents should be considered irritants, as they all have the potential to cause inflammation, pain, or irritation. Table 9 reviews the responsibilities of oncology nurses in the administration of antineoplastic therapy. By adhering to these guidelines, nurses play a crucial role in ensuring the safe and effective administration of antineoplastic therapy, while also prioritizing patient safety and care quality (ACS, 2019; Nettina & Nelson-Tuttle, 2024; Siegel et al., 2024).
Table 9
Standard of Care Guidelines for Administering IV Antineoplastic Therapy
Before Administration |
|
During Administration |
|
After Administration |
|
(ACS, 2019, 2024; Olsen et al., 2023; ONS, 2025a; Siegel et al., 2024)
Antineoplastic Therapy Side Effects
Because chemotherapy is designed to target rapidly dividing cells, it commonly affects tissues with high cellular turnover, such as the GI tract, skin, hair follicles, and bone marrow. Common adverse effects include bone marrow suppression (leading to cytopenias), nausea and vomiting, diarrhea, fatigue, alopecia, and oral mucositis. Patient responses to chemotherapy vary, and not all agents carry the same toxicity profile. Comprehensive patient assessment and education are essential to facilitate early recognition and effective management of side effects. Proactive interventions, such as premedication regimens for nausea and individualized symptom management strategies, can significantly improve patient outcomes. Oncology nurses play a pivotal role in mitigating chemotherapy-related toxicities through expert symptom management, patient advocacy, and evidence-based interventions. By leveraging a range of supportive care measures, nurses can enhance patient comfort, adherence to treatment, and overall quality of life. Table 10 discusses some of the most common chemotherapy side effects and critical teaching points (ACS, 2019; Siegel et al., 2024).
Table 10
Chemotherapy Side Effects and Key Teaching Points
Generalized Fatigue |
|
Bone Marrow Suppression |
Neutropenia (Low White Blood Cell Count)
Thrombocytopenia (Low Platelet Count)
Anemia (Low Red Blood Cell Count)
|
Integumentary System (Skin, Hair, Nails) |
|
Gastrointestinal System |
|
Genitourinary System |
|
Neurologic System |
|
Cardiovascular System |
|
Vascular System |
|
Pulmonary System |
|
Reproductive System and Sexual Health |
|
Psychosocial and Psychiatric Health |
|
(ACS, 2019; Maloney-Newton et al., 2023; Nettina & Nelson-Tuttle, 2024; Siegel et al., 2024)
Oral Cancer Drugs
An oral cancer drug is any medication taken by mouth (in liquid, tablet, or capsule formulation) to treat cancer and includes chemotherapy, targeted therapies, and some types of hormonal treatments. Oral medications offer the convenience of at-home administration without sacrificing strength or effectiveness when compared to injectables. However, with the increased flexibility come unique safety challenges. Nurses must ensure patients understand how to handle and dispose of these drugs safely. One of the most challenging issues with oral cancer treatment is poor adherence, which substantially impacts the drug’s efficacy, safety, side effects, and toxicities. Oral cancer medications are prescribed at defined intervals based on the drug's mechanism of action, half-life (i.e., the amount of time it takes for 50% of the drug to be excreted from the body), and side effect profile. Oncology nurses must educate patients on the significance of taking the medication consistently as prescribed to ensure a constant level of the drug remains in their body to target the cancer cells effectively. Even a slight increase or decrease in the dose level can impair the drug's efficacy or lead to superfluous side effects. These medications can quickly become dangerous if not taken as prescribed. Patients must be counseled not to crush, chew, or split oral cancer pills, as this can affect how the medication works (Huff, 2020; Maloney-Newton et al., 2023).
Establishing a routine can help patients remain consistent with their medication dosing schedule. Some strategies may include filling pillboxes each week, setting pill reminders on smartphones or tablets, enrolling in electronic medication reminders through a pharmacy, or using a simple paper pill diary to avoid overdosing. Oral cancer drugs are potentially hazardous and require special precautions for safe handling, especially for caregivers. According to the ONS Guidelines to Support Patient Adherence to Oral Anti-cancer (OAC) Medications, nurses should educate patients and caregivers on drug safety before patients receive OAC medications. These evidence-based guidelines emphasize the importance of providing patients with comprehensive symptom management and adherence strategies. By equipping patients with the necessary tools and knowledge, oncology nurses can support patients in effectively managing their treatment regimens. Table 11 provides an overview of the safe handling of OAC medications and core components for patient/caregiver education (Belcher et al., 2022; Huff, 2020; Maloney-Newton et al., 2023).
Table 11
Safe Handling of OAC Medications
Storage Guidelines |
|
|
|
Disposal and Exposure Guidelines |
|
(Belcher et al., 2022; Huff, 2020; Maloney-Newton et al., 2023; ONS, 2024)
Targeted Therapies
Targeted therapies have transformed cancer treatment by offering a more precise approach to attacking cancer cells while minimizing harm to normal tissues. Unlike traditional chemotherapy, targeted therapies work by blocking specific molecules that drive cancer growth and progression. Targeted therapies interfere with cancer growth by focusing on specific molecular targets involved in tumor development. These targets may include mutated genes, overexpressed proteins, or signaling pathways that contribute to uncontrolled cell proliferation. Targeted therapies are classified into several categories based on their mechanism of action, including hormonal therapies, monoclonal antibodies, tyrosine kinase inhibitors (TKIs), and angiogenesis inhibitors (Maloney-Newton et al., 2023; NCI, 2022; Siegel et al., 2024).
In 2003, the completion of the Human Genome Project marked a dramatic shift in the understanding of cancer and other diseases. Researchers mapped the entire human genetic code and discovered that every human cell contains 20,000 to 30,000 genes. As a result, novel approaches to treating cancer and new drug discovery have exploded over the last 2 decades. Targeted agents are crafted to attack specific parts of cancer cells. Numerous proteins located on the cellular membranes (i.e., growth factor receptors) connect the external and internal cellular environments and are essential for cell growth and development. Alterations in genes lead to changes in cellular proteins, stimulating the disruption of normal processes, inducing malfunction, and enabling cancer growth. Targeted therapies impede cancer growth through unique and distinctive pathways. Some targeted therapies focus on the external components and functioning of the cell, whereas others use small molecules to enter the cell and disrupt its function. There are various mechanisms by which targeted therapies function, such as:
- Blocking or turning off chemical signals that tell cancer cells to grow and divide
- Altering proteins within the cancer cells
- Starving the tumor by cutting off its blood supply
- Helping the immune system destroy cancer cells
- Carrying toxins or poison to the cancer cells directly to kill them without harming healthy cells
- Depriving the cancer of the hormones it needs to grow (NCI, 2022; National Human Genome Research Institute, 2024; Thomson et al., 2023)
Oncology nurses play a pivotal role in administering targeted therapies, monitoring patients for adverse effects, and educating patients on treatment adherence. Although targeted therapies are often better tolerated than traditional chemotherapy, they come with unique toxicities. Some of the most common side effects include skin reactions (e.g., rash, dryness, sensitivity to sunlight), hypertension, proteinuria, diarrhea, nausea, cardiotoxicity, and hepatotoxicity. Oncology nurses should encourage patients to adhere strictly to their prescribed regimen, assess for early signs of toxicity, collaborate with the health care team to manage adverse effects, and educate patients about potential drug interactions with over-the-counter (OTC) medications and supplements. By understanding the mechanism, benefits, and risks of these treatments, oncology nurses can optimize patient safety, enhance adherence, and improve treatment outcomes in cancer care (Belcher et al., 2022; NCI, 2022; ONS, 2024).
Hormonal Therapies
Hormones drive certain types of cancer growth. Hormonal therapies are targeted agents that block circulating hormones from reaching cancer cells or prevent the body from producing the hormones altogether. The most common hormone-dependent cancers include breast, prostate, uterine, ovarian, and neuroendocrine. For breast cancer, hormonal therapy is often prescribed long-term, averaging 5 to 10 years; however, it may extend longer for patients with recurrent, advanced, or stage IV malignancies. Side effects of hormonal therapy can prominently impact sexual health and the reproductive system and vary based on biological sex. Patients assigned female at birth may experience vasomotor symptoms of menopause, such as hot flashes, night sweats, loss of libido, weight gain, and vaginal dryness. Other common side effects may include joint aches or pains, mood changes, hair loss, and bone thinning (i.e., osteopenia, osteoporosis). Patients assigned male at birth may experience hot flashes, impotence (i.e., an inability to have or maintain an erection), shrinking of the testicles, gynecomastia (i.e., breast tissue enlargement), and bone thinning. Due to the risk of bone thinning on hormonal therapy, patients should be counseled on the importance of following a calcium-rich diet with at least 1,200 mg of dietary calcium daily and engaging in regular weight-bearing exercises. Patients who cannot get the recommended amount of calcium in their diet should consider calcium supplementation (Nettina & Nelson-Tuttle, 2024; Siegel et al., 2024).
Monoclonal Antibodies
Monoclonal antibodies are artificial proteins manufactured to behave like natural human antibodies. They attach to targets on the surface of cancer cells and inject their contents inside, causing them to die. Cells that do not have the target remain unharmed. Antibodies are part of the adaptive immune system. The body creates antibodies in response to an antigen (e.g., bacteria). The antibodies attach to the antigen to mark it for destruction by the immune system. In the laboratory, scientists analyze specific antigens on the surface of cancer cells to determine a protein match for the antigen. Then, using protein specimens from animals and humans, they create a unique antibody that will attach to the target antigen as a key fits into a lock. This technology allows treatment to target specific cells, causing less toxicity to healthy cells. Monoclonal antibodies work on cancer cells in the same way natural antibodies function—by recognizing and binding to the target cells and then alerting other cells in the immune system to the presence of the cancer cells. This type of treatment can only be offered for cancers in which antigens and their respective antibodies have been identified (ACS, 2025d; Siegel et al., 2024; Tsao et al., 2021).
The nomenclature of monoclonal antibodies provides clues to their composition and function. Monoclonal antibodies always end with the stem -mab. The term -mab is used when receptor targets are overexpressed on the outer layer of the cells. Monoclonal antibodies have an additional layer of classification within their sub stem, representing how the drug is comprised. For example, murine monoclonal antibodies end in -momab (e.g., ibritumomab [Zevalin]). Murines are composed of mouse proteins and include conjugated antibodies, which are physically attached to antitumor agents such as radioisotopes, chemotherapy, toxic drugs, and other biologic agents. Murines have the highest risk of HSRs due to their high mouse content. Conjugate monoclonal antibodies present the highest risk for bone marrow suppression and additional side effects since they are physically attached to a toxic or poisonous agent. Chimeric monoclonals end with -ximab (e.g., rituximab [Rituxan]). Chimerics are primarily comprised of mouse proteins with a small human protein component. Therefore, chimerics have a relatively high risk of HSR. Humanized monoclonals, which end in -zumab (e.g., trastuzumab [Herceptin]), include a small number of mouse proteins, and the rest are human components. Humanized agents induce less severe HSRs. Fully human monoclonals end with -mumab (i.e., panitumumab [Vectibix]). Since these options are fully human, HSRs are rare. Certain types of monoclonal antibodies include additional information in their naming classification that denotes their primary target. For example, the li in ipilimumab (Yervoy) identifies the immune system as the target. The tu in rituximab (Rituxan) indicates the target is the tumor. The ci in bevacizumab (Avastin) identifies the circulatory system as the drug’s target (ACS, 2025d; Maloney-Newton, 2023; Siegel et al., 2024; Tsao et al., 2021). While each monoclonal antibody has a unique side effect profile, the most common are infusion reactions, which include flu-like symptoms, rash, pruritus, headaches, joint aches, nausea, diarrhea, and hypotension. Patients are usually premedicated with acetaminophen (Tylenol) and diphenhydramine (Benadryl) to prevent HSR and infusion reactions (ACS, 2025d; Maloney-Newton, 2023; Siegel et al., 2024; Tsao et al., 2021).
Antibody Drug Conjugates (ADCs)
Antibody drug conjugates (ADCs) combine monoclonal antibodies with the cytotoxic potency of chemotherapy. The mechanism of action involves three key steps. First, the monoclonal antibody selectively binds to a specific antigen in cancer cells (e.g., HER2, CD30, or TROP-2). Second, the ADC is internalized by the cancer cell, where the cytotoxic drug (payload) is released. Third, the cytotoxic payload disrupts essential cellular functions, typically by damaging DNA or inhibiting microtubules, leading to cancer cell death while sparing normal cells. ADCs are used in the treatment of various cancers, and each has a distinct target. For example, brentuximab vedotin (Adcetris) is used for Hodgkin lymphoma. It is comprised of an antibody that targets the CD30 antigen (found on lymphocytes) and is attached to a chemo drug monomethyl auristatin E (MMAE). In breast cancer, ado-trastuzumab emtansine (Kadcyla) is comprised of an antibody that targets the HER2 protein and is attached to a chemo drug called DM1. Since there is a powerful chemotherapy component to these drugs, common side effects include bone marrow suppression (neutropenia, anemia, thrombocytopenia), fatigue, nausea, and specific toxicities based on the cytotoxic payload (e.g., interstitial lung disease with ado-trastuzumab emtansine [Kadcyla] or peripheral neuropathy with brentuximab vedotin [Adcetris]) (ACS, 2025d; Khongorzul et al., 2020).
Bispecific Monoclonal Antibodies
Bispecific monoclonal antibodies are an innovative class of cancer treatment engineered to bind two distinct antigens to enhance their therapeutic efficacy. A common design involves one arm targeting a tumor-associated antigen and the other engaging a T-cell surface protein, such as CD3. This dual engagement facilitates the formation of an immunological synapse, directing cytotoxic T-lymphocytes to recognize and eliminate cancer cells. Clinically, bispecific monoclonal antibodies have shown promise in treating various malignancies. Blinatumomab (Blincyto) targets CD19 on B-cells and CD3 on T-cells and is approved for certain types of B-cell acute lymphoblastic leukemia. Similarly, teclistamab (Tecvayli) binds to B-cell maturation antigen (BCMA) and CD3, offering a novel approach for relapsed or refractory MM. Nurses must remain vigilant for potential side effects associated with these drugs as cytokine release syndrome (CRS) is common (ACS, 2025d; Salvaris et al., 2021). CRS is a systemic inflammatory response caused by rapid immune activation and characterized by fever, hypotension, and respiratory distress. CRS can also cause neurotoxicity, presenting as confusion, headache, or seizures. Prompt recognition and management of these reactions are crucial to ensure patient safety (refer to Table 12) (ACS, 2025d; Salvaris et al., 2021).
Tyrosine Kinase Inhibitors (TKIs)
Tyrosine kinase inhibitors (TKIs) are a critical class of targeted cancer therapies that block signaling pathways essential for tumor growth and survival. Tyrosine kinases are enzymes responsible for activating various proteins involved in cell division, proliferation, and survival. In many cancers, these enzymes become overactive due to genetic mutations, leading to uncontrolled tumor growth. TKIs work by binding to the appropriate receptor, thereby inhibiting their activation and disrupting downstream signaling pathways that promote cancer progression. TKIs are commonly used in cancers driven by aberrant tyrosine kinase activity, such as chronic myeloid leukemia (CML), non-small cell lung cancer (NSCLC), renal cell carcinoma, and GI stromal tumors (GISTs). Examples of TKIs include imatinib (Gleevec), erlotinib (Tarceva), gefitinib (Iressa), and osimertinib (Tagrisso). While TKIs are often administered orally and provide a more targeted approach compared to chemotherapy, they are known for causing moderate-to-severe skin rashes, diarrhea, liver dysfunction, hypertension, and cardiotoxicity. TKIs require close monitoring of liver enzymes, blood pressure, and cardiac function. Meticulous assessment of any food or drug interactions is needed as many TKIs are metabolized via the cytochrome P450 system (NCI, 2022; ONS, 2024; Thomson et al., 2023).
Angiogenesis Inhibitors
Angiogenesis inhibitors prevent the formation of new blood vessels (i.e., angiogenesis) that tumors need for growth and metastasis. Tumors stimulate angiogenesis by releasing vascular endothelial growth factor (VEGF) and other pro-angiogenic signals, allowing them to develop a blood supply to receive oxygen and nutrients. Angiogenesis inhibitors, such as bevacizumab (Avastin) and aflibercept (Zaltrap), work by targeting VEGF or its receptors, preventing new blood vessel formation and effectively "starving" the tumor. These agents are widely used in colorectal cancer, lung cancer, renal cell carcinoma, glioblastoma, and other solid tumors. Angiogenesis inhibitors also carry unique toxicities, including hypertension, proteinuria, impaired wound healing, bleeding, and GI perforation. Nurses should monitor blood pressure, assess for signs of bleeding or clotting, and ensure appropriate wound-healing precautions before surgical procedures. Additionally, patient education on recognizing symptoms such as severe headaches, sudden onset of severe abdominal pain, or unusual bruising is essential to prevent complications (Liu et al., 2023; Maloney-Newton et al., 2023).
Immunotherapy
While targeted therapies have significantly improved survival rates and quality of life for many cancer types, they continue to evolve with the advancement of precision medicine and biomarker-driven treatments. The integration of targeted therapy with immunotherapy and combination regimens is expanding treatment options for patients with various malignancies. Immunotherapy uses the immune system to identify cancer cells and attack them like an infection, virus, or other potential threat. Immunotherapy produces antitumor effects by rousing natural defense mechanisms, priming the immune system’s sensitivity to cancer cells, and helping it learn how to identify, attack, and kill them. The connection between cancer and the immune system was discovered about 100 years ago by Dr. William Coley, who is known as the father of immunotherapy. Dr. Coley found that malignant tumors disappeared in patients who contracted streptococcal infections. Immune checkpoints are proteins that act as “brakes” on a normally functioning immune system. Immune checkpoint inhibitors target these proteins, block them, and allow the immune system to attack the cancer cells by taking off the brakes. Some forms of immunotherapy are so highly specialized that they only target a single receptor on the surface of tumor cells. Although immunotherapy is among the most promising approaches since the development of chemotherapy, it is not equally effective against all cancers at this stage in development. Immunotherapies include checkpoint inhibitors and chimeric antigen receptor (CAR) T-cell therapy (NCI, 2023a; Siegel et al., 2024).
Given the widespread use of immunotherapies, oncology nurses must understand their unique side effect profiles and toxicities. Adverse reactions and toxicities are graded according to the NCI’s Common Terminology Criteria for Adverse Events (CTCAE). Currently, version 5 scale is available, but version 6 is expected to be released in 2025 (NCI, 2025b). Removing the immune system's brakes increases the risk of autoimmune-like symptoms and systemic inflammation. Inflammation of any organ system is possible and can progress to life-threatening situations if not promptly recognized and managed. For example, inflammation in the GI tract (colitis) can initially present as abdominal pain, cramping, and diarrhea. If untreated, colitis can become fatal. Other examples include endocrinopathy (adrenal insufficiency or hypothyroidism), pneumonitis (inflammation of lung tissue), hepatitis (inflammation of the liver), pancreatitis (inflammation of the pancreas), and uveitis (inflammation of the eye). Patients with clinical signs of hepatitis or transaminitis (elevated liver enzymes) should avoid excessive use of acetaminophen (Tylenol), which can exacerbate these conditions. If diagnosed early, most immune-related adverse events (irAEs) are reversible with interruption of the offending therapy and administration of a prolonged corticosteroid taper. However, toxicities must be graded appropriately, monitored cautiously, and managed per guidelines formulated by manufacturers in collaboration with the US Food & Drug Administration (FDA). Severe irAEs should prompt discontinuation of the immunotherapy and long-term corticosteroids. In extreme cases, immunosuppressive agents such as infliximab (Remicade) are given to manage symptoms (NCI, 2023a; Schneider et al., 2021; Siegel et al., 2024).
Chimeric Antigen Receptor (CAR) T-cell Therapy
Chimeric antigen receptor (CAR) T-cell therapy is a groundbreaking form of immunotherapy that harnesses the body’s immune system to fight cancer. CAR T-cell therapy involves genetically modifying a patient’s T-cells to recognize and attack cancer cells more effectively. The process begins with collecting the patient’s T-cells, which are then engineered in a laboratory to express a CAR that specifically targets a cancer-associated antigen, such as CD19, in B-cell malignancies. Once modified, the CAR T-cells are expanded and reinfused into the patient, where they seek out and destroy cancer cells. CAR T-cell therapy is primarily approved for hematologic malignancies, including relapsed or refractory B-cell acute lymphoblastic leukemia (ALL), large B-cell lymphoma (LBCL), mantle cell lymphoma (MCL), and MM. Some types of CAR T-cell therapies include tisagenlecleucel (Kymriah), axicabtagene ciloleucel (Yescarta), idecabtagene vicleucel (Abecma). CAR T-cell therapy is particularly beneficial for patients who have failed multiple lines of conventional treatment, such as chemotherapy or stem cell transplantation. Unlike traditional therapies, CAR T-cells persist in the body and provide long-term immune surveillance, reducing the risk of relapse (Baer, 2021; Siegel et al., 2024).
While CAR T-cell therapy has demonstrated high response rates, it carries unique risks. As the CAR-T cells multiply in the body, they release large amounts of chemicals called cytokines into the bloodstream, causing many symptoms and potential complications, including CRS and immune effector cell-associated neurotoxicity syndrome (ICANS). According to ASCO guidance, the incidence of CRS ranges from 57% to 93%, depending on the bispecific agent used (Santomasso et al., 2021). ICANS affects the nervous system, causing neurotoxicity, manifesting as headaches, cognitive changes, confusion, agitation, tremors, seizures, and speech and balance difficulties. Patients are typically hospitalized for the infusion and monitored closely. Oncology nurses play a vital role in monitoring patients for these toxicities, providing supportive care, and educating patients on post-infusion expectations. As research continues to expand CAR T-cell applications, it remains a promising advancement in cancer therapy, offering hope to patients with otherwise limited treatment options (ACS, 2024; Baer, 2021; Morris et al., 2021; Schneider et al., 2021; Siegel et al., 2024; Xiao et al., 2021).
Hypersensitivity Reaction (HSR) and Cytokine Reaction Syndrome (CRS)
Hypersensitivity reaction (HSR) is defined as a symptomatic interaction between antibodies and allergens that causes an exaggerated and harmful response in the body. In other words, HSRs occur when a foreign substance overstimulates the immune system and forms antibodies that cause an immune response. HSRs range from mild to life-threatening in severity and symptoms and can include anaphylactoid reactions. All cancer therapies pose a risk for HSRs, but they are most common with monoclonal antibodies and certain types of chemotherapy. HSRs can occur during the initial chemotherapy infusion or subsequent administrations of the same agent. Most HSRs arise during the first 15 min of the infusion, but reactions can occur outside this time frame. Therefore, oncology nurses must monitor vigilantly for signs of HSR and ensure they are prepared to intervene immediately. Refer to Table 12 for an overview of the clinical manifestations and management of HSRs. Nurses should also be familiar with their institution's specific chemotherapy HSR protocols and policies (Baer, 2021; Morris et al., 2021; Nettina & Nelson-Tuttle, 2024; Santomasso et al., 2021; Siegel et al., 2024).
Cytokine reaction syndrome (CRS) is caused by the rapid release of cytokines from cancer cells during cell lysis following contact with the drug. Clinical manifestations may vary from mild, flu-like symptoms to severe, life-threatening manifestations. Respiratory symptoms may initially develop with a mild cough and tachypnea but can rapidly progress to acute respiratory distress syndrome (ARDS) with dyspnea, hypoxemia, and a chest x-ray revealing bilateral opacities. ARDS may necessitate mechanical ventilation. Patients with severe CRS can develop cardiac dysfunction with a reduced ejection fraction, vascular leakage with peripheral and pulmonary edema, and renal failure. Laboratory abnormalities commonly include cytopenias (neutropenia, anemia, thrombocytopenia), impaired kidney function, elevated liver enzymes, and unbalanced coagulation parameters. Most patients are premedicated with acetaminophen (Tylenol) and diphenhydramine (Benadryl) to reduce the risk of infusion reactions. Slowing down the infusion rate can also reduce the risk of prolonged rigors. Institutions should have policies outlining the importance of slowly titrating the infusion rate of monoclonal antibodies to reduce the risk of rigors, chills, and fevers. The nursing management of CRS has distinct differences from the typical HSR management, which further emphasizes the importance of the oncology nurse's knowledge base and skill when administering these medications. Table 12 compares the features and management of HSR and CRS (Baer, 2021; Morris et al., 2021; Nettina & Nelson-Tuttle, 2024; Santomasso et al., 2021; Siegel et al., 2024; Xiao et al., 2021).
Table 12
HSR Vs. CRS
| HSR | CRS |
Common Cause | Chemotherapy/cytotoxic therapies | Targeted agents (immunotherapy, monoclonal antibodies, bispecific antibodies, CAR T-cell therapy) |
Risks |
| |
Risk Reduction | Premedication with corticosteroids, antipyretics, antihistamines (histamine 1-/histamine 2-receptor antagonists) | |
Safety | Confirm emergency medications and equipment are available before starting chemotherapy treatment. | |
Clinical Signs |
|
|
Management
Early recognition and intervention are key.
|
|
|
Anaphylaxis |
| |
(Backler, 2020; Baer, 2021; Dickman, 2020; Morris et al., 2021; Nettina & Nelson-Tuttle, 2024; Siegel et al., 2024; Xiao et al., 2021)
Before administering a cytotoxic, monoclonal, or immunotherapy, the nurse should inform the patient and their family about the potential for immediate complications. The patient should be instructed to report any signs or symptoms that may indicate an HSR, including flushing, warmth, chills, itching, redness, discomfort, chest pain, shortness of breath, or nonspecific symptoms such as impending doom or anxiety. While Table 12 provides guidance on the most common signs and symptoms of HSR and CRS, it is important to understand that these reactions can also present in various other ways. Therefore, it is essential to educate each patient and their family to report any abnormalities during an infusion. Delayed reactions or symptoms occurring after the infusion and once the patient arrives home are less common but have been reported (Backler, 2020; Dickman, 2020; Morris et al., 2021; Nettina & Nelson-Tuttle, 2024; Siegel et al., 2024; Xiao et al., 2021).
Sexual Health and Cancer
Cancer and its treatments can have a profound impact on various aspects of sexuality, including libido, sexual function, self-image, and overall body perception. These effects stem from both physical and psychological changes caused by cancer treatments. Certain medication therapies may disrupt hormone levels or affect nerve function, leading to decreased sexual desire or difficulties with sexual performance. Additionally, emotional factors such as anxiety, depression, fatigue, and pain can further contribute to a reduced interest in sexual activity. Sexuality is an essential aspect of well-being and can play a significant role in helping individuals cope with the emotional and psychological burden of cancer. However, many patients struggle to discuss sexual health concerns, often experiencing feelings of embarrassment, shame, or anxiety. While they may have questions or fears about how cancer treatment affects their sexual function, they frequently hesitate to bring up these concerns with their healthcare providers. Recognizing the importance of addressing sexual health as part of comprehensive cancer care can help ensure that patients receive the support and guidance they need to navigate these changes and improve their quality of life (ACS, 2020c).
According to Taylor and colleagues (2020), sexual dysfunction is a common yet often overlooked side effect of cancer treatment among survivors. In a survey of 405 cancer survivors, 87% of patients experienced changes in sexual function or desire due to treatment, yet only 27.9% were formally asked about their sexual health by healthcare providers. The researchers also found that survivors who were assigned female at birth were less likely to receive attention for their sexuality concerns when compared to their male counterparts (Taylor et al., 2020). Oncology nurses can often develop strong rapport and therapeutic relationships with patients throughout their disease trajectory. Therefore, they are in a unique position to initiate conversations on sensitive topics such as sexuality. Nurses should approach these topics with empathy, compassion, and no judgment, inviting patients to express their concerns comfortably and ask honest questions. Patients should be counseled on the impact of cancer treatment on their sexuality and forewarned that when sexual changes do occur, they typically do not improve right away. Treatment-related sexual changes may be long term or permanent. Patients with menstrual cycles may endure irregular bleeding while on cancer therapy but must be advised that it may still be possible to conceive a child during treatment. Pregnancy is contraindicated while on anti-cancer therapy due to the risk of fetal harm; therefore, patients and their partners must be counseled on taking necessary precautions to prevent pregnancy. Patients of childbearing age desiring fertility preservation must be counseled on the potential for infertility due to premature ovarian failure or oligospermia related to treatment. These patients should be advised on fertility preservation options and referred to reproductive health specialists and/or sperm banking before starting therapy (ACS, 2020c; Katz et al., 2022; Maloney-Newton et al., 2023).
The most common sexual health issue for patients assigned female at birth with cancer is menopause, which may be induced by surgery to remove the ovaries due to cancer, chemotherapy, radiation therapy, or hormone-blocking agents. Treatment-induced menopause can be temporary or permanent, depending on the type of treatment and the patient's age when treatment was received. Patients who endure premature menopause due to cancer therapy are at risk for sexual and vaginal complications. Some of these symptoms may include vaginal atrophy (thinning, drying, and inflammation of the vaginal walls due to a reduction in estrogen) and dyspareunia (pain during intercourse). Other symptoms include difficulty with sexual arousal, a loss of libido, and vasomotor symptoms such as hot flashes and night sweats. In addition, many patients report mood changes due to the abrupt loss of hormones, describing feelings of anxiety, sadness, loss, and a lack of interest in sexual contact with their partners. Patients who are biologically male may experience erectile dysfunction, an inability to achieve or maintain an erection, fertility problems related to low testosterone levels, and premature or delayed ejaculation. Physical limitations, such as Peyronie's disease (curvature of the penis during an erection), can occur due to specific treatments for prostate cancer. Patients should be counseled that finding the most helpful remedy may take time and requires patience and open communication with partners. Both psychological and physical factors can cause sexual changes. Nurses can help by offering practical, realistic, and cost-effective strategies and interventions to alleviate some of these potential adverse effects or help connect patients with appropriate resources (ACS, 2020c; Katz et al., 2022; Maloney-Newton et al., 2023). Table 13 cites some key teaching points and interventions for some of the most common symptoms and sexual alterations experienced by patients with cancer.
Table 13
Sexual Health and Cancer: Key Teaching Points
General Counseling |
Psychosocial and/or psychosexual counseling should be offered to all patients with cancer to improve body image, intimacy, relationship issues, and overall sexual health, function, and satisfaction. Any identifiable medical and treatable contributing factors should be addressed first. |
Vaginal Atrophy |
Water-based vaginal lubricants, gels, or creams may be applied two to three times a week, regardless of sexual activity. These are estrogen-free, provide symptom relief, and help the vaginal tissue restore its natural moisture. Vaginal estrogen is generally contraindicated for patients with hormone-driven cancers. |
Vaginal Stenosis |
Vaginal stenosis can occur following radiation therapy to the region. A vaginal dilator is a tube-shaped device used to stretch the vagina (increase the elasticity of the vaginal mucosa) after radiation therapy. It prevents vaginal agglutination (adhesion of vaginal labia to each other) and lessens discomfort with sexual activity and pelvic exams. |
Vasomotor Symptoms |
Antidepressants can help manage hot flashes (e.g., venlafaxine [Effexor]). Treatments that help patients cope with stress and anxiety may also lessen hot flashes, such as hypnosis and acupuncture. |
Erectile Dysfunction (ED) |
Oral medications such as sildenafil (Viagra) or tadalafil (Cialis) may be prescribed for symptom management. Assistive devices (e.g., vacuum pumps) are available if oral medications are ineffective. In addition, it is essential to address psychological components that may be contributing to ED. |
General Precautions |
Herbs and dietary supplements should be used with caution. For example, black cohosh is an herb often marketed to manage symptoms of menopause, such as reducing hot flashes. However, this drug should not be taken by patients with a history of estrogen-receptor-positive (hormone-positive) cancers due to estrogenic effects. In addition, patients should avoid petroleum jelly-based products (Vaseline) or skin lotion as a lubricant for the vagina or penis. These can damage condoms and increase the risk of yeast infections. Vaginal oils, gels, or creams containing fragrances, flavors, or herbal ingredients should be avoided since they irritate the vaginal mucosa. |
(ACS, 2020c; Maloney-Newton et al., 2023; Katz et al., 2022)
Safety and Exposure
In addition to patient safety, cytotoxic drugs can be equally hazardous to nurses and other health care workers, so it is critical to adhere to standards and practices of dangerous drug handling to minimize any occupational exposure. Exposure to hazardous medication is linked to an increased risk for several malignancies, and exposure can occur through various sources, including workplace surface contamination (ONS, 2024, 2025a). According to Siegel and colleagues (2024), oncology nurses must wear appropriate PPE whenever there is a risk of chemotherapy being released into the environment. Exposure risk occurs during chemotherapy preparation, spiking or priming IV tubing, and handling body fluids or chemotherapy spills. These guidelines also describe hazardous drug handling as posing reproductive risks, so health care workers who are pregnant, breastfeeding, or trying to conceive must notify their employer. These individuals should not be handling hazardous medications such as chemotherapy. Chemotherapy medications must be mixed and spiked/primed under an approved filtered hood to reduce the risk of aerosolized exposure. Gloves tested for use with hazardous drugs are required, and reusing gloves is prohibited. Nurses should wear disposable, lint-free gowns made of low-permeability fabric when administering chemotherapy, and spill kits should be available in all areas where chemotherapy is stored, prepared, and administered. Gloves and gowns should be discarded in leak-proof containers marked as contaminated or hazardous waste. Linens or clothes contaminated with chemotherapy or bodily fluids from patients who have received chemotherapy within 48 hr should be contained in labeled hazardous waste bags. If any chemotherapy medication spills on clothes in the clinic, the items should be thrown away or double-bagged in plastic for transport home. The clothing must then be washed separately in hot water with regular detergent (ONS, 2024, 2025a; Siegel et al., 2024).
Nursing Implications in Oncologic Emergencies
Early recognition and prompt intervention of oncologic emergencies are critical to patients' quality of life and survival. The symptoms of oncologic emergencies may be obvious or subtle in presentation and potentially overlooked, contributing to increased morbidity and mortality. Oncology nurses are vital to improving patient outcomes when an oncologic emergency occurs to limit devastating functional losses, preserve the quality of life, and thwart progression to a life-threatening emergency. Eight of the most common oncologic emergencies are outlined below in Table 14 (Maloney-Newton et al., 2023).
Table 14
Oncologic Emergencies
Hypercalcemia of Malignancy (HCM) |
|
Spinal Cord Compression (SCC) |
|
Cardiac Tamponade |
|
Superior Vena Cava Syndrome (SVCS) |
|
Disseminated Intravascular Coagulation (DIC) |
|
Tumor Lysis Syndrome (TLS) |
|
Syndrome of Inappropriate Antidiuretic Hormone (SIADH) |
|
Febrile Neutropenia |
|
(Brydges, & Brydges, 2021; Maloney-Newton et al., 2023; Rothberg et al., 2022; Siegel et al., 2024)
Survivorship Care
According to the ACS (2022), the term “cancer survivor” refers to any person with a history of cancer from the time of diagnosis through the remainder of their life. There are an estimated 18 million cancer survivors in the United States as of January 1, 2022, and these numbers are projected to increase to over 26 million by 2040. Currently, about two-thirds of survivors are 65 years or older, and an estimated 73% of survivors in the United States will be 65 years or older by 2040. With a growing population of cancer survivors, nurses with specialized oncologic education and training are at the height of current and projected nursing demands (ACS, 2022b).
There has been a national target toward ensuring survivorship care planning becomes the standard of care since the Institute of Medicine (IOM)'s 2006 report entitled From Cancer Patient to Cancer Survivor: Lost in Transition. The report exposed the unmet needs of the growing population of cancer survivors, endorsing a call to action for every survivor to receive an individualized post-treatment care plan (IOM, 2006). In collaboration with the Department of Veterans Affairs (VA) and other Health and Human Services agencies, the NCI (n.d.) has developed the National Standards for Cancer Survivorship Care. These standards aim to enhance the quality of care for cancer survivors by outlining essential health system policies and processes, as well as methods for evaluating care quality. The initiative addresses the need for consistent, comprehensive survivorship care across various health care settings. The standards are designed to guide health systems in assessing and improving their survivorship programs, ensuring that survivors receive coordinated and effective care throughout their journey (ACS, 2022b).
The goals of cancer survivorship focus on the prevention of recurrent and new cancers, surveillance for cancer spread or recurrence; assessment of late medical and psychological effects of therapy, adherence, and interventions for consequences of cancer and its treatment; and coordination between oncologists, specialty providers, and primary care physicians. Composed of guidelines for monitoring and maintaining health, survivorship care planning strives to improve the quality of care and long-term outcomes of survivors. These recommendations reinforce the need for survivors to maintain a healthy weight, consume a well-balanced diet, and engage in regular physical exercise, regardless of tumor type. Cancer survivorship literature demonstrates that physical inactivity, poor nutrition, and an elevated BMI are the most critical risk factors (aside from tobacco use) for cancer recurrence, morbidity, and premature death after curative treatment (ACS, 2020a, 2022; NCI, n.d.).
Oncology nurses are uniquely positioned to use the diagnosis and treatment of cancer as an opportunity to educate patients on cancer survivorship, initiate healthy lifestyle counseling, and offer guidance on interventions to prevent cancer. Many patients have difficulty adjusting to life after cancer treatment, as cancer can cause long-term physical and psychological consequences. Nurses should provide education regarding the signs and symptoms of cancer recurrence, identification of late side effects, and information on adopting healthier lifestyles. In addition, adolescents and young adult cancer survivors are prone to developing second cancers, especially lung cancer. Therefore, patients must be educated on the risk of secondary malignancies to ensure they receive adequate surveillance and screening long-term (Palmer et al., 2021). Nurses help coordinate referrals to specialists and ensure each patient's primary care physician receives a copy of their survivorship care plan to promote continuity of care within the health care community. Despite the successes in cancer treatment, providers must also address the needs of survivors who experience the deleterious consequences of cancer treatment and help them restore their health as they transition to survivorship (ACS, 2020a; 2022; NCI, n.d.).
Refer to the Cancer Survivorship NursingCE course for more information on this topic.
End-of-Life (EOL) Issues in Cancer Care
Palliative cancer care addresses each person as a whole, striving to promote quality of life and relieve suffering throughout the disease trajectory, not just at the end of life (EOL). Palliative care should be a standard component of cancer care for all patients. Best practice guidelines recommend that palliative care services be initiated at diagnosis and used throughout treatment. The goals are to prevent or manage cancer symptoms and side effects of treatment, provide comfort, and preserve the quality of life. Palliative care also addresses cancer's psychological, social, and spiritual complications. Whereas palliative can begin at any point along the cancer continuum, hospice care starts toward the EOL when curative treatment is no longer the goal. Curative treatments are withdrawn, and the focus shifts to promoting comfort through the EOL to support a comfortable, peaceful, and pain-free dying process. Hospice eligibility begins when life expectancy is 6 months or fewer. Despite the distinctions between these care domains, they also overlap in a few ways. Palliative care helps patients develop care goals and advanced directives. Palliative care teams support patients and families as they learn to cope with different concerns and emotional issues in considering a hospice transition. Early referrals of patients to palliative care and hospice improve symptoms, quality of life, and survival (Chen et al., 2024; NCI, 2024d; Nettina & Nelson-Tuttle, 2024).
EOL issues should be addressed early in the disease trajectory, readdressed as the patient's clinical status changes, and concentrated on their care goals. A prospective, longitudinal study by Chen and colleagues (2024) examines how palliative care consultations influence the quality of EOL care for patients with terminal cancer. The study included 174 cancer patients divided into two groups: 65 patients received palliative care consultations, and 109 patients did not. Researchers evaluated EOL quality using the Quality of Dying and Death (QODD) scale at two-time points within the last 6 months of life. The findings indicated that patients who had palliative care consultations experienced significantly higher overall QODD scores, particularly in areas related to preparation for death and honoring treatment preferences. The study suggests integrating palliative care services can improve patients' perceptions of their EOL experience. It highlights the importance of early referrals and tailored interventions to enhance the quality of EOL care (Chen et al., 2024).
Oncology nurses are essential drivers of these conversations, which should be avoided during life-threatening events when the patient and family feel distressed and pressured to decide. As advocates, nurses encourage patients to express their preferences regarding EOL care to their medical team and family. Appropriate legal documents, such as advance directives, medical orders for life-sustaining treatment, health care proxy, and durable power of attorney, should support wishes. Nurses are critical in educating patients and families about these vital decisions, explaining options, ensuring decisions align with care goals, and assisting patients and families with making decisions about withholding or withdrawing life-sustaining therapies. While preparing for EOL and making decisions about treatment and preferences can be challenging and distressing for patients and their families, failing to plan for these events can be even more devastating. Patients without predetermined plans for a transition to EOL endure increased psychological distress and are routinely subjected to medical treatments incongruent with their preferences. In addition, families and caregivers experience heightened emotional pain accompanying the decision-making process and endure a more difficult bereavement period. As a result, the cost of care and utilization of burdensome and expensive health care services and resources offering minimal therapeutic benefits are also heightened. Each state outlines how individual rights determining one’s health care treatment are guaranteed. In addition, nurses serve fundamental roles in ensuring symptoms are managed, connecting patients to necessary resources, and implementing measures that promote quality of life. Nurses make referrals for respite care, counseling, pastoral care, and bereavement services (Chen et al., 2024; NCI, 2024d; Nettina & Nelson-Tuttle, 2024).
Oncology nurses have several ethical, legal, and professional responsibilities. They must ensure patient safety, protect patients from harm, verify hazardous medications for errors, and adhere to the guidelines for safe handling, delivery, and disposal of cytotoxic drugs. Nurses have a right to feel competent in their roles and the procedures delineated by their employing organization. They are ethically and morally bound by their position in advocacy, protecting patients' rights, and ensuring patients have a voice and are educated to make informed decisions. Oncology nurses are commonly faced with moral dilemmas and endure personal distress in this challenging field. Regardless of their personal beliefs, they must support patient choices and uphold patient wishes, even when family members disagree. Finally, oncology nurses are champions for patients and families opting to forgo further treatment; oncology nurses promote the decision to transition to hospice as courageous, not weak. Continuing education is essential for nurses to remain current in their knowledge of evidence-based practice, reduce legal liability, and provide high-quality care. Oncology nurses face various legal and ethical dilemmas when delivering care, particularly with evolving technological advancements, a high emphasis on innovative devices and electronic health records and changing state and federal laws. Ethical dilemmas may include medical treatment that extends life without considering its quality, disparities in care goals between patients and their families, fertility preservation, withdrawing care at the end of life, and medical futility. Most hospitals have ethics committees and ethics consultation services to assist staff, patients, and families in navigating these challenging scenarios. Ethical principles can guide oncology nurses in complicated clinical dilemmas (NCI, 2024d; Maloney-Newton et al., 2023; Nettina & Nelson-Tuttle, 2024).
Refer to the Palliative Care for RNs and LPNs and End-of-Life Care and Pain Management (with Ethical Issues) NursingCE courses for more information on these topics.
The Financial Burden of Cancer Care
Beyond the sweeping life impacts of a cancer diagnosis, the high cost of treatment can be equally catastrophic for patients and their families. As novel agents and new oral drugs become available, the price increases. One of the most significant barriers to successful cancer treatment today is not the proper treatment but the patient's access to it: Access is limited by cost. Oral therapies offer several advantages over traditional intravenous therapies. They should be less expensive due to the lower cost of self-administration of the medication, yet the opposite is true. Oral cancer drugs are costly and may not be covered entirely by insurance and prescription plans. The financial burden is among the most common reasons for non-compliance with oral medications. Most private insurers consider oral anti-cancer medications to be a prescription drug benefit, using a tiered structure that increases each patient's cost-sharing responsibility as the price of the medication increases. As a result, patients on oral anti-cancer treatment face high out-of-pocket costs and often need to decide between financial ruin and continued treatment. The economic burden of oral cancer treatment leads to delays in treatment initiation, contributes to non-compliance, and provokes premature discontinuation of therapy. These have negative consequences on treatment benefits, quality of life, and survival. Studies show that many patients opt to forego treatment altogether due to the substantial cost burden and inaccessibility (Iragorri et al., 2021; NCI, 2024c).
A systematic literature review by Iragorri and colleagues (2021) explores the financial strain that cancer patients experience due to out-of-pocket (OOP) health care costs. It identifies that these expenses vary depending on factors such as cancer type, patient demographics, and geographic location. As described in the article, individuals with breast or colorectal cancer may spend an estimated $200 per month, whereas families of pediatric cancer patients often face significantly higher costs, averaging $800 monthly. In the United States, cancer medications alone cost an average of $288 per month. The study further highlights that OOP costs account for a significant proportion of patients' annual income—42% in low- and middle-income countries and 16% in high-income nations. The researchers emphasize the urgent need for health care systems to expand coverage for both medical and non-medical expenses to reduce the financial burden on patients and improve access to care (Iragorri et al., 2021).
Oncology nurses should encourage patients to speak to their healthcare providers if they have difficulty affording their medication before stopping. Some manufacturers offer copay assistance programs or have grants to fund lower-cost or free drugs (e.g., compassionate use programs or need-based cost programs). In addition, some states have passed laws that require insurance companies to cover oral cancer medications as they would cover other treatments. Oncology nurses should help patients fight high medical costs by connecting them with available resources. The Association of Community Cancer Centers (ACCC, n.d.) maintains the Patient Assistance and Reimbursement Guide on its website. This is a digital resource providing the latest information on financial support programs for cancer medications. It helps health care professionals and patients navigate assistance options to ease the cost of cancer treatment. Users can search by product or manufacturer and apply filters to refine results. Each listing includes program details, eligibility requirements, application instructions, and direct links to external resources and contact information. Oncology nurses should provide patients with reputable and reliable financial resources to help them receive the medicines they need and reduce some of the financial toxicity of cancer. These resources extend beyond co-pay assistance and include drug discount cards, rebates, patient advocate programs, aid with housing expenses, and even electric bills for those in need who are actively undergoing cancer treatment (Iragorri et al., 2021; NCI, 2024c).
Refer to the Financial Toxicity in Cancer Care NursingCE course for more information.
References
American Cancer Society. (2019). How chemotherapy drugs work. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/chemotherapy/how-chemotherapy-drugs-work.html
American Cancer Society. (2020a). American Cancer Society guideline for diet and physical activity for cancer prevention. CA: A Cancer Journal for Clinicians, 70(4), 245–271. https://doi.org/10.3322/caac.21591
American Cancer Society. (2020b). Diet and physical activity: What’s the cancer connection? https://www.cancer.org/cancer/risk-prevention/diet-physical-activity/diet-and-physical-activity.html
American Cancer Society. (2020c). How cancer and cancer treatment can affect sexuality. https://www.cancer.org/cancer/managing-cancer/side-effects/fertility-and-sexual-side-effects/how-cancer-affects-sexuality.html
American Cancer Society. (2020d). Signs and symptoms of cancer. https://www.cancer.org/treatment/understanding-your-diagnosis/signs-and-symptoms-of-cancer.html
American Cancer Society. (2022a). Cancer facts & figures for African American/Black people 2022–2024. https://www.cancer.org/research/cancer-facts-statistics/cancer-facts-figures-for-african-americans.html
American Cancer Society. (2022b). Cancer treatment & survivorship facts & figures (2022–2024). https://www.cancer.org/research/cancer-facts-statistics/survivor-facts-figures.html
American Cancer Society. (2023a). American Cancer Society guidelines for the early detection of cancer. https://www.cancer.org/healthy/find-cancer-early/american-cancer-society-guidelines-for-the-early-detection-of-cancer.html
American Cancer Society. (2023b). American Cancer Society recommendations for the early detection of breast cancer. https://www.cancer.org/cancer/types/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html
American Cancer Society. (2023c). Viruses that can lead to cancer. https://www.cancer.org/cancer/risk-prevention/infections/infections-that-can-lead-to-cancer/viruses.html
American Cancer Society. (2024). CAR T-cell therapy and its side effects.
https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/car-t-cell1.html
American Cancer Society. (2025a). Cancer facts & figures 2025. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2025-cancer-facts-figures.html
American Cancer Society. (2025b). Cancer statistics center: 2025 estimates. https://cancerstatisticscenter.cancer.org/
American Cancer Society. (2025c). Key statistics for endometrial cancer. https://www.cancer.org/cancer/types/endometrial-cancer/about/key-statistics.html
American Cancer Society. (2025d). Monoclonal antibodies and their side effects. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/monoclonal-antibodies.html
Amjad, M. T., Chidharla, A., & Kasi, A. (2023). Cancer chemotherapy. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK564367/
Association of Community Cancer Centers. (n.d.). Patient assistance & reimbursement guide. Retrieved February 6, 2025, from https://www.accc-cancer.org/home/learn/financial-advocacy/patient-assistance-guide
Backler, C. (2020). STAT: Anaphylaxis. Clinical Journal of Oncology Nursing, 24(3). https://doi.org/10.1188/20.CJON.336
Baer, B. (2021). CAR T-cell therapy. Clinical Journal of Oncology Nursing, 25(3), 255–258. https://doi.org/10.1188/21.CJON.255-258
Belcher, S. M., Mackler, E., Muluneh, B., Ginex, P., Anderson, M. K., Bettencourt, E., DasGupta, R. K., Elliott, J., Hall, E., Karlin, M., Kostoff, D., Marshall, V. K., Millisor, V. E., Molnar, M., Schneider, S. M., Tipton, J., Yackzan. S., LeFebvre, K. B., Sivakumaran, K., Waseem, H., & Morgan, R. L. (2022). ONS guidelines to support patient adherence to oral anticancer medications. ONF, 49(4), 279–295. https://doi.org/10.1188/22.ONF.279-295
Bodelon, C., Sung, H., Mitchell, E. L., Deubler, E. L., Newton, C. C., Jemal, A., Teras, L. R., & Patel, A. V. (2024). Excess body weight and the risk of second primary cancers among cancer survivors. JAMA Network Open, 7(9), e2433132. https://doi.org/10.1001/jamanetworkopen.2024.33132
Brydges, N., & Brydges, G. J. (2021). Oncologic emergencies. AACN Advanced Critical Care, 32(3), 306–314. https://doi.org/10.4037/aacnacc2021832
Centers for Disease Control and Prevention. (2024a). Burden of cigarette use in the U.S. https://www.cdc.gov/tobacco/campaign/tips/resources/data/cigarette-smoking-in-united-states.html
Centers for Disease Control and Prevention. (2024b). HPV vaccination. https://www.cdc.gov/hpv/vaccines/index.html
Centers for Disease Control and Prevention. (2024c). Managing risk for hereditary breast, ovarian, and related cancers. https://www.cdc.gov/breast-ovarian-cancer-hereditary/managing-risk/index.html
Centers for Disease Control and Prevention. (2025). Obesity and cancer. https://www.cdc.gov/cancer/risk-factors/obesity.html
Chen, S. T., Chen, S. C., Lee, H. S., & Chen, C. H. (2024). Associations between palliative-care consultations and end-of-life quality in cancer patients’ last 6 months. Support Care Cancer, 32(9), 606. https://doi.org/10.1007/s00520-024-08814-7
Degrande, F. A., Marta, G. N., Alves, T. M., Ferreira, G. B., Dumaszak, F. V., Carvalho, H. A., & Hanna, S. A. (2023). Deep inspiration breath hold: Dosimetric benefits to decrease cardiac dose during postoperative radiation therapy for breast cancer patients. Rep Pract Oncol Radiother, 28(2), 172–180. https://doi.org/10.5603/RPOR.a2023.0027
Dickman, E. (2020). STAT: Cytokine release syndrome. Clinical Journal of Oncology Nursing, 24(4). https://doi.org/10.1188/20.CJON.456
Greer, J. A., Applebaum, A. J., Jacobsen, J. C., Temel, J. S., & Jackson, V. A. (2020). Understanding and addressing the role of coping in palliative care for patients with advanced cancer. Journal of Clinical Oncology, 38(9). https://doi.org/10.1200/JCO.19.00013
Huff, C. (2020). Oral chemotherapy: A home safety educational framework for healthcare providers, patients, and caregivers. Clinical Journal of Oncology Nursing, 24(1), 22–30. https://doi.org/10.1188/20.CJON.22-30
Institute of Medicine and National Research Council. (2006). From cancer patient to cancer survivor: Lost in transition. The National Academies Press. https://doi.org/10.17226/11468
Iragorri, N., de Oliveira, C., Fitzgerald, N., & Essue, B. (2021). The out-of-pocket cost burden of cancer care: A systematic literature review. Curr Oncol, 28(2), 1216–1248. https://doi.org/10.3390/curroncol28020117
Katz, A., Agrawal, L. S., & Sirohi, B. (2022). Sexuality after cancer as an unmet need: Addressing disparities, achieving equality. 42, 1–7. https://doi.org/10.1200/EDBK_100032
Khan, S. U., Fatima, K., Aisha, S., & Malik, F. (2024). Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun Signal, 22(1), 109. https://doi.org/10.1186/s12964-023-01302-1
Khanna, L., Prasad, S. R., Yedururi, S., Parameswaran, A. M., Marcal, L. P., Sandrasegaran, K., Tirumani, S. H., Menias, C. O., & Katabathina, V. S. (2021). Second malignancies are radiation therapy: Update on pathogenesis and cross-sectional imaging findings. RadioGraphics, 41(3). https://doi.org/10.1148/rg.2021200171
Khongorzul, P., Ling, C. J., Khan, F. U., Ihsan, A. U., & Zhang, J. (2020). Antibody-drug conjugate: A comprehensive review. Molecular Cancer Research, 18(1), 3–19. https://doi.org/10.1158/1541-7786.MCR-19-0582
Kokts-Porietis, R. L., Elmrayed, S., Brenner, D. R., & Friedenreich, C. M. (2021). Obesity and mortality among endometrial cancer survivors: A systematic review and meta-analysis. Obesity Reviews, 22(12). https://doi.org/10.1111/obr.13337
Li, J., Peng, F., Huang, H., & Cai, Z. (2022). Trends in the risk of second primary malignancies after non-Hodgkin’s lymphoma. American Journal of Cancer Research, 12(6), 2863–2875. https://pmc.ncbi.nlm.nih.gov/articles/PMC9251676/
Liu, Z., Chen, H., Zheng, L., Sun, L., & Shi, L. (2023). Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduction and Targeted Therapy, 8(198). 1–39. https://doi.org/10.1038/s41392-023-01460-1
Longo, D. L. (2019). Harrison’s hematology and oncology (3rd ed.). McGraw-Hill Education.
Maani, E. V., & Maani, C. V. (2022). Radiation therapy. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK537036/
Maloney-Newton, S., Hickey, M., & Brandt, J. M. (2023). Mosby’s oncology nursing advisor (3rd ed.). Elsevier
McQuestion, M., Drapek, L. C., & Witt, M. E. (2021). Manual for radiation oncology nursing practice and education (5th ed.). Oncology Nursing Society
Miller, K. D., Nogueira, L., Devasia, T., Mariotto, A. B., Yabroff, K. R., Jemal, A., Kramer, J., & Siegel, R. L. (2022). Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin, 72(5), 409–436. https://doi.org/10.3322/caac.21731
Morris, E. C., Neelapu, S., Giavridis, T., & Sadelain, M. (2021). Cytokine release syndrome and associated neurotoxicity in cancer immunity therapy. Nature Reviews Immunology, 22, 85–96. https://doi.org/10.1038/s41577-021-00547-6
National Cancer Institute. (n.d.). National standards for cancer survivorship care. Retrieved February 8, 2025, from https://cancercontrol.cancer.gov/ocs/special-focus-areas/national-standards-cancer-survivorship-care
National Cancer Institute. (2021). What is cancer? https://www.cancer.gov/about-cancer/understanding/what-is-cancer
National Cancer Institute. (2022). Targeted cancer therapies. https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet
National Cancer Institute. (2023a). Immunotherapy side effects. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/side-effects
National Cancer Institute. (2023b). Major categories of chemotherapy agents. https://training.seer.cancer.gov/treatment/chemotherapy/types/major-categories.html
National Cancer Institute. (2023c). Tumor markers. https://www.cancer.gov/about-cancer/diagnosis-staging/diagnosis/tumor-markers-fact-sheet
National Cancer Institute. (2024a). BRCA gene changes: Cancer risk and genetic testing. https://www.cancer.gov/about-cancer/causes-prevention/genetics/brca-fact-sheet
National Cancer Institute. (2024b). Cancer statistics. https://www.cancer.gov/about-cancer/understanding/statistics
National Cancer Institute. (2024c). Financial toxicity (financial distress) and cancer treatment (PDQ®): Patient version. https://www.cancer.gov/about-cancer/managing-care/track-care-costs/financial-toxicity-pdq
National Cancer Institute. (2024d). Planning the transition to end-of-life care in advanced cancer (PDQ®): Health professional version. https://www.cancer.gov/about-cancer/advanced-cancer/planning/end-of-life-hp-pdq
National Cancer Institute. (2025a). Cancer disparities. https://www.cancer.gov/about-cancer/understanding/disparities
National Cancer Institute. (2025b). Common terminology criteria for adverse events (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm
National Comprehensive Cancer Network. (2025). NCCN guidelines treatment by cancer type. https://www.nccn.org/guidelines/category_1
National Human Genome Research Institute. (2024). Fact sheet: Human genome project. https://www.genome.gov/about-genomics/educational-resources/fact-sheets/human-genome-project
Nettina, S. M., & Nelson-Tuttle, C. (2024). Lippincott manual of nursing practice (12th ed.). Wolters Kluwer.
Nussinov, R., Tsai, C., & Jang, H. (2021). Anticancer drug resistance: An update and perspective. Drug Resistance Updates, 59(100796), 1–12. https://doi.org/10.1016/j.drup.2021.100796
Olsen, M., LeFebvre, K., Walker, S., & Dunphy, E. P. (2023). Chemotherapy and immunotherapy guidelines and recommendations for practice (2nd ed.). Oncology Nursing Society.
Oncology Nursing Society. (2024). Generalist oncology nurse competencies. https://www.ons.org/sites/default/files/2025-01/generalist-oncology-nurse-competencies-1.pdf
Oncology Nursing Society. (2025a). ONS/ONCC chemotherapy immunotherapy certificate course. https://www.ons.org/store/courses/onsoncc-chemotherapy-immunotherapy-certificatetm
Oncology Nursing Society. (2025b). ONS/ONCC radiation therapy certificate course. https://www.ons.org/store/courses/onsoncc-radiation-therapy-certificatetm
Palmer, J. D., Tsang, D. S., Tinkle, C. L., Olch, A. J., Kremer, L. C. M., Ronchkers, C. M., Gibbs, I. C., & Constine, L. S. (2021). Late effects of radiation therapy in pediatric patients and survivorship. Pediatric Blood & Cancer, 68(S2). https://doi.org/10.1002/pbc.28349
Rothberg, B. E., Quest, T. E., Yeung, S. J., Pelosof, L. C., Gerber, D. E., Seltzer, J. A., Bischof, J. J., Thomas, C. R., Akhter, N., Mamtani, M., Stutman, R. E., Baugh, C. W., Anantharaman, V., Pettit, N. R., Klotz, A. D., Gibbs, M. A., & Kyriacou, D. N. (2022). Oncologic emergencies and urgencies: A comprehensive review. CA: A Cancer Journal for Clinicians, 72(6), 570–593. https://doi.org/10.3322/caac.21727
Sagnic, S. (2021). Obesity and endometrial cancer. In V. Rao, & L. Rao (Eds.), Role of obesity in human health and disease. IntechOpen. https://doi.org/10.5772/intechopen.99827
Salvaris, R., Ong, J., & Gregory, G. P. (2021). Bispecific antibodies: A review of development, clinical efficacy and toxicity in B-cell lymphomas. Journal of Personalized Medicine, 11(5), 355. https://doi.org/10.3390/jpm11050355
Santomasso, B. D., Nastoupil, L. J., Adkins, S., Lacchetti, C., Schneider, B. J., Anadkat, M., Atkins, m. B., Brassil, K. J., Caterino, J. M., Chau, I., Davies, M. J., Ernstoff, M. S., Fecher, L., Funchain, P., Jaiyesimi, I., Mammen, J. S., Naidoo, J., Naing, A., Phillips, T., Porter, L. D., Reichner, C.A., Seigel, C., … & Ghosh, M. (2021). Management of immune-related adverse events in patients treated with chimeric antigen receptor t-cell therapy: ASCO guideline. Journal of Clinical Oncology, 39(35), 3978–3992. https://doi.org/10.1200/JCO.21.01992
Schneider, B. J., Naidoo, J., Santomasso, B. D., Lacchetti, C., Adkins, S., Anadkat, M., Atkins, M. B., Brassil, K. J., Caterino, J. M., Chau, I., Davies, M. J., Ernstoff, M. S., Fecher, L., Ghosh, M., Jaiyesimi, I., Mammen, J. S., Naing, A., Nastoupil, L., & Bollin, K. (2021). Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. Journal of Clinical Oncology, 39(36), 4073–4137. https://doi.org/10.1200/JCO.21.01440
Siegel, R. D., LeFebvre, K. B., Temin, S., Barbarotta, L., Bowman, R. M., Chan, A., Dougherty, D. W., Ganio, M., Hunter, B., Klein, M., Miller, T. P., Mulvey, T. M., Ouzts, A., Polovich, M., Salazar-Abshire, M., Stenstrup, E. Z., Sydenstricker, C. M., Tsai, S., & Olsen, M. M. (2024). Antineoplastic therapy administration safety standards for adult and pediatric oncology: ASCO-ONS Standards. ONF, 51(4), 297–320. https://doi.org/10.1188/24.ONF.297-320
Smrz, S. A., Calo, C., Fisher, J. L., & Salani, R. (2021). An ecological evaluation of the increasing incidence of endometrial cancer and the obesity epidemic. American Journal of Obstetrics & Gynecology, 224(5). https://doi.org/10.1016/j.ajog.2020.10.042
Taylor, J., Ruggerio, M., Maity, A., Ko, K., Greenberger, B., Donofree, D., Sherif, K., Lazar, M., Jaslow, R., Richard, S., Mitchell, E., Anne, P. R., Trabulsi, E., Leader, A., & Simone, N. L. (2020). Sexual health toxicity in cancer survivors: Is there a gender disparity in physician evaluation and intervention? International Journal of Radiation Oncology, 108(3), S136. 10.1016/j.ijrobp.2020.07.872
Thomson, R. J., Moshirfar, M., & Ronquillo, Y. (2023). Tyrosine kinase inhibitors. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK563322/
Tsao, L., Force, J., & Hartman, Z. C. (2021). Mechanisms of therapeutic antitumor monoclonal antibodies. Cancer Research, 81(18), 4641–4651. https://doi.org/10.1158/0008-5472.CAN-21-1109
Xiao, X., Huang, S., Chen, S., Wang, Y., Sun, Q., Xu, X., & Li, Y. (2021). Mechanisms of cytokine release syndrome and neurotoxicity of CAR T-cell therapy and associated prevention and management strategies. J Exp Clin Cancer Res, 18(40), 367. https://doi.org/10.1186/s13046-021-02148-6
Powered by Froala Editor
Single Course Cost: $38.00
Add to CartSave while Futhering your Education
Oncology Nursing CE course is offered in the packages below.- 69 ANCC Hours
- 23 Courses
- 537 ANCC Hours
- 194 Courses