About this course:
This learning module reviews relevant terminology and explores current research on best practices and related wound care for pressure injuries (PIs) caused by medical devices or hospitalization.
Course preview
Pressure Injuries from Medical Devices and Hospitalization
This learning module reviews relevant terminology and explores current research on best practices and related wound care for pressure injuries (PIs) caused by medical devices or hospitalization.
After this learning module, the learner will be prepared to:
- define the incidence and prevalence of PIs from hospitalization
- review the pathophysiology of the skin related to the incidence of PIs
- understand PI risk from medical device use
- discuss the impact of hospitalization on PI risk
- explore national patient safety goals related to PIs
- describe the management of PIs based on current clinical guidelines
Epidemiology and Statistics
PIs are localized damaged skin and soft tissue areas caused by unrelieved pressure, friction, shearing, and moisture. They often result from immobility or the use of a medical device. Despite the nationwide focus on reducing their incidence, PIs continue to be a significant problem in acute care hospitals and long-term care facilities. Each year, more than 2.5 million patients in the U.S. are affected by hospital-acquired PIs (HAPIs), formerly described as pressure ulcers or pressure sores, resulting in 60,000 deaths. In the U.S., it is estimated that treating a single PI can cost between $20,000 up to $150,000, resulting in costs associated with HAPIs up to $26.8 billion in the U.S. (National Pressure Injury Advisory Panel [NPIAP], n.d.-a; Sim et al., 2024; Tomlinson et al., 2024; Wassel et al., 2020). A HAPI can result in significant patient harm, leading to costly treatments, increased pain, extended hospitalization, and increased morbidity and mortality. Overall estimated incidence rates of HAPIs vary from 5% to 15%, with the occurrence rate in intensive care units (ICUs) being the highest. The International Pressure Ulcer Prevalence Survey reported that rates had declined slightly between 2006 and 2015 but have plateaued since that time. True prevalence is difficult to estimate, possibly due to post-discharge PIs that develop due to shorter hospital stays. The Incidence of HAPIs remains high without any significant, notable improvement (Berlowitz, 2024; Mondragon & Zito, 2024; Siotos et al., 2022; The Joint Commission [TJC], 2022; Tomlinson et al., 2024).
The Centers for Medicare and Medicaid Services (CMS) does not provide reimbursement for HAPIs. CMS has deemed stage III and IV PIs preventable and classifies them as never events. In addition to refusing payment or reimbursement for the treatment of these PIs, CMS can also penalize facilities with a 1% reduction in overall reimbursement for hospitals whose rate falls into the bottom 25% of hospitals nationwide (Agency for Healthcare Research and Quality [AHRQ], 2014; Berlowitz, 2023a; CMS, 2024; Mondragon & Zito, 2024).
Anatomy and Physiology of the Skin
The skin is the largest human organ, accounting for approximately 20% of the total body weight. The skin's primary function is to protect the internal organs and structures from biological invasion, ultraviolet radiation, fluid loss, and physical damage. Additional skin functions are providing thermoregulation through sweating and blood flow regulation, synthesizing vitamin D, relaying sensation from nerve endings, excreting salts and small amounts of waste products, and providing aesthetics and communication. The skin comprises the epidermis (i.e., outer layer), the dermis (i.e., middle layer), and the hypodermis (i.e., the deepest or subcutaneous layer; see Figure 1). The skin's health influences overall health and has a profound psychological significance because it identifies the individual with unique facial and body characteristics. Self-image may be enhanced or deterred by society's standards for one's appearance (Rogers & Brashers, 2023).
Figure 1
Structure of the Human Skin
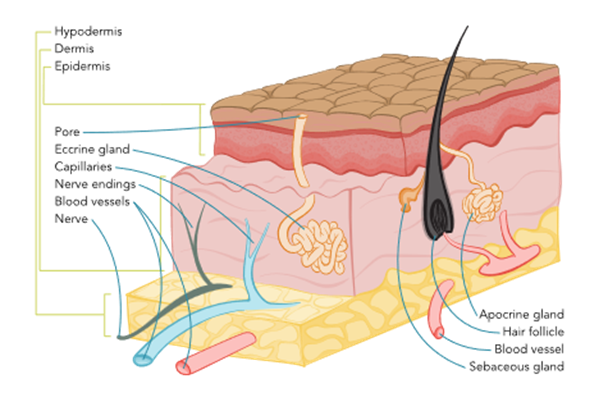
The epidermis acts as a defensive barrier that constantly renews itself by shedding the superficial layer (stratum corneum). It is composed primarily of keratinocytes embedded in a lipid matrix. The epidermis is slightly acidic, with a pH of 4.5 to 6 and comprises five layers: the stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale. Each layer has a specific function and plays a role in the healing process of wounds (Rogers & Brashers, 2023).
The stratum corneum is composed of tough superficial sheets of cornified cells. This layer is also waterproof. Forming the outermost layer, it consists of fibrous dead cells that help regulate pH and temperature and provide a protective role. It is very thin, measuring only 0.05 mm on the eyelids and 1.5 mm on the soles of the feet and palms of the hands, but it can become thicker with frequent pressure or friction (e.g., calluses or corns). This layer is continuously being replaced and helps with the skin's ability to repair itself. The stratum lucidum consists of layers of cells containing eleidin, which become keratin as cells move up to the stratum corneum layer. The stratum lucidum is only found in palmoplantar skin (on the soles of the feet and palms of the hands) to provide extra protection since these areas are exposed to more significant deterioration. The stratum granulosum is the layer where keratinocytes lose their nuclei, flatten, and die. Keratinization (i.e., the increased production of the protein keratin) occurs in this layer and helps reduce water loss from the epidermis. The stratum spinosum is 8 to 10 cells thick and contains living cells with spiny processes called desmosomes. This layer also contains Langerhans cells (a type of dendritic cell), which can initiate an immune response. The stratum basale (basal layer) is also known as the basement membrane and is the lowest layer of the epidermis. This layer is one cell thick and forms a border between the epidermis and dermis. It constantly makes new keratinocytes that move up to the surface, flattening during the process and replacing cells shed from the stratum corneum. This process takes between 28 and 35 days. Cells are continuously dividing for the ongoing rejuvenation of the skin. Melanocytes are also produced in the stratum basale layer (Rogers & Brashers, 2023).
The dermis is the layer below the epidermis. It is 1 to 4 mm thick and composed of different types of connective tissue: collagen, elastin, reticulin, and a gel-like ground substance. The primary role of the dermis is to support and provide nutrition to the epidermis. This layer consists primarily of collagen, a tough, fibrous protein that helps skin resist tearing. The dermis is resilient and elastic, allowing the skin to stretch with body movement. The dermis houses the nerves, sensory receptors, blood vessels, lymphatics, hair follicles, sebaceous glands, and sweat glands (Rogers & Brashers, 2023).
The third layer of skin is the hypodermis or subcutaneous tissue. This layer connects the dermis to the underlying muscle. This layer comprises blood vessels, adipose tissue, and connective tissue that support the dermis. Macrophages, fibroblasts, nerves, and hair follicle roots are also found within this layer. The fat stored in this layer provides internal structures with additional protection and insulation against the cold (Rogers & Brashers, 2023).
Pathophysiology
PIs are formed when the pressure applied to the skin and soft tissues is above a certain threshold for a...
...purchase below to continue the course
Figure 2
Placement of Pressure Injuries
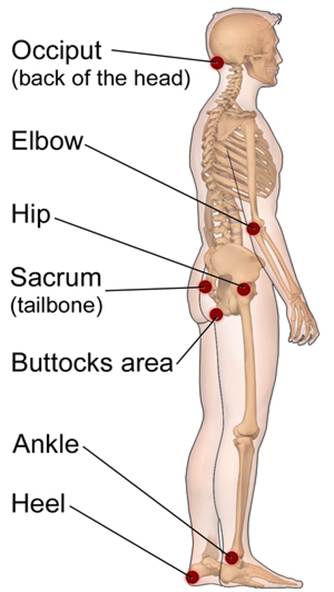
While the skin is visible during an inspection, the underlying muscle tissue is damaged first in PIs. Muscle tissue requires increased oxygen and nutrients when compared to surface tissues. Only two hours of continuous pressure can damage the underlying muscle and tissue, creating a wound in the shape of an inverted cone from the surface down to the muscle. Individuals with darker-pigmented skin are at a higher risk of PIs due to less visible early signs of skin damage (Kirman, 2024; Rogers & Brashers, 2023).
Staging Pressure Injuries
The NPIAP updated its staging system for PIs in 2016 (see Figure 3 and Table 1). The system includes four main stages of PI, ranging from I to IV. PIs can only be numerically staged if the tissue injury can be visualized or palpated. The NPIAP added unstageable pressure and deep tissue injuries to describe injuries that cannot be visualized due to slough, eschar, or color changes. Once deep tissue injuries evolve or an unstageable PI is debrided, the injury can be staged on the numeric scale. The stages are not considered progressive (either backward or forward), and downstaging is not recommended. The system is used to describe a wound at a specific time and is meant to be used as a communication tool among healthcare providers and various disciplines to drive the plan of care (NPIAP, 2019).
Figure 3
Stages of Pressure Injuries
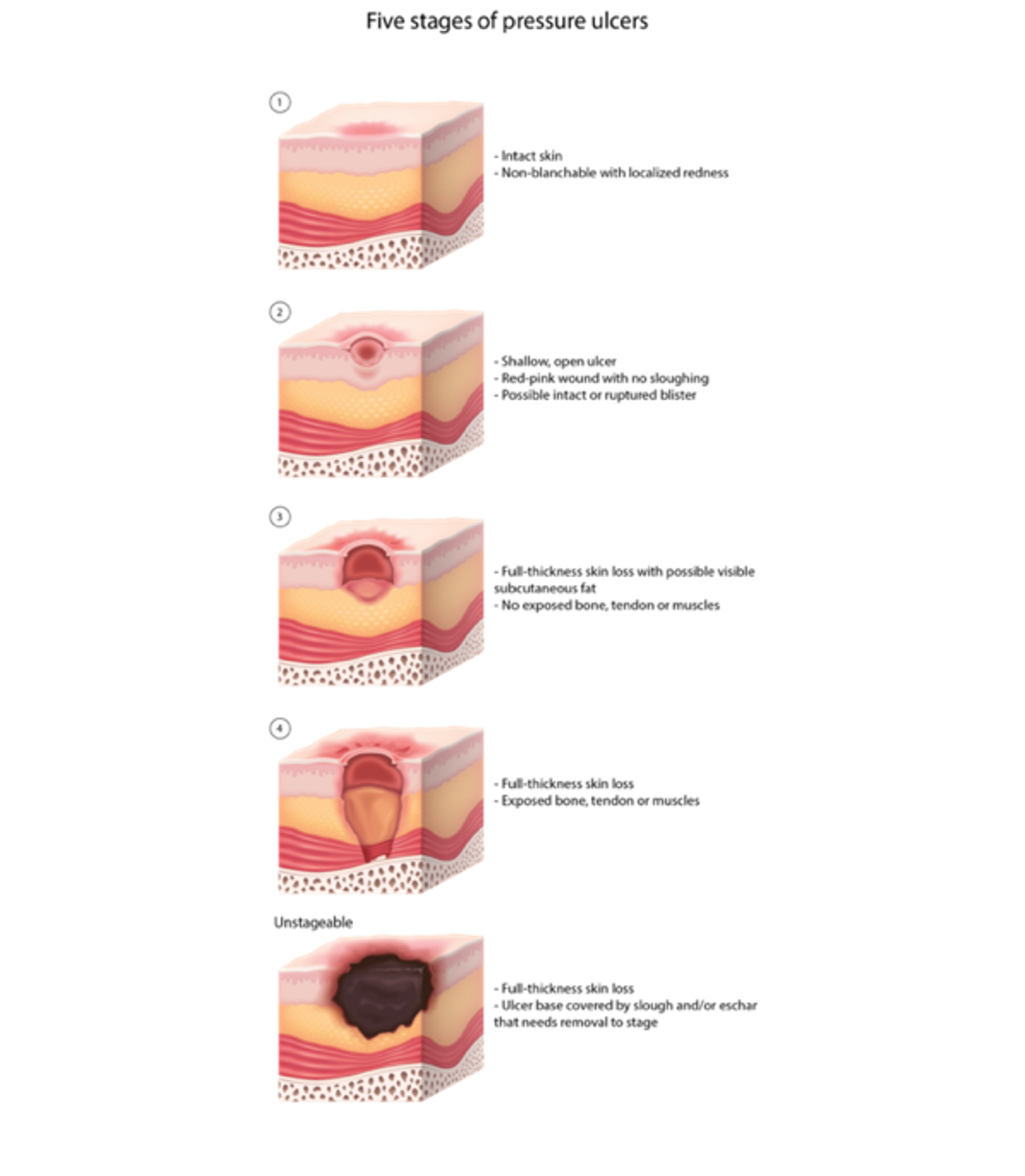
Table 1
Pressure Injury Staging
Classification | Description |
Stage I PI: non-blanchable erythema | These injuries are characterized by intact skin with a localized area of non-blanchable erythema that may appear differently in darkly pigmented skin. Erythema is localized usually over a bony prominence. Changes in sensation, temperature, or firmness may precede visual changes. The area may be painful. Color changes do not include purple or maroon discoloration; these may indicate deep tissue PI. |
Stage II PI: partial-thickness skin loss | These partial-thickness wounds contain exposed dermis. The wound bed is viable, pink or red, and moist. This type of wound may also present as an intact or ruptured serum-filled blister. Adipose (fat) and deeper tissues are not visible. Granulation tissue, slough, and eschar are not present. These injuries commonly result from adverse microclimate and shear in the skin over the pelvis or behind the heel. This stage should not be used to describe moisture-associated skin damage (MASD), including incontinence-associated dermatitis (IAD), intertriginous dermatitis (ITD), medical adhesive-related skin injury (MARSI), or traumatic wounds (skin tears, burns, and abrasions). |
Stage III PI: full-thickness skin loss | In these injuries, full-thickness loss of skin, adipose (fat) is visible in the wound, and granulation tissue and epibole (rolled wound edges) are often present. Slough or eschar may be visible. The depth of tissue damage varies by anatomical location; areas of significant adiposity can develop deep wounds. Undermining and tunneling can occur. Fascia, muscle, tendon, ligament, cartilage, or bone are not exposed. If slough or eschar obscures the extent of tissue loss, the wound would be classified as an unstageable PI. |
Stage IV PI: full-thickness tissue loss | This wound involves full-thickness skin and tissue loss with exposed or palpable muscle, tendon, cartilage, or bone. Slough or eschar may be visible on some parts of the wound bed. If slough or eschar obscures the extent of tissue loss, the wound is classified as an unstageable PI. Epibole, undermining, and tunneling often occurs. Depth varies by anatomical location. The damage can extend into the fascia or joint capsule, increasing osteomyelitis risk. |
Unstageable | This type of wound involves full-thickness skin and tissue loss in which the extent of tissue damage is obscured by slough or eschar. A stage III or IV PI will be revealed if slough or eschar is removed. Stable eschar (i.e., dry, adherent, and intact without erythema or fluctuance) on the heel or ischemic limb should not be softened or removed. |
Suspected deep tissue PI (DTPI) | This wound involves intact skin or a blood-filled blister with a localized area of persistent non-blanchable deep red, maroon, and purple color. Pain and temperature changes often precede skin color changes. Discoloration may appear differently in darkly pigmented skin. This injury results from intense or prolonged pressure and shear forces at the bone-muscle interface. The wound may evolve rapidly to reveal the extent of tissue injury or resolve without tissue loss. Do not use DTPI to describe vascular, traumatic, neuropathic, or dermatologic conditions. |
(NPIAP, 2019)
The NPIAP released Clinical Practice Guidelines in 2019 for use by healthcare professionals (HCPs) globally, which were developed to create evidence-based guidelines relating to the prevention and treatment of PIs. An update to these guidelines is scheduled for release in 2025. Within the guidelines, the staging of medical device-related PIs (MDRPIs) and mucosal membrane PIs is addressed (see Table 2 below; NPIAP, n.d.-a).
Table 2
Extended Staging
Etiology | Staging |
MDRPI | MDRPIs should be staged using the aforementioned NPIAP staging system. |
Mucosal membrane PI | Due to the anatomy and location of mucosal membrane PIs, these wounds cannot be staged. |
(NPIAP, 2019)
Risk Factors
General
The literature has identified over a hundred factors that increase the risk of a HAPI, but the most cited risk is patient immobility. Any patient can develop a HAPI, but their incidence is higher in unique populations, including patients receiving palliative care, those with spinal cord injuries, neonates and children, and individuals in critical condition. HAPIs are also highest in patients in critical care and long-term care settings (NPIAP, 2019). Other leading risk factors include the following:
- Decreased activity level or immobility (e.g. hip fracture, stroke)
- History of HAPI, vasopressor use, anemia, shock, sepsis, or fluid and electrolyte imbalances
- Changes in sensory perception (e.g. spinal cord injury, neuropathy)
- Length of stay and stay in ICU in a health care facility
- Incontinence (urinary or bowel)
- Malnutrition
- Dermatitis associated with moisture
- Smoking
- Cellulitis
- Chronic kidney disease
- Congestive heart failure
- Dementia
- Diabetes mellitus
- Immunodeficiency
- Neoplasm
- Obstructive sleep apnea
- Spinal instability (Kim et al., 2022; NPIAP, 2019; Wassel et al., 2020)
Older adults have the highest rate of PIs. Although advanced age alone is a contributing factor, the risk increases with comorbidities such as decreased mobility and activity level, impaired skin status, decreased oxygenation and perfusion, or increased skin moisture. Patients with mobility limitations, especially those confined to a bed or chair, have a higher risk of PI development (NPIAP, 2019). Available risk tools examine contributing factors, including but not limited to:
- Activity descriptors such as chairfast or bedfast
- Factors affecting mobility, including weakness or paralysis
- Increased friction or shear due to the inability to reposition
- Mobility and activity related to activities of daily living (NPIAP, 2019)
Perfusion and oxygenation directly relate to the risk of developing a PI. Vascular disease, hypertension, diabetes, smoking, and edema cause decreased circulation to the skin and increase the risk of tissue ischemia, leading to injury and reduced healing. Skin moisture is a risk factor for PI development across the literature. While a certain amount of hydration is necessary for skin condition and function, excess moisture affects the skin's mechanical properties and barrier function. Common sources of excess moisture include diaphoresis and fecal or urinary incontinence. Nutritional deficits are associated with altered skin tolerance, morphology of the tissues, physiology, ability for repair, and thermal properties. Each of these factors can increase the risk of PI (NPIAP, 2019). Specific risk factors to assess include:
- Decreased or increased mid-arm circumference
- Food intake descriptors
- Recent weight loss
- Body mass index (BMI) below 18 or above 30
- Malnutrition (NPIAP, 2019)
Decreased sensory perception occurs with spinal cord injuries or other neurological disorders. Like older adults, these individuals are at increased risk when comorbidities exist. Coexisting factors such as immobility and altered pathophysiology increase the likelihood of PIs in these patients. Altered hematological values of particular concern include lymphocytopenia, hypoalbuminemia, anemia, or an elevated C-reactive protein. Additional concerns are urea and electrolyte imbalances. A creatinine level above 1 mg/dL is associated with a higher risk of PI development. An increase or significant decrease in body temperature can lead to an increased risk of injury to the skin as it affects its susceptibility and tolerance. A patient with sustained fever or hypothermia is at an increased risk of developing a HAPI. Poor mental or physical health can also lead to PI (NPIAP, 2019). Risk factors in this category include:
- Extended hospitalizations
- Acute injuries
- Chronic illnesses including AIDS, diabetes, or respiratory conditions
- Chronic wounds
- Medication use
- Mental health conditions, including dementia
- Recent surgery (NPIAP, 2019)
Medical Device Risk Factors
In 2016, the NPIAP redefined PIs and included medical devices as a source of PIs. An MDRPI is a PI that results from using a device designed and applied for diagnostic or therapeutic purposes. Medical devices are an integral part of treating patients in the acute care setting. Nearly all hospitalized patients require at least one medical device, which increases the risk of injury. Patients in the critical care setting have an even higher risk of developing an MDRPI due to the increased number of medical devices needed to treat these patients (Weber et al., 2021). Approximately 30% of all PIs result from medical device use, and 80% of HAPIs are classified as MDRPIs. The wound typically takes on the pattern or shape of the offending medical device and should be staged using the NPIAP staging system (see Table 5; Medline, 2024). Poorly fitting or improperly positioned fixation devices used to secure a medical device can render the patient vulnerable to an MDRPI. Many MDRPIs are included in reports related to the specific device rather than in PI reports; thus, the number of MDRPIs may be far higher than is currently thought. The risk of developing these injuries is more challenging to predict as well. While the available skin assessment tools may be predictive of PIs, they may not be predictive of MDRPIs (Lee & Choi, 2024; Medline, 2024; NPIAP, 2019; Weber et al., 2021).
Like the general risk factors listed above, the risk factors for MDRPIs include impaired sensation, poor perfusion, altered tissue tolerance, poor nutritional status, or moisture under the device (TJC, 2018). Medical devices associated with PIs include, but are not limited to, the following:
- Oxygen delivery systems (nasal cannula, CPAP, BiPAP, non-rebreather, endotracheal tube)
- Orthopedic devices (including braces and casts)
- Abdominal binders
- Cervical collars
- Compression stockings
- Enteral feeding tubes
- Encephalogram electrode
- Indwelling urinary catheters
- Intravenous catheters (arterial and venous)
- Pulse oximeters
- Restraints
- Blood pressure cuffs
- Tracheostomy ties or securement devices (Lee & Choi, 2024, Table 2; NPIAP, 2019; Pittman & Gillespie, 2020; TJC, 2018; Weber et al., 2021)
Patient-specific risk factors for developing an MDRPI from one of the previously listed medical devices include:
- Extended length of stay (LOS)
- Receiving vasopressors
- Low Braden Scale score
- Dependence on mechanical ventilation
- Advanced age
- Concurrent HAPI not attributable to a medical device
- Receiving enteral nutrition
- Critical illness (Weber et al., 2021)
Although MDRPIs can occur in any hospital setting that uses medical devices, patients in critical care units such as ICUs are at higher risk due to exposure to a greater number of medical devices. However, because there is a significant risk for MDRPIs among many patient populations, the nurse's clinical judgment and visual inspection are vital to protect all patients. Policies and procedures focusing on the increased assessment of medical device locations are needed to prevent MDRPIs. Early intervention with repositioning devices at regular intervals, adding protective layers between the skin and medical devices where possible, using foam securement devices to provide padding and prevent friction, and carefully monitoring the moisture associated with devices can decrease the incidence or severity of MDRPIs in hospitalized patients. Multiple studies have reported that nasal oxygen delivery devices cause MDRPIs most often, followed by casts or splints. A large prevalence study revealed that MDRPIs occur quicker than non-MDRPIs following admission to a facility (Lee & Choi, 2024; NPIAP, 2019; TJC, 2018; Weber et al., 2021).
Children and neonates are a population that is also at increased risk for MDRPIs. Skin immaturity has been identified as a significant risk factor for PIs in neonates. For neonates under 30 weeks of gestation, the stratum corneum is not fully developed, and for neonates over 30 weeks of gestation, the stratum corneum is only two to three cells thick. Their skin is extremely fragile, provides an inadequate barrier, and is, therefore, highly susceptible to breakdown. Children and neonates are also at higher risk for HAPIs due to their size and weight (NPIAP, n.d.-a, 2019).
Current skin assessment tools may not accurately identify the risk from medical devices, as the focus of these tools is the presence of risk factors such as nutritional status, age, or illness. Additionally, patients can be at risk for an MDRPI even where these factors are not present. Early detection is challenging as the skin beneath medical devices can be difficult to assess. Injuries from medical devices can occur in any patient without proper assessment, prevention, or early intervention. Modification of existing tools or the development of new tools to identify risk or actual injury from medical devices may be needed, considering the limitations of current skin assessment tools. A robust exploration into quality improvement (QI) initiatives should be considered by health care organizations that include higher levels of vigilance in assessment, product selection, and efficient reporting strategies. QI initiatives should focus on a more explicit recognition of population or situational risk factors associated with medical devices (Lee & Choi, 2024).
In 2016, the NPIAP also addressed mucosal membrane PIs. These PIs are found on mucosal membranes following medical device use. Staging is not possible with these PIs due to the location and anatomy of the tissue. Mucosal membrane PIs are found in the gastrointestinal (GI) tract, nasal passages, urinary tract, and tracheal lining (NPIAP, n.d.-b).
Prone Positioning
During the COVID-19 pandemic, up to 20% of infected patients required hospitalization, and 5% to 8% of those needed care in the ICU due to the development of acute respiratory distress syndrome (ARDS) and the need for mechanical ventilation (Anesi, 2024). As the treatment for COVID-19 evolved, specific interventions used for patients with ARDS were implemented. One such intervention was to place the patient in a prone position. This positioning increases oxygenation and decreases mortality in patients with ARDS. However, it increases the incidence of PIs on the face and anterior weight-bearing regions. Research revealed that patients with ARDS were placed in prone positioning approximately 9 hours per session with an average total of 18 hours of prone positioning daily. A 2022 meta-analysis of ten systematic reviews found that up to half of all patients placed in the prone position developed PIs (Patton et al., 2022).
Skin Assessment
To properly determine HAPI risk, the HCP must complete a thorough head-to-toe skin assessment. Elements of a comprehensive skin assessment include assessing temperature, color, moisture, turgor, edema, and integrity. To accurately assess skin temperature, the HCP should use the dorsal side of the hand instead of the palmar side to touch the skin and determine if the skin is warm or cool. It is essential to assess symmetrical body areas bilaterally to compare if temperature changes are unilateral or bilateral. While assessing the skin for temperature changes, the HCP should also evaluate the same areas for dryness or moisture. Macerated skin from excessive moisture may feel soft or boggy. Determine if the moisture or dryness is localized to one location or if it is generalized. Evaluate for odors. To determine skin color changes, the HCP must assess the skin under natural or halogen lighting, not fluorescent, which gives a bluish tone to darker skin. If needed, a penlight can illuminate hard-to-see areas such as the sacrum. Note the patient's baseline skin tone to determine if changes have occurred; for symmetrical body parts, assess the area bilaterally to determine if color changes are unilateral. For any areas with color change, depress the site to determine if the discoloration is blanchable or non-blanchable. Assessing skin turgor includes briefly pinching the skin near the clavicle or back of the hand and releasing the skin. A typical finding consists of the skin returning in 0 to 1 second. When the skin remains upright, it is known as tenting, which is an abnormal finding indicative of dehydration or connective tissue disease. An older adult’s skin may take up to 20 seconds to return to normal without the presence of dehydration due to elasticity changes. Skin integrity is vital to a thorough skin assessment. The HCP should assess the entire body, including under medical devices, for the loss of skin integrity, such as cracks, openings, excoriations, or lesions. A comprehensive skin assessment is critical for the proper risk assessment of an individual (AHRQ, 2014; Morgan, n.d.).
Preexisting PIs or any alterations in skin condition indicate a higher risk for the worsening or development of subsequent injuries. If the patient's acuity and the length of hospital stay increase, the opportunity for injury to the skin also rises. Maintaining good nutritional status, managing medical needs, and encouraging mobility are all actions that reduce the risk of injury. Identifying risk allows for early intervention, which can improve outcomes. Once an injury has occurred, staging skin injuries is essential to direct the most appropriate management based on evidence and current guidelines (Lee & Choi, 2024; NPIAP, 2019).
Risk Assessment Tools
The elements of a comprehensive skin tool include skin temperature, color, moisture, turgor, integrity, and patient mobility. Many tools are available to the health care team, yet the most commonly utilized in the U.S. are the Braden and Norton Scales. Both scales have high levels of validity and reliability in identifying high-risk patients. The HCP should use a skin assessment tool to measure the risk of skin injury as soon as possible after admission and repeat per policy (AHRQ, 2014; Lee & Choi, 2024).
Braden Scale
The Braden Scale has been in use since 1988. It is the most used tool for predicting PIs and skin breakdown in hospitals and long-term care facilities (Huang et al., 2021; TJC, 2022). The Braden Scale estimates the risk for PIs by assessing the following factors as described in Table 3.
Table 3
Braden Pressure Injury Risk Assessment
Sensory Perception | 1. Completely Limited: | 2. Very Limited: | 3. Slightly Limited: | 4. No Impairment: |
Moisture | 1. Constantly Moist: | 2. Very Moist: | 3. Occasionally Moist: | 4. Rarely Moist: |
Activity | 1. Bedfast: | 2. Chairfast: | 3. Walks Occasionally: | 4. Walks Frequently: Walks outside the room at least twice a day and inside room at least once every two hoursduring waking hours. |
Mobility | 1. Completely Immobile: | 2. Very Limited: | 3. Slightly Limited: | 4. No Limitations: Makes major and frequent changes in position without assistance. |
Nutrition | 1. Very Poor: | 2. Probably Inadequate: | 3. Adequate: | 4. Excellent: Eats most of every meal. Never refuses a meal. Usually eats a total of four or more servings of meat and dairy products. Occasionally eats between meals. Does not require supplementation. |
Friction and Shear | 1. Problem: | 2. Potential Problem: | 3. No Apparent Problem: |
(AHRQ, 2014, section 3D; Bergstrom et al., 1987)
Table 4 is the abbreviated Braden Scale that is used in facilities. Each category is given a score of 1 to 4 (except friction and shear, which is scored 1 to 3). The individual scores are added to determine the individual's risk for skin-related issues during admission. Total scores may range from 6 to 23. The higher the score, the lower the risk for PIs. A score of 18 or less indicates an at-risk status for developing a PI (see Table 5). HCPs should keep in mind that patients could potentially have a low-risk total score yet have an increased risk in a single category; thus, a review of the information may give opportunities to avoid injury and should be considered when developing a care plan. After determining overall risk, interventions are instituted accordingly to decrease the risk and prevent the development of skin breakdown (AHRQ, 2014, section 3D; Bergstrom et al., 1987; Hovan, 2021; NPIAP, 2019).
Table 4
Abbreviated Braden Scale
Category | Scoring |
Sensory Perception | 1 - Completely limited 2 - Very limited 3 - Slightly limited 4 - No impairment |
Moisture | 1 - Constantly moist 2 - Very moist 3 - Occasionally moist 4 - Rarely moist |
Activity Level | 1 - Bedfast 2 - Chairfast 3 - Walks occasionally 4 - Walks frequently |
Mobility | 1 - Completely immobile 2 - Very limited 3 - Slightly limited 4 - No limitations |
Nutrition | 1 - Very poor 2 - Probably inadequate 3 - Adequate 4 - Excellent |
Friction and Shear | 1 - Problem 2 - Potential problem 3 - No apparent problem |
(AHRQ, 2014; Bergstrom et al., 1987)
Table 5
Braden Scale Scoring
Score | Risk |
19 to 23 | No risk |
15 to 18 | Mild risk |
13 to 14 | Moderate risk |
Less than 9 | Severe risk |
(Hovan, 2021)
Norton Scale
The Norton Scale was created in 1962 in England. It was the first-ever designed PI risk assessment tool. It is mainly used for older adults in long-term care settings. The scale is easy to use and effectively recognizes the risk of PIs or other skin concerns. The Norton Scale has five categories: physical, mental, activity, mobility, and incontinence. Within each category, the patient is given a score of 1 to 4 based on characteristics defined by the scale (see Table 6). The scores for each of the five categories are added together with a sum between 5 and 20 (see Table 7). Like the Braden score, the lower the Norton score, the higher the risk. A score of 14 or less indicates the patient is at risk of developing a PI (AHRQ, 2014; Spinal Cord Injury Research Evidence [SCIRE], n.d.).
Table 6
Norton Scale
Category | Scoring |
Physical condition | 1 - Very bad 2 - Poor 3 - Fair 4 - Good |
Mental condition | 1 - Stuporous 2 - Confused 3 - Apathetic 4 - Alert |
Activity | 1 - Bedfast 2 - Chairbound 3 - Walks with help 4 - Ambulant |
Mobility | 1 - Immobile 2 - Very limited 3 - Slightly impaired 4 - Full |
Incontinence | 1 - Urinary and fecal 2 - Usually urinary 3 - Occasional 4 - None |
(AHRQ, 2014, section 3E; SCIRE, n.d.)
Table 7
Norton Scale Scoring
Score | Risk |
18 to 20 | Low risk |
14 to 18 | Medium risk |
10 to 14 | High risk |
Less than 10 | Very high risk |
(SCIRE, n.d.)
The Braden Scale is considered more precise than the Norton Scale due to the broader range of clinical factors; therefore, it is more widely used. When using a risk assessment scale, it is essential to include a comprehensive risk assessment of modifiable and nonmodifiable risk factors not included in the risk assessment, including those outlined previously in the section on general and medical device risk factors (NPIAP, 2019; SCIRE, n.d.).
The NPIAP (2019) recommends that a full skin assessment using a validated assessment tool such as the Braden Scale or Norton Scale is completed as soon as possible but no more than eight hours after admission. In contrast, the National Database of Nursing Quality Indicators recommends that the skin assessment be completed within 24 hours of admission to identify any pre-existing injuries and assess the risk for future injury (Edsberg et al., 2022). The nurse should also remember that risk can exist without a high score on the skin assessment tool. Any change in condition or acuity that could increase the risk requires a repeat assessment. Along with each risk assessment, a comprehensive skin assessment with documentation should be completed and maintained in the medical record. While there is no universally accepted risk assessment, each facility should have a structured risk assessment that is consistently applied. Each institution should also have a risk-based prevention plan for patients identified as being at risk for developing PIs. The physical assessment and skin risk assessment tool results determine the risk for PI (NPIAP, 2019).
Prevention
Severe PIs are considered never events, critical medical errors that should never happen. CMS classifies HAPIs as preventable and no longer reimburses hospital systems for treating/managing them (Bowman et al., 2023; Edsberg et al., 2022).
Intervention Bundles
Various interventions have been discussed in the literature to reduce the number of HAPIs in today's clinical setting, including intervention bundles. A bundled intervention is a "structured way of improving the processes of care and patient outcomes; a small, straightforward set of evidence-based practices-generally three to five-that, when performed collectively and reliably, have been proven to improve patient outcomes" (Institute for Healthcare Improvement [IHI], 2012, para. 1). Examples of bundled interventions developed for patients at risk for PIs include the SKIN or pressure injury prevention (PIP) bundles (AHRQ, 2014, section 3.1; Caldwell, 2025; Rivera et al., 2020). The acronym "SKIN" stands for:
- Surface selection
- Keep turning patients
- Incontinence management
- Nutrition (Caldwell, 2025)
By applying the SKIN or PIP bundle, the bedside nurse is the first line of defense in PI prevention. The SKIN or PIP bundles focus on caring for the patient to decrease the risk and prevent injuries. An evidence-based initiative for implementation of a PIP bundle in 2017-2018 demonstrated a significant reduction in PIs. Understanding how the different components of a bundle are related is essential to effectively following a clinical pathway to implement a bundle successfully. Clinical pathways provide a guide for each step. The benefits of clinical pathways include enhanced standardization of care between health care facilities, provision of evidence-based care, improved patient outcomes, improved care planning, and enhanced interdisciplinary collaboration (AHRQ, 2014; Caldwell, 2025; Rivera et al., 2020).
Regardless of the acronym, aspects of an intervention bundle, or policies and procedures of the facility, those responsible for patient care should work to utilize the tools available for assessment and prevention. HCPs should be familiar with the latest guidelines for PI prevention and implement evidence-based care for their patients. A multi-pronged approach to PI prevention is the best practice. Organizations typically adopt multiple strategies to decrease the risk of HAPIs. Patient repositioning and pressure support devices are key measures to reduce PIs. In addition, the following supportive interventions may help assist health care facilities in meeting their goals to prevent PIs (Berlowitz, 2023b; Rivera et al., 2020):
- Improve mobility
- Improve skin perfusion
- Provide proper skin care
- Minimize excess temperature and moisture
- Provide multilayer foam dressings
- Correct malnutrition
- Consider use of continuous bedside pressure mapping
Organizations must determine the best methods based on the guidelines and the patient population they serve. Budget, workforce, and resources, in addition to national guidelines, drive the policies and procedures for patient care (Berlowitz, 2023b; NPIAP, 2019).
MDRPI Prevention
The NPIAP (2019) guidelines recommend that medical devices be selected and adjusted individually to exert the least amount of pressure or shearing forces. For instance, HCPs should use the softest device or securement products that promote the slightest friction or shearing force for any tubing, such as an endotracheal tubes, urinary catheters, or tracheostomy tubes. Medical devices such as helmets, halo vests, or restrictive devices should fit correctly to avoid excessive pressure. All devices should be applied according to the manufacturer's specifications to prevent unintended injury and liability to the facility. The focus should be on securing any medical device appropriately and using padding or cushioning against the tissue. Recommendations for the assessment of skin surrounding or beneath a medical device and prevention of MDRPIs include:
- Increase the frequency of skin assessments and observe for increased edema or irritation.
- Educate the patient and family on risks related to the medical device, including recognizing and reporting injuries or concerns.
- If an injury occurs, classify the MDRPI using the International NPIAP Pressure Classification system (see Table 1).
- Remove devices as quickly as appropriate for the medical condition.
- Maintain proper skincare under and around the medical device, keeping the area clean and dry.
- Reposition the patient regularly to decrease pressure from the medical device.
- Reposition or rotate the position of the medical device regularly, when possible, to avoid ongoing pressure in the same area.
- Provide cushioning or supportive devices to decrease pressure on the underlying tissue.
- Prophylactic dressings for pressure, such as a soft foam silicone dressing, may reduce the risk of skin breakdown. The NPIAP guidelines have further information on the ideal characteristics of prophylactic dressings, including the ability to assess skin under the dressing, ease of removal and reapplication, location of the device on the patient's body, or ability to manage body fluids around the dressing (Berlowitz, 2023b; Medline, 2024; NPIAP, 2019).
National Patient Safety Goals
TJC endorses the National Patient Safety Goals (NPSGs), which are standards addressing the highest-priority patient safety issues in all health care settings. The prevention of HAPIs is included in the NPSGs, which note that each patient's risk should be identified, and action should be taken to address any PIs discovered during the skin assessment. It also states that most PIs can be avoided through preventative measures, and damage can be decreased if injuries are identified early, preferably at stage I. The health care organization is expected to have a written plan to identify risk and prevent PIs, perform an initial skin assessment that identifies risk for PIs, use a valid skin risk assessment tool such as the Braden or Norton scales, reassess patient skin at specified intervals as determined by the organizational policies, and take actions appropriate for the risks identified during the evaluation that prevents injury to the patient and protects them from external mechanical forces. Also, there should be education for staff that identifies risk factors for PIs and prevention techniques as defined by the organization based on the latest evidence and guidelines (AHRQ, 2014, 2017; Shaikh, 2024; TJC, 2025).
Management of Pressure Injuries
The NPIAP (2019) Clinical Practice Guideline of Interventions for treating PIs examines wound bed preparation. This clinical concept is a holistic, systematic approach to evaluating and treating wounds that allow a natural progression toward wound healing. The goal of wound bed preparation is "to promote a well-vascularized wound bed, free from nonviable tissue and excess exudate, and with a reduced bacterial burden and reduced edema, which is optimal for the development of healthy granulation tissue" (NPIAP, 2019). The acronym TIMERS describes the components of wound bed preparation as follows.
- Tissue management
- Infection and inflammation control
- Moisture balance
- Epithelial edge advancement
- Repair and regeneration
- Social factors and factors related to the individual (NPIAP, 2019)
Routine assessment and wound care driven by TIMERS will remove the barriers that delay routine healing in chronic wounds. Wound cleansing removes remnants of old dressings and decreases the number of bacteria in the wound bed. Cleansing should be gentle to avoid damaging new tissue growth. An aseptic technique with sterile products should be utilized for immunocompromised patients or if the wound enters a sterile body cavity. Normal saline is recommended for wound cleansing for clean PIs or those without any signs of infection. Antimicrobial cleansing solutions should be used for wounds with confirmed or suspected infection. Wounds with sinus tracts or tunneling should be cleansed with caution as some of the solution could be retained in the wound, causing further injury. Wound cleansing should be done using sufficient pressure to adequately cleanse the wound but avoid damaging tissue or driving bacteria further into the wound. All cleansing solutions should be single-patient use and disposed of properly to prevent cross-contamination. The surrounding skin should also be cleansed, taking particular care of wounds on the coccyx or perineal areas (Mayer et al., 2024; NPIAP, 2019).
Debridement
Debridement is needed for wounds that have other particles or devitalized tissue remaining after cleansing, such as slough, debris, tissue that is necrotic, biofilm, and micro-organisms. Debridement of non-viable tissue and the associated bacterial and cellular burden stimulates healthy tissue growth by creating an environment that is optimal for wound healing. Studies have shown that debridement eliminates barriers to wound healing, yet 60% of wounds are not debrided when needed. When debridement is needed, multiple factors should be considered. The procedure can be excruciating, and pain management should be part of the care plan. Devitalized tissue that is thick or leathery and discolored, including yellow, green, tan, grey, brown, or black, should be debrided to promote healing. Bacteria grow in necrotic tissue and further delay wound healing. However, the patient's overall condition and ability to tolerate the debridement should be considered. Indications for debridement include the presence of biofilm (collection of one or more types of organisms that form a slimy build-up), delayed wound healing, or failure to respond to standard wound care (Mayer et al., 2024; NPIAP, 2019). Debridement of PIs can utilize the following techniques.
- Surgical debridement is performed by a specialty or primary HCP. Surgical debridement removes necrotic tissue, cellular debris, wound exudate, or eschar from the wound bed to facilitate healing. This invasive procedure uses sharp and sterile instruments to excise the nonviable materials from the wound bed. For wounds requiring this intervention, the interdisciplinary team must support the procedure and perform subsequent wound care and assessments (Mayer et al., 2024).
- Mechanical debridement removes exudate, necrotic or infected material, and foreign bodies from the wound bed. After cleansing a wound, a wet-to-dry dressing (i.e., gauze pads soaked in saline) can be applied to the wound bed. After the dressing has dried, it is removed. The goal is to pull away the top layer of tissue within the wound with the dried dressing. The disadvantage of a wet-to-dry dressing is that it is non-selective; the entire top layer of the wound is removed without discerning between viable and nonviable tissue. A 2024 international consensus document on debridement best practices determined this method should be the last choice when no alternative is available due to the associated pain and harm. Debridement pads are preferred over traditional gauze pads. Other methods of mechanical debridement that are recommended include hydrotherapy, pulsative lavage, ultrasonic debridement, and negative pressure wound therapy and instillation with dwell time (NPWTi-d; Manna et al., 2023; Mayer et al., 2024).
- Hydrosurgical debridement is performed in an operating room (OR) with a high-power waterjet and suction. In comparison to moist dressings, hydrotherapy debridement is well tolerated by patients who are sensitive to pain. It is faster and more selective than a blade with a straight edge. It can also be done at the bedside with a micro waterjet, and special training is not required.
- Pulsatile lavage combines intermittent lavage with suction to loosen and remove nonviable tissue from the wound bed. Although this is fast and effective, caution must be taken not to damage underlying structures such as blood vessels, bones, and tendons. Pulsatile lavage is also costly because the equipment is single use only.
- Ultrasonic debridement uses low- or high-frequency ultrasonic waves that create acoustic energy to remove nonviable tissue from the wound bed. When used in conjunction with standard wound care, ultrasonic debridement can safely and effectively remove necrotic tissue and promote wound healing. This type of debridement is extremely helpful with cavity wounds.
- NPWTi-d is a mechanical debridement technique that uses a reticulated open-cell foam (ROCF) that combines the instillation of normal saline or other solution with a debriding foam that has mechanical debriding effects. It is a gentle wound-healing approach that can be useful in patients who are unable to tolerate sharp/surgical debridement.
- Mechanical debridement can be excruciating for the patient, so pre-medication with analgesics is indicated to increase patient comfort. Patients on anticoagulant medications or with underlying coagulopathy may have significant bleeding with mechanical debridement, so these individuals require extra caution and care (Berlowitz, 2023a; Mayer et al., 2024).
- Biologic debridement, otherwise known as maggot debridement therapy, uses sterile, medical-grade larvae of the Lucilia sericata species of the greenbottle blowfly to remove necrotic tissue from a wound bed. It is beneficial in large wounds needing painless removal of necrotic tissue. Proteolytic enzymes are released from the larvae to dissolve necrotic tissue. The wound bed should be cleaned, and sterile larvae are then applied to the wound bed and covered with a mesh-like, air-permeable dressing for one to three days, after which the dressing and larvae are removed. If further debridement is needed, the process can be repeated. Wounds should never be allowed to close over the larvae, and they should not be left in the wound bed if they die, as this increases the risk of allergic reaction or infection. Larvae used for this treatment are considered contaminated and should be disposed of properly by sealing them in a plastic bag and placing them in a biohazard container for incineration (Manna et al., 2023; Nowak et al., 2022; Thomas et al., 2021; Wernick et al., 2023).
- Enzymatic or chemical debridement dissolves necrotic tissue, cellular debris, wound exudate, and foreign materials using commercial enzyme products. One commonly used medication in the U.S. is collagenase (Santyl) ointment. The active ingredient is from the bacterium Clostridium histolyticum, which breaks down collagen in necrotic tissue. Patients with wounds that are heavily infected are not candidates for this treatment. Products with silver and Dakin solution are contraindicated for use in conjunction with collagenase (Avila-Rodríguez et al., 2020; Manna et al., 2023; Mayer et al., 2024; Nowak et al., 2022).
- Autolytic debridement is the most gentle, conservative form of debridement and uses the body's enzymes and healing processes to rehydrate, soften, liquefy, and then expel the necrotic tissue from the wound. Occlusive dressings are often used to keep the body's fluids in the wound bed and maintain a moist healing environment, such as absorptive hydrofibers, alginate dressings, hydrocolloid dressings, and amorphous hydrogel. This should not be attempted in infected wounds, those that need to be urgently debrided for best outcomes, or wounds with large amounts of necrotic tissue, undermining, or tunneling. Immunocompromised patients should not undergo autolytic debridement (Avila-Rodríguez et al., 2020; Manna et al., 2023; Nowak et al., 2022).
Infection
Bacteria are present on all skin surfaces, and when the skin's integrity is breached, bacteria present on the surface can enter the wound. When the bacterial presence increases and wound damage occurs, an infection is present. Healthy individuals can usually avoid developing an infection due to their immune system's defense response, yet an immunocompromised patient will have a decreased ability to fight the bacteria. "The number of micro-organisms and their effect on the host can be categorized in the following stages: contamination, colonization, local infection, spreading infection, and systematic infection" (NPIAP, 2019, p. 251). Micro-organisms may multiply, invade, or damage tissues in or around the wound bed, delay healing, and cause systemic responses. Infection is present if a PI is not healing due to the bacteria in the wound bed. Biofilms may be the source of wound infection or delay healing. “Biofilm is a complex community of microorganisms embedded in a protective matrix called the extracellular polymeric substance, which is composed of polysaccharides, proteins, extracellular DNA and metal ions such as magnesium, calcium, and iron” (Mayer et al., 2024, para. 14). To enhance wound healing, the biofilms must be removed by debridement and prevented from reforming by using antiseptics and antimicrobial dressings (NPIAP, 2019). Other signs or symptoms of infection are:
• Erythema that extends past the wound edges
• Induration of the tissue
• An increase or change in pain
- A change in temperature
• Purulent drainage and malodor
• An increase in wound size
• Systemic reactions including fever, malaise, lymph node enlargement, confusion, or anorexia (particularly in the older adult; Armstrong & Meyr, 2023; NPIAP, 2019)
To properly manage wound infection, wound cultures may be required to determine the appropriate treatment based on the organism involved. If a biofilm is recognized, a tissue biopsy may be necessary. Other health care team members should be notified of signs and symptoms of infection, including dieticians, nurses, and providers, such as vascular and wound care specialists. Poor nutritional status, lack of glycemic control, certain medications, and inadequate circulation are potential causes of an infection. Any deficits in these areas should be further explored and managed by the health care team (NPIAP, 2019).
Treatment of infected wounds can include systemic antibiotics, topical antiseptics, silver-containing dressings and pastes, medical-grade honey, enzyme alginogels, or topical antibiotics (NPIAP, 2019). Wound dressings should be selected based on the following:
• Ability to keep the wound bed moist
• Need to address the bacterial burden
• Type and amount of wound exudate
• Condition of the tissue in the wound bed
• Status of the surrounding skin
• Injury stage and location
• Presence of tunneling or undermining
• Goals of the patient and the health care team (NPIAP, 2019)
Antibiotic therapy is indicated for wounds that are more severe than a local, superficial infection. Therapy includes intravenous (IV) and/or oral antibiotics to elicit systemic effects. Topical antibiotic use should be limited to situations in which the benefits outweigh the risks of side effects and bacterial resistance. For mild infections without signs of systemic involvement, amoxicillin-clavulanate (Augmentin) plus either trimethoprim-sulfamethoxazole (Bactrim) or doxycycline (Vibramycin) would be appropriate. If amoxicillin-clavulanate (Augmentin) is not feasible due to allergy or intolerability, levofloxacin (Levaquin) can be used instead. For more severe infections with systemic involvement, parenteral regimens are preferred, such as vancomycin (Vancocin) plus ceftazidime (Fortaz) or cefepime (Maxipime) plus metronidazole (Flagyl), which can be given orally due to high bioavailability. Alternative options include a carbapenem such as meropenem (Merrem) plus vancomycin (Vancocin). Pseudomonal coverage can be ensured with ciprofloxacin (Cipro), levofloxacin (Levaquin), ceftazidime (Fortaz), cefepime (Maxipime), or meropenem (Merrem). Most patients can be switched to oral therapy once a clinical response (i.e., resolution of systemic signs and symptoms) is observed. Specific side effects of antibiotics are dependent on their administration route and classification. General side effects of IV antibiotics include swelling, redness, and pain at the injection site. Common side effects of oral antibiotics include diarrhea, nausea, bloating, and abdominal pain (NPIAP, 2019; Singhal, 2021; Tleyjeh & Berlowitz, 2024).
Dressings
The proper dressing for a wound will support healing at a faster rate. Dressing types will often need to change throughout the healing stages, and the HCP will need to assess the injury and adjust the type of dressing throughout the trajectory of care. Unfortunately, the cost is essential to consider in determining wound care products. Comfort and usability are also crucial factors, as the patient or family members may be performing the wound care once the patient is discharged from the health care facility. Staff workload and patient compliance should be considered when determining the frequency of dressing changes. Newer treatment modalities require less frequent changes and can stay in place for longer with optimal results (NPIAP, 2019).
- Hydrocolloid dressings are moisture-retentive dressings used to protect wounds with a small to moderate amount of drainage. These are often used to treat non-infected stage I through stage IV PIs, abrasions, and necrotic wounds. Hydrocolloids can function as a secondary dressing with alginates or wound fillers that need to remain in the wound bed for an extended time. Do not use hydrocolloids with wound that produce excessive exudate. Do not cover enzymatic debriding agents, gels, or ointments with hydrocolloid dressings. Examples of hydrocolloid dressings are DuoDerm or Tegasorb (NPIAP, 2019; Weller et al., 2020).
- Hydrogel dressings contain a high water/glycerin content within a gel base and are used to provide moisture to the wound bed. The moist environment facilitates the debridement of necrotic tissue and tissue granulation. The amorphous hydrogel can be applied in a layer over the wound surface, or a hydrogel-impregnated gauze can be used to fill dead spaces in deep wounds such as stage III or IV PIs. These dressings should not be used with moderate to heavy wound exudate or when the goal of care is to maintain dry eschar. Amorphous hydrogel dressings should be used on injuries that are not infected but are granulating. Gravity-dependent body areas, such as the lower legs, benefit from amorphous hydrogel dressings. Hydrogel sheets can treat dry bed wounds and are often used on non-moving or nondependent body surfaces. Hydrogel dressings can contain allergens such as iodine, silver, or sodium carboxymethyl cellulose and should not be used in patients with known sensitivities or allergies to these products. Examples of hydrogel dressings are Hydrosorb or Solugel (NPIAP, 2019; Weller et al., 2020).
- Alginate dressings are used for highly exudative wounds and contain alginic acid from seaweed covered in calcium/sodium salts. These dressings are highly absorbent, absorbing up to 20 times their weight in exudate. Alginate dressings may also contain controlled-release ionic silver. These dressings interact with the serum to form a hydrophilic gel in the wound bed. They may be utilized concurrently with infection treatment but have minimal antimicrobial properties and should not be used as a primary treatment for infected wounds. Alginate dressings should be irrigated first to aid in removal and then gently removed. Examples of alginate dressings are Aligisite, Curasorb, Tegaderm Alginate, or Kaltostat (NPIAP, 2019; Weller et al., 2020).
- Foam dressings can be constructed from either foam that draws in fluid and physically expands as it retains the drainage or pseudo-foam that contains absorbent materials such as viscose and acrylate fibers designed to hold extra fluid. These are best for wounds with moderate to heavy drainage. They can be used as a primary or secondary dressing on wounds and remain in place for up to seven days. Most are non-adhesive and can easily be used on those with allergies to adhesives. Antimicrobial foam dressings can be used on infected wounds. Foam dressings are best for stage II or III PIs with heavy drainage. Examples of foam dressings are Aquacel Foam or Aquacel Extra (Weller et al., 2020).
- Gauze and non-woven dressings have been used for wound care for decades, but with the advancement of new materials designed to accelerate healing, these materials are rarely used in modern-day wound care. Daily dressing changes are required with these materials to manage drainage effectively. Gauze dressings can cause further tissue damage in open PIs, particularly if they are removed dry. The 2019 NPIAP guideline recommends avoiding wet-to-dry dressings in PIs. It is acceptable to use gauze dressings when other moisture-retentive dressings are not available; however, wound bed moisture must be maintained. Further, the gauze dressing can be used as a cover or loose packing for deep-pressure wounds. Only use saline-moistened gauze when other forms of moisture-retentive dressings are not available. Multiple layers of gauze should not be used to control drainage as that can serve as a source of infection. Multiple studies have shown that other dressings have higher success rates with wound healing, and gauze dressings can promote infection or delay wound healing (Mayer et al., 2024; NPIAP, 2019).
- Bioactive dressings improve wound healing and are derived from natural sources, proteins, or tissues. These products are especially useful in stage II and III PIs. Examples are collagen dressings and medical-grade honey (Wood, 2024).
- Silver-impregnated dressings are appropriate in infected wounds heavily colonized with bacteria (NPIAP, 2019).
- Cadexomer iodine dressings are useful in cases of excessive wound drainage. However, these should be used with extreme caution and avoided in individuals with renal failure, thyroid disorders, or known iodine sensitivity (NPIAP, 2019).
- Silicone dressings are used as a wound contact layer to promote atraumatic dressing changes or prevent peri-wound injury around fragile tissue. They will not chemically interact with the wound and are easy to remove (NPIAP, 2019; Weller et al., 2020).
- Collagen matrix dressings can be incorporated to treat stage III and IV PIs. The application of collagen has been shown to promote wound healing in at least one study, but the cost of the product is high (NPIAP, 2019).
Hyperbaric Oxygen Therapy
Hyperbaric oxygen therapy (HBOT) may be used on chronic wounds to promote wound healing. Injuries that are not healing are typically hypoxic, and increasing oxygen tension and pressure by various methods can stimulate healing. HBOT promotes muscle and nerve regeneration by stimulating angiogenesis or developing new blood vessels. The treatments can be done on limbs placed in a limb-encasing device or a full-body chamber. HBOT has been in use for over 40 years and should be part of an interdisciplinary team approach with a comprehensive plan of care for wound healing that includes strategies for extensive vessel disease, glycemic abnormalities, nutritional deficiencies, infection, and the presence of necrotic tissue. Relative contraindications for systemic HBOT are asthma or COPD, claustrophobia, recent ear or thoracic surgery, eustachian tube dysfunction, asymptomatic pulmonary blebs or bullae noted on chest radiographs, seizure history, and upper respiratory infections. Medications could also have potential adverse effects with conjunctive use, such as bleomycin (Blenoxane) and doxorubicin (Rubex), but evidence is sparse. An untreated pneumothorax is the only absolute contraindication to HBOT (Manaker, 2024; Wernick et al., 2023).
Negative Pressure Wound Therapy
Negative pressure wound therapy (NPWT), also called vacuum-assisted wound closure, applies suction or negative pressure to the wound bed. This therapy promotes wound healing by removing excess drainage, stimulating vascularization, and supporting the closure of wound edges or margins. Negative pressure creates mechanical stress that promotes growth factor expressions, angiogenesis, and granulation tissue growth. The negative pressure opens the capillary beds and draws blood to the wound area, reduces edema and bacterial colonization, and provides a moist wound bed that promotes healing. This treatment is often used on deep or full-thickness stage III or IV PIs. Pain is commonly reported as an adverse reaction and should be proactively managed during treatment. This treatment should not be used in individuals with vital structures that are exposed or the presence of tissue that is malignant. Caution is advised in use with patients who have ischemic wounds, infectious wounds, devitalized tissue or fragile skin, an allergy to adhesives, or are at increased risk of bleeding as life-threatening hemorrhage could result (Gestring, 2025; Wernick et al., 2023).
Pain Control
Pain is an often overlooked aspect of PIs and wound care. Although initial pain can indicate an issue with the skin, it can become chronic and debilitating. A considerable number of patients report that the experience of wound care is as painful as the initial injury. Pain can increase patient fear, anxiety, and depression, negatively impacting wound healing. A patient with a wound may experience diverse types of pain. The injury itself may cause neuropathic pain, wound treatments may cause nociceptive pain, and anticipatory pain can be a factor for patients undergoing painful wound treatments. Factors that affect pain are the depth of the wound, the structures involved, infection, and other concurrent injuries or conditions. The nurse should complete a pain assessment to determine all aspects of the pain, including location, duration, exacerbating and relieving factors, quality, and severity, using a patient-appropriate pain scale. Premedicate patients with analgesics before debridement or other wound care that may incite pain. The HCP must time medication administration according to the expected onset of action and duration to ensure coverage during dressing changes or debridement (Mayer et al., 2024; NPIAP, 2019). There are also nonpharmacologic interventions that the HCP can use to reduce pain and increase patient comfort during wound care activities.
- Distractions can help decrease pain, including watching television, deep breathing, listening to music, playing games, or reading a book.
- The HCP should provide education and information about wound care activities.
- The HCP should space out care to give the patient uninterrupted rest time in a quiet environment.
- Music therapy or other guided imagery techniques may reduce anxiety.
- The patient should be positioned for comfort during any procedures or dressing changes.
- Dressings should be soaked at least 30 min prior to dressing change.
- When possible, the number of dressing changes should be limited by using dressings that can remain in place for more extended periods.
- Dressing types, tapes, and adhesives that do not adhere to the wound bed or pull at the wound should be utilized.
- The patient should be allowed to choose times for treatments or dressing changes whenever possible to create a feeling of independence or control and decrease anxiety (Admassie et al., 2022; Brown, 2023; NPIAP, 2019).
Future Opportunities
Future opportunities in the prevention and promotion of wound healing in PIs include but are not limited to biological dressings comprised of animal materials, human skin cells, plant materials, metals, synthetic materials, or a mixture of these, artificial intelligence (AI) and machine learning (ML), and 3D and 4D technology. Examples of biologic dressings are metal-organic framework hydrogel composites (MOF-HCs), skin substitutes, xenografts, and allografts. MOF-HCs have significant advantages over traditional wound care products due to high porosity and functionality (drug release and improved antibacterial activity) combined with moisture-retention and biocompatibility properties, allowing for exceptional therapeutic efficacy. The application of AI and ML can help with risk and wound assessments, monitoring changes, smart dressings with sensors, remote wound care with telehealth, noninvasive imaging, enhanced education, and increased communication. In addition, 3D printing technology has produced biocompatible scaffolds, custom dressings, and systems for drug delivery. Looking forward, 4D printing technology and bioelectronics may offer personalized and precise strategies for treatment. Current challenges include prohibitive cost and regulatory requirements, and more research is needed to support the widespread use of these promising innovations (Afruzi et al., 2025; BioStem Technologies, 2024; Dweekat et al., 2023; NPIAP, 2019; Sachdeo et al., 2024).
Another treatment that is gaining popularity for various ailments is platelet-rich plasma (PRP). To make PRP, blood is drawn from the patient and centrifuged to create the PRP. The PRP is then administered by injection to the injured area. PRP may present opportunities for escalated wound healing with a lower risk of compatibility or adverse reactions. Current investigations for the recovery of PIs include other growth factors, such as recombinant platelet-derived growth factor, basic fibroblast growth factor, epidermal growth factor, transforming growth factor-beta, or granulocyte-macrophage colony-stimulating factor. Stage III and IV PIs with delayed wound healing may benefit from a recombinant platelet-derived growth factor. These treatments are in the early stages of use and are not first-line treatments. The downsides are the risk for tissue hyperplasia or increased alloimmunity, along with their high cost and sparse insurance coverage. Nevertheless, these treatments demonstrate opportunities for improved healing of persistent chronic wounds (NPIAP, 2019; Patel et al., 2023; Pretorius et al., 2023).
Summary
PIs are costly to both the organization and the patient. Litigation is at an all-time high for PIs, and care related to PIs for these patients is not reimbursable. Education for all HCPs is needed to ensure that all interdisciplinary team members are aware of the risks, appropriate interventions, and the overall cost to the organization of an HAPI. Studies have shown that the prevention of PIs is multifaceted, including care bundles, improved nursing practices, and educational programs. Nurses are frontline advocates who can promote appropriate staffing, preventative devices, and other tools needed to deliver safe and effective care to decrease the risk of PIs (Caldwell, 2025; Kandula, 2025; NPIAP, 2019).
References
Admassie, B. M., Ferede, Y. A., Tegegne, B. A., Lema, G F., & Admass, B. A. (2022). Wound-related procedural pain management in a resource limited setting: Systematic review. International Journal of Surgery Open, 47, 100549. https://doi.org/10.1016/j.ijso.2022.100549
Afruzi, F. H., Abdouss, M., Zare, E. N., Ghomi, E. R., Mahmoudi, S., & Neisany, R. E. (2025). Metal-organic framework-hydrogel composites as emerging platforms for enhanced wound healing applications: Material design, therapeutic strategies, and future prospects. Coordination Chemistry Reviews, 524(1). https://doi.org/10.1016/j.ccr.2024.216330
Agency for Healthcare Research and Quality. (2014). Preventing pressure ulcers in hospitals. https://www.ahrq.gov/patient-safety/settings/hospital/resource/pressureulcer/tool/index.html
Agency for Healthcare Research and Quality. (2017). Pressure injury prevention in hospitals training program. https://www.ahrq.gov/professionals/systems/hospital/pressureinjurypxtraining/index.html
Anesi, G. L. (2024). COVID-19: Epidemiology, clinical features, and prognosis of the critically ill adult. UpToDate. Retrieved February 28, 2025, from https://www.uptodate.com/contents/covid-19-epidemiology-clinical-features-and-prognosis-of-the-critically-ill-adult
Armstrong, D. G., & Meyr, A. J. (2023). Clinical assessment of chronic wounds. UpToDate. Retrieved February 25, 2025, from https://www.uptodate.com/contents/clinical-assessment-of-chronic-wounds
Avila-Rodríguez, M. I., Meléndez-Martínez, D., Licona-Cassani, C., Aguilar-Yañez, J. M., Benavides, J., & Lorena Sánchez, M. (2020). Practical context of enzymatic treatment for wound healing: A secreted protease approach (Review). Biomedical Reports, 13(1), 3–14. https://doi.org/10.3892/br.2020.1300
Bergstrom, N., Braden, B. J., Laguzza, A., & Holman, V. (1987). The Braden Scale for predicting pressure sore risk. Nursing Research, 36(4), 205-210. https://pubmed.ncbi.nlm.nih.gov/3299278/
Berlowitz, D. (2023a). Clinical staging and general management of pressure-induced skin and soft tissue injury. UpToDate. Retrieved February 25, 2025, from https://www.uptodate.com/contents/clinical-staging-and-general-management-of-pressure-induced-skin-and-soft-tissue-injury
Berlowitz, D. (2023b). Prevention of pressure-induced skin and soft tissue injury. UpToDate. Retrieved February 25, 2025, from https://www.uptodate.com/contents/prevention-of-pressure-induced-skin-and-soft-tissue-injury
Berlowitz, D. (2024). Epidemiology, pathogenesis, and risk assessment of pressure-induced skin and soft tissue injury. UpToDate. Retrieved February 25, 2025, from https://www.uptodate.com/contents/epidemiology-pathogenesis-and-risk-assessment-of-pressure-induced-skin-and-soft-tissue-injury
BioStem Technologies. (2024). The future of chronic wound care: What to expect in 2025. https://biostemtechnologies.com/the-future-of-chronic-wound-care-what-to-expect-in-2025/
Bowman, C. L., De Gorter, R., Zaslow, J., Fortier, J. H., & Garber, G. (2023). Identifying a list of healthcare 'never events' to effect system change: A systematic review and narrative synthesis. BMJ open quality, 12(2), e002264. https://doi.org/10.1136/bmjoq-2023-002264
Brown, A. (2023). Assessing and managing wound pain. Practice Nursing, 34(1). https://www.practicenursing.com/content/clinical-focus/assessing-and-managing-wound-pain
Caldwell, S. (2025). Reducing hospital-acquired pressure injuries in a cardiothoracic intensive care unit. Critical Care Nurse, 45(1), 12-20. https://doi.org/10.4037/ccn2025980
Centers for Medicare and Medicaid Services. (2024). Hospital-acquired condition reduction program. https://www.cms.gov/medicare/quality/value-based-programs/hospital-acquired-conditions
Dweekat, O. Y., Lam, S. S., & McGrath, L. (2023). Machine learning techniques, applications, and potential future opportunities in pressure injuries (bedsores) management: A systematic review. International Journal of Environmental Research and Public Health, 20(1), 796. https://doi.org/10.3390/ijerph20010796
Edsberg, L. E., Cox, J., Koloms, K., & VanGilder-Freese, C. A. (2022). Implementation of pressure injury prevention strategies in acute care: Results From the 2018–2019 International Pressure Injury Prevalence Survey. Journal of Wound, Ostomy, and Continence Nursing, 49(3), 211–219. https://doi.org/10.1097/WON.0000000000000878
Gestring, M. (2025). Negative pressure wound therapy. UpToDate. Retrieved February 25, 2025, from https://www.uptodate.com/contents/negative-pressure-wound-therapy
Hovan, H. (2021). Understanding the Braden Scale: Focus on sensory perception (Part 1). Wound Source. https://www.woundsource.com/blog/understanding-braden-scale-focus-sensory-perception-part-1
Huang, C., Ma, Y., Wang, C., Jiang, M., Yuet Foon, L., Lv, L., & Han, L. (2021). Predictive validity of the Braden scale for pressure injury risk assessment in adults: A systematic review and meta-analysis. Nursing Open, 8(5), 2194–2207. https://doi.org/10.1002/nop2.792
Institute for Healthcare Improvement. (2012). What is a bundle? http://www.ihi.org/resources/Pages/ImprovementStories/WhatIsaBundle.aspx
The Joint Commission. (2018). Quick safety 43: Managing medical device-related pressure injuries. https://www.jointcommission.org/resources/news-and-multimedia/newsletters/newsletters/quick-safety/quick-safety-43-managing-medical-devicerelated-pressure-injuries/
The Joint Commission. (2022). Quick safety issue 25: Preventing pressure injuries (updated March 2022). https://www.jointcommission.org/resources/news-and-multimedia/newsletters/newsletters/quick-safety/quick-safety-issue-25-preventing-pressure-injuries/
The Joint Commission. (2025). Hospital: 2025 national patient safety goals. https://www.jointcommission.org/standards/national-patient-safety-goals/hospital-national-patient-safety-goals
Tomlinson C, Edwards P, Pfeifer L. (2024). Preventing hospital-acquired pressure injuries. American Nurse Journal, 19(1), 06-09. doi:10.51256/anj012406 https://www.myamericannurse.com/preventing-hospital-acquired-pressure-injuries/
Kandula, U. R. (2025). Impact of multifaceted interventions on pressure injury prevention: A systematic review. BMC Nursing, 24, 11. https://doi.org/10.1186/s12912-024-02558-9
Kim, P., Aribindi, V. K., Shui, A. M., Deshpande, S. S., Rangarajan, S., Schorger, K., Aldrich, M., & Lee, H. (2022). Risk factors for hospital-acquired pressure injury in adult critical care patients. American Journal of Critical Care, 31(1), 42–50. https://doi.org/10.4037/ajcc2022657
Kirman, C. N. (2024). Pressure injuries (pressure ulcers) and wound care. Medscape. https://emedicine.medscape.com/article/190115-overview
Lee, H., & Choi, S. (2024). Protocols and their effects for medical device-related pressure injury prevention among critically ill patients: A systematic review. BMC Nursing, 23, 403. https://doi.org/10.1186/s12912-024-02080-y
Manaker, S. (2024). Hyperbaric oxygen therapy. UpToDate. Retrieved February 25, 2025, from https://www.uptodate.com/contents/hyperbaric-oxygen-therapy
Manna, B., Nahirniak, P., & Morrison, C. A. (2023). Wound debridement. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK507882/
Mayer, D. O., Tettlebach, W. H., Ciprandi, G., Downie, F., Hampton, J., Hodgson, H., Lazaro-Martinez, J. L., Probst, A., Schultz, G., Stürmer, E. K., Parnham, A., Frescos, N., Stang, D., Holloway, S., & Percival, S. L. (2024). Best practice for wound debridement. Journal of Wound Care, 33(Sub6b), S1–S32. https://doi.org/10.12968/jowc.2024.33.Sup6b.S1
Medline. (2024). Medical device related pressure injury prevention. https://www.medline.com/strategies/skin-health/medical-device-related-pressure-injury-prevention/
Mondragon, N., & Zito, P. M. (2024). Pressure injury. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK557868/
Morgan, N. (n.d.). Comprehensive skin assessment. Wound Care Advisor. Retrieved February 25, 2025, from https://woundcareadvisor.com/comprehensive-skin-assessment-vol4-no4/
National Pressure Injury Advisory Panel. (n.d.-a). International guidelines. Retrieved February 25, 2025, from https://npiap.com/page/InternationalGuidelines
National Pressure Injury Advisory Panel. (n.d.-b). NPIAP pressure injury stages. Retrieved February 25, 2025, from https://cdn.ymaws.com/npiap.com/resource/resmgr/online_store/npiap_pressure_injury_stages.pdf
National Pressure Injury Advisory Panel. (2019). Prevention and treatment of pressure ulcers/injuries: Clinical practice guideline. https://www.biosanas.com.br/uploads/outros/artigos_cientificos/127/956e02196892d7140b9bb3cdf116d13b.pdf
Nowak, M., Mehrholz, D., Barańska-Rybak, W., & Nowicki, R. J. (2022). Wound debridement products and techniques: Clinical examples and literature review. Advances in Dermatology and Allergology, 39(3), 479–490. https://doi.org/10.5114/ada.2022.117572
Patel, H., Pundkar, A., Shrivastava, S., Chandanwale, R., & Jaiswal, A. M. (2023). A comprehensive review on platelet-rich plasma activation: A key player in accelerating skin wound healing. Cureus, 15(11), e48943. https://doi.org/10.7759/cureus.48943
Patton, D., Latimer, S., Avsar, P., Walker, R. M., Moore, Z., Gillespie, B. M., O’Connor, T., Nugent, L., Budri, A., MPhil, N. O. B., & Chaboyer, W. (2022). The effect of prone positioning on pressure injury incidence in adult intensive care unit patients: A meta-review of systematic reviews. Australian Critical Care, (35)6, 714-722. https://doi.org/10.1016/j.aucc.2021.10.003
Pittman, J., & Gillespie, C. (2020). Medical device-related pressure injuries. Critical Care Nursing Clinics of North America, 32, 4, 533–542. https://doi.org/10.1016/j.cnc.2020.08.004
Pretorius, J., Habash, M., Ghobrial, B., Alnajjar, R., & Ellanti, P. (2023). Current status and advancements in platelet-rich plasma therapy. Cureus, 15(10), e47176. https://doi.org/10.7759/cureus.47176
Rivera, J., Donohoe, E., Deady-Rooney, M., Douglas, M., & Samaniego, N. (2020). Implementing a pressure injury prevention bundle to decrease hospital-acquired pressure injuries in an adult critical care unit: An evidence-based, pilot initiative. Wound Management & Prevention, 66(10), 20–28. https://www.hmpgloballearningnetwork.com/site/wmp/article/implementing-pressure-injury-prevention-bundle-decrease-hospital-acquired-pressure-injuries
Rogers, J. L. and Brashers, V. L. (Eds.). (2023). McCance and Huether's pathophysiology: The biologic basis for disease in adults and children (9th ed.). Elsevier.
Sachdeo, R. A., Khanwelkar, C., & Shete, A. (2024). 3D printing in wound healing: Innovations, applications, and future directions. Cureus, 16(12), e75331. https://doi.org/10.7759/cureus.75331
Shaikh, U. (2024). National patient safety goals. Patient Safety Network, AHRQ. https://psnet.ahrq.gov/primer/national-patient-safety-goals
Sim, W., Clara Michelle, T. H. S., Lim, N. Q. B. I., Loh, V., Chua, C. W. X., Er, J., Er, J., Phan, P., & Choi, E. C. E. (2024). Why do pressure injuries still occur? A multicenter qualitative study of nurses and caregivers. JAAD International, 17, 29–36. https://doi.org/10.1016/j.jdin.2024.05.006
Singhal, H. (2021). Wound infection medication. Medscape. https://emedicine.medscape.com/article/188988-medication#2
Siotos, C., Bonett, A. M., Damoulakis, G., Becerra, A. Z., Kokosis, G., Hood, K., Dorafshar, A. H., & Shenaq, D. S. (2022). Burden of pressure injuries: Findings from the global burden of disease study. Eplasty, 22, e19.
Spinal Cord Injury Research Evidence. (n.d.). The Norton pressure sore risk-assessment scale scoring system. Retrieved February 25, 2025, from https://scireproject.com/outcome/norton-pressure-ulcer-risk-scale/
Thomas, D. C., Tsu, C. L., Nain, R. A., Arsat, N., Fun, S. S., & Sahid Nik Lah, N. A. (2021). The role of debridement in wound bed preparation in chronic wound: A narrative review. Annals of Medicine and Surgery (2012), 71, 102876. https://doi.org/10.1016/j.amsu.2021.102876
Tleyjeh, I. M., & Berlowitz, D. (2024). Infectious complications of pressure-induced skin and soft tissue injury. UpToDate. Retrieved February 25, 2025, from https://www.uptodate.com/contents/infectious-complications-of-pressure-induced-skin-and-soft-tissue-injury
Wassel, C. L., Delhougne, G., Gayle, J. A., Dreyfus, J., & Larson, B. (2020). Risk of readmissions, mortality, and hospital-acquired conditions across hospital-acquired pressure injury (HAPI) stages in a US National Hospital Discharge database. International Wound Journal, 17(6), 1924–1934. https://doi.org/10.1111/iwj.13482
Weber, P., Weaver, L., & Miller, C. (2021). Risk factors associated with medical device-related pressure injuries in the adult intensive care patient: a scoping review. Wound Practice and Research, 29(4), 219-225. https://doi.org/10.33235/wpr.29.4.219-225
Weller, C. D., Team, V., & Sussman, G. (2020). First-line interactive wound dressing update: A comprehensive review of the evidence. Frontiers in Pharmacology, 11, 155. https://doi.org/10.3389/fphar.2020.00155
Wernick, B., Nahirniak, P., & Stawicki, S. P. (2023). Impaired wound healing. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK482254/
Wood, B. (2024). Skin grafts and biologic skin substitutes. Medscape. https://emedicine.medscape.com/article/1295109-overview