About this course:
This 4-hour course reviews the indications for tracheostomy placement, including the anatomy and pathophysiology of the respiratory system and the use of mechanical ventilation. In addition, this course reviews various aspects of tracheostomy placement, including timing considerations, types of procedures, and post-placement complications. Finally, this course outlines postoperative tracheostomy management and care, including tracheostomy cleaning, suctioning, and patient education.
Course preview
This 4-hour course reviews the indications for tracheostomy placement, including the anatomy and pathophysiology of the respiratory system and the use of mechanical ventilation. In addition, this course reviews various aspects of tracheostomy placement, including timing considerations, types of procedures, and post-placement complications. Finally, this course outlines postoperative tracheostomy management and care, including tracheostomy cleaning, suctioning, and patient education.
After this activity, learners will be prepared to:
- Discuss the indications for tracheostomy placement and contributing factors, including mechanical ventilation
- Describe the anatomy and physiology of the respiratory system
- Describe the various aspects of tracheostomy placement, including timing considerations and types of tracheostomy procedures
- Discuss various tracheostomy-related complications, including prevention and treatment strategies
- Describe the principles of routine tracheostomy management and care, including tracheostomy cleaning and suctioning
- Discuss postoperative tracheostomy-related patient education for discharge planning
Healthcare providers (HCPs) are responsible for offering high-quality, evidence-based care to optimize patient outcomes. As new treatments emerge, people are living longer, healthier lives. As the US population ages, more people live with chronic health conditions. Because more people are managing their complex chronic conditions, increasing numbers of patients are admitted to critical care units (Dirkes & Kozlowski, 2019). Traditionally, HCPs working with critically ill patients have focused on stabilizing immediate, life-threatening cardiopulmonary symptoms. Mechanical ventilation (MV) is frequently used for critically ill patients requiring airway protection or respiratory support. MV delivers positive pressure to the lungs through a tracheostomy or endotracheal (ET) tube. Many patients will successfully wean off the mechanical ventilator once respiratory support is no longer needed. However, patients who cannot wean will require temporary or permanent tracheostomy placement. HCPs caring for patients requiring MV and subsequent tracheostomy placement must be able to describe the pathophysiology of the respiratory system and identify appropriate indications for tracheostomy placement (Hyzy & McSparron, 2021a).
Background
The National Center for Chronic Disease Prevention and Health Promotion (2021) defines chronic diseases as conditions that last more than 1 year and require ongoing medical attention and/or limit activities of daily living (ADLs). Chronic disease is the leading cause of death and disability in the US. An estimated 6 out of 10 American adults have at least one chronic disease, and 4 out of 10 have two or more chronic diseases. Chronic conditions such as heart disease, cancer, chronic lung disease, diabetes mellitus (DM), Alzheimer's disease, and chronic kidney disease (CKD) significantly contribute to the $3.8 trillion spent on US healthcare costs annually (National Center for Chronic Disease Prevention and Health Promotion, 2021).
The current life expectancy for adults in the US is 78.8 years (Centers for Disease Control and Prevention [CDC], 2021). More advanced medical treatments will be necessary as the population lives longer with complex chronic conditions. Over 5 million patients are admitted to intensive care units (ICUs) in the US annually for intensive or invasive monitoring, including airway, breathing, or circulation support; stabilization of acute or life-threatening medical problems; and comprehensive management of an injury or illness (Society of Critical Care Medicine, n.d.). Although the ICU patient population is heterogeneous, the most common indications for admission include cardiac, respiratory, and neurological conditions. Respiratory failure with ventilator support is among the top 5 reasons for ICU admission for adults. In addition, the most common technological support required in an ICU is MV, accounting for 20% to 40% of admissions in the US. Annual critical care costs exceed $82 billion. ICU costs per day were estimated to be $4,300 in 2010, representing a 61% increase since 2000 (Society of Critical Care Medicine, n.d.). The number of patients admitted to the ICU for respiratory support is expected to rise further due to the coronavirus (COVID-19) pandemic, with rates of MV among these patients ranging from 29% to 89% across countries (Wunsch, 2020).
MV is a common cause of ICU admission for patients requiring airway protection or respiratory support (Mora Carpio & Mora, 2021). By performing the work of breathing (WOB) and gas exchange, MV can fully or partially replace the functions of spontaneous breathing for patients with respiratory failure. During MV, a predetermined air mixture (i.e., oxygen and other gases) is forced into the central airways and travels into the alveoli. As a result, the lungs inflate, causing intra-alveolar pressure to increase. The ventilator stops forcing air into the central airways when a termination signal occurs, usually from increased flow or pressure. Expiration passively happens as the central airway pressure decreases, with air flowing from the higher-pressure alveoli to the lower-pressure central airways (Hyzy & McSparron, 2021a).
HCPs caring for patients requiring MV must understand the following ventilator-related concepts (Hinkle & Cheever, 2018; Mora Caprio & Mora, 2021; Respiratory Therapy Zone, 2021):
- Ventilation is the movement and exchange of gases (oxygen and carbon dioxide) between the lungs and the air. A ventilator forces oxygen into the lungs while carbon dioxide is removed from the body during exhalation. During MV, the carbon dioxide in a patient’s blood can be modified by changing the tidal volume (TV) or respiratory rate (RR).
- Oxygenation of mechanically ventilated patients, which boosts the oxygen supply to the lungs, can be achieved by increasing the fraction of inspired oxygen (FiO2) or the positive end-expiratory pressure (PEEP).
- TV is the volume of air moved in and out of the lungs with each respiratory cycle.
- PEEP refers to the positive pressure applied by the ventilator at the end of each respiratory cycle. In mechanically ventilated patients, the pressure will remain greater than the atmospheric pressure to prevent the alveoli from collapsing.
- Auto-PEEP (also known as intrinsic PEEP) refers to the residual pressure (i.e., above the atmospheric pressure) in the alveoli at the end of exhalation. Air trapping can occur due to impedance (obstruction, narrowing) during expiration or limited expiration time/duration.
- FiO2 is the percentage of oxygen in the air mixture that the ventilator delivers to a patient.
- Flow refers to the speed at which the ventilator delivers breaths, measured in liters per minute (LPM).
- Compliance is the elasticity and expandability of the lungs and chest wall determined by the change in volume divided by the change in pressure.
- Trigger sensitivity refers to the ventilator's sensitivity control to determine how much effort (negative pressure) a patient must generate to trigger a breath. Sensitivity is usually between -1 and -2 cm H2O. If the sensitivity is too high, it will cause the ventilator to initiate auto-triggering, increasing the rate of breaths. Conversely, if the sensitivity is too low, the patient may struggle to initiate a breath.
- Hypoxia refers to a lack of oxygen and decreased partial pressure of arterial oxygen (PaO2).
- Hypercarbia refers to elevated c
...purchase below to continue the course
HCPs will see more patients requiring prolonged mechanical ventilation (PMV) as more patients receive MV. The Centers for Medicare and Medicaid Services (CMS) define PMV as more than 21 days of MV for at least 6 hours per day. An estimated 300,000 patients require MV each year in the US. Of these patients, between 4% and 13% require PMV, which means between 7,250 and 11,400 patients undergo PMV at any time. Successful ventilator weaning occurs when a patient has 7 consecutive days without the use of ventilatory support (Villalba et al., 2019). HCPs should frequently assess patients for weaning readiness to prevent complications associated with MV. PMV is associated with increased length of stay (LOS), healthcare costs, ventilator-associated events (VAEs), morbidity, and mortality. Therefore, the prevention of PMV and VAEs is essential for HCPs to optimize patient outcomes (Jarrett et al., 2016; King Han, 2020).
A tracheostomy is a surgical opening created in the anterior neck to insert a tube into the patient's trachea (windpipe). The tracheostomy tube is inserted through the surgical opening (stoma), below the vocal cords, to allow air into the patient's lungs. The placement of a tracheostomy can be either temporary (e.g., for acute respiratory failure) or permanent (e.g., for neuromuscular disease), depending on the underlying disease processes. Although some tracheostomy placements occur emergently (i.e., for airway obstruction), most are done in a controlled surgical or ICU setting when patients cannot wean from the mechanical ventilator (Mayo Clinic, 2019; Unitek College, 2021). Although many patients requiring MV will successfully wean from the ventilator, some will require PMV. For these patients, an elective tracheostomy is often performed to reduce the risk of respiratory injury or other complications of prolonged ET tube intubation, including VAEs, sinusitis, and tracheal stenosis (Andriolo et al., 2015). An estimated 8% to 13% of patients requiring advanced respiratory support, including MV, will need a tracheostomy. These rates have recently increased due to the COVID-19 pandemic, with tracheostomies rising from 16% to 61% in patients requiring advanced respiratory support (National Heart, Lung, and Blood Institute, n.d.; Williams & McGrath, 2021). HCPs caring for patients requiring a tracheostomy must understand the following terms (Unitek College, 2021):
- A stoma is the opening in the trachea that connects the body to the outside environment.
- A tracheostomy tube is a curved, hollow tube of rubber or plastic inserted into the stoma. The tracheostomy tube consists of an outer tube (placed into the stomal opening) and an inner tube that fits into the outer tube and can be removed for cleaning. A flat, plastic plate (flange) lies against the patient's neck and is attached to the outer tube.
- Tracheal suctioning refers to suctioning to clear mucus and secretions from the trachea.
- Decannulation refers to removing a tracheostomy tube when it is no longer needed.
- A cuff is an inflatable air reservoir that helps hold the tracheostomy in place and provides an airway seal.
- Fenestration refers to a hole on the curve of the outer tracheostomy tube that enhances airflow in and out of the trachea.
- A speaking valve (i.e., tracheostomy button or cap) covers the tracheostomy tube opening, creating a seal that facilitates speech and swallowing.
Anatomy and Pathophysiology of the Respiratory System
The respiratory system comprises the upper and lower respiratory tracts responsible for moving air in and out of the lungs (see Figure 1). The upper respiratory tract warms and filters inspired air, while the lower respiratory tract is responsible for gas exchange. Gas exchange involves delivering oxygen through the bloodstream to the tissues and eliminating carbon dioxide during expiration. The upper respiratory tract includes the nose, paranasal sinuses, pharynx, tonsils, adenoids, larynx, and trachea. The nose provides a passageway for air to move to and from the lungs, filtering impurities and humidifying and warming the air during inhalation. As air enters the nostrils, the nasal mucosa (i.e., the large surface of moist, warm, highly vascular, ciliated mucous membranes) traps dust and organisms in the inhaled air. The paranasal sinuses are four pairs of bony cavities (i.e., frontal, ethmoid, sphenoid, and maxillary) connected by a series of ducts that drain into the nasal cavity. The pharynx (throat) is a tubelike structure that connects the oral and nasal cavities to the larynx. The tonsil and adenoids encircle the throat and help guard the body against the invasion of organisms (Hinkle & Cheever, 2018).
The larynx (voice-box) is a cartilaginous organ that connects the pharynx and trachea. The larynx consists of the epiglottis (i.e., the flap of cartilage that covers the opening to the larynx during swallowing) and the glottis (i.e., the opening between the vocal cords in the larynx). Although the primary function of the larynx is vocalization (i.e., when the vocal cords produce sound by muscular movements of the ligaments), it also protects the lungs from foreign substances by facilitating coughing (Hinkle & Cheever, 2018). The trachea serves as the passage between the larynx and the right and left main stem bronchi, which enter the lungs through an opening called the hilus. The trachea is composed of smooth muscle and cartilage rings spaced at regular intervals, preventing the trachea from collapsing. The cartilaginous rings form a C-shape, with the posterior surface having an incomplete connection (Hinkle & Cheever, 2018).
The lower respiratory tract contains the lungs, including the bronchial and alveolar structures needed for gas exchange. The lungs are paired elastic structures enclosed in an airtight thoracic cage. The right lung is divided into upper, middle, and lower lobes, while the left lung is divided into the upper and lower lobes. The lungs and the thoracic cavity are lined with a serous membrane (pleura) to provide lubrication, allowing smooth movement of the lungs within the thoracic cavity during inspiration and expiration. The bronchi within the lungs divide into the lobar, segmental, and subsegmental branches and the bronchioles. The lobar and segmental bronchi facilitate adequate postural drainage. The subsegmental bronchi are surrounded by connective tissue that contains arteries, lymphatics, and nerves. The bronchioles are the final branches in the lungs and contain no cartilage. Bronchiole patency depends on the elastic recoil of the surrounding smooth muscle and the alveolar pressure. Bronchi and bronchioles contain cilia that propel mucus and foreign substances toward the larynx. Finally, the lungs comprise 300 million alveoli, including type I cells (providing a barrier between the air and the alveolar surface) and type II cells (producing surfactant). Surfactant reduces surface tension, allowing effective lung function (Hinkle & Cheever, 2018).
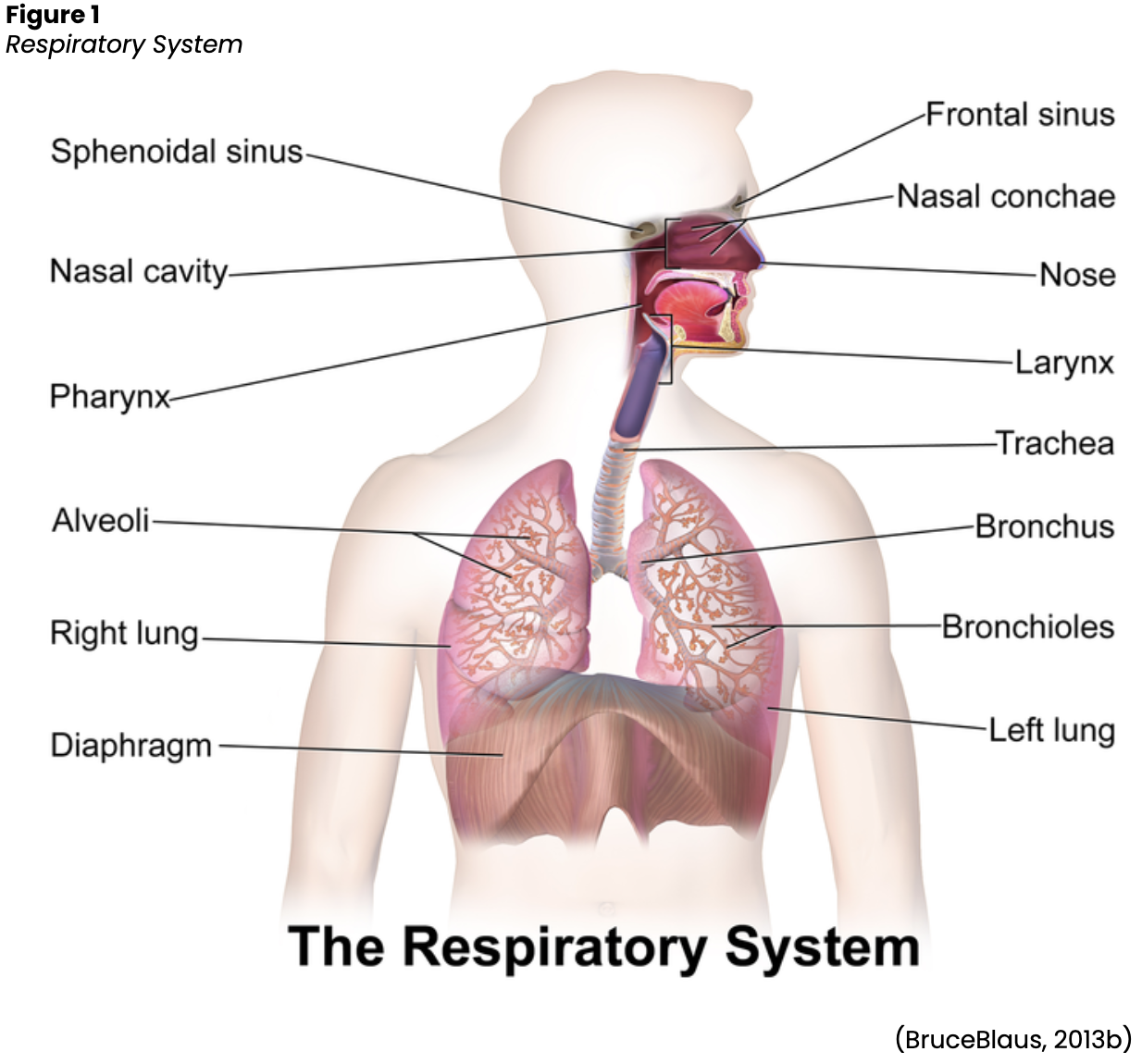
The respiratory system facilitates life-sustaining processes, including oxygen transport, respiration, ventilation, and gas exchange. Human cells rely on the oxidation of carbohydrates, fats, and proteins to produce energy. Without a continuous supply of oxygen, cells in the brain, heart, and other essential organs cannot survive. Oxygen is transported to, and carbon dioxide is removed from, the circulating blood through the thin walls of the capillaries. Oxygen diffuses through capillary walls to the interstitial fluid and eventually to the cells. Carbon dioxide diffuses in the opposite direction from the cells to the blood. After these tissue capillary exchanges, blood enters the systemic venous circulation and travels to the pulmonary circulation. The oxygen concentration in the alveoli is higher than the concentration in the blood; therefore, oxygen diffuses from the alveoli to the blood. Similarly, carbon dioxide has a higher concentration in the blood than in the alveoli, so it diffuses from the blood to the alveoli (see Figure 2; Hinkle & Cheever, 2018).

Ventilation
Ventilation requires movement of the walls of the thoracic cage and the diaphragm. The thoracic cage and diaphragm movements alternate by increasing and decreasing the chest’s capacity. When the chest capacity increases, air enters the trachea, passes through the bronchi and bronchioles, and inflates the alveoli in the lungs (inspiration). As the thoracic cavity expands, the pressure inside the thorax drops below the atmospheric pressure; air flows from a region of higher pressure to lower pressure. During expiration, the diaphragm relaxes and the lungs recoil, decreasing the size of the thoracic cavity. As the pressure in the alveoli increases, air flows from the lungs to the atmosphere. The inspiratory phase of respiration requires active energy, while the expiratory phase is passive (Hinkle & Cheever, 2018).
Besides air-pressure variances, airway resistance, lung compliance, and lung volumes can also impact ventilation. The size or diameter of the airway, lung volumes, and airflow velocity determine airway resistance. Any process that changes the diameter of the airway will affect airway resistance and the rate of airflow. A more significant respiratory effort will be required to achieve normal ventilation with increased airway resistance. Compliance allows the thoracic cavity to expand (i.e., change volume) based on air-pressure variances. Alveolar surface tension and the amount of connective tissue and water in the lungs determine lung compliance. Standard compliance allows the lungs and thorax to stretch easily when pressure is applied. Increased compliance occurs when the lungs have lost their elastic recoil and become permanently overextended (e.g., in chronic obstructive pulmonary disease [COPD]). Decreased compliance occurs when the lungs become stiff, requiring increased energy expenditure by the patient to achieve normal ventilation levels. Acute respiratory distress syndrome (ARDS), pulmonary edema, pleural effusion, morbid obesity, pneumothorax, and pulmonary fibrosis are associated with decreased lung compliance (Hinkle & Cheever, 2018).
Perfusion
Gas exchange depends on pulmonary diffusion and perfusion. Pulmonary diffusion is how oxygen and carbon dioxide are exchanged in the body due to the differences in gas concentrations in the alveoli and capillaries. The alveolar-capillary membrane is ideal for diffusion due to its large, thin surface area, allowing for gas exchange from areas of high concentration to low concentration. Pulmonary perfusion refers to the actual blood flow through the pulmonary vasculature and is determined by pulmonary artery pressure, gravity, and alveolar pressure. The right ventricle pumps blood into the lungs through the pulmonary artery. The pulmonary artery supplies both lungs by dividing into the right and left branches. The normal range for systolic blood pressure in the pulmonary artery is about 20 to 30 mm Hg, and the diastolic pressure is 5 to 15 mm Hg, making pulmonary circulation a low-pressure system. Due to these lower pressures, the pulmonary vasculature can accommodate varying amounts of blood flow. Alveolar pressure also influences perfusion, as pulmonary capillaries lie between adjacent alveoli. Therefore, if the alveolar pressure is high, the capillaries are squeezed, impacting perfusion (Hinkle & Cheever, 2018).
Adequate gas exchange depends on effective ventilation and perfusion, resulting in an adequate ventilation-perfusion (V/Q) ratio. Four types of V/Q states can occur in the lungs: normal V/Q ratio, low V/Q ratio, high V/Q ratio, and absence of ventilation and perfusion. In a healthy lung, the V/Q ratio is 1:1, indicating equal amounts of blood and gas moving through the alveoli. Low V/Q ratios (shunt) happen when perfusion (Q) exceeds ventilation (V). When blood bypasses the alveoli without gas exchange, shunting results in hypoxia as the blood is delivered to the body’s tissues without obtaining the necessary oxygen. Low V/Q ratios can occur with obstruction of the distal airways (e.g., tumor, atelectasis, pneumonia, mucus plug). High V/Q ratios (dead space) develop when ventilation exceeds perfusion, resulting in an inadequate blood supply for gas exchange in the alveoli (e.g., pulmonary emboli [PE], pulmonary infarction, cardiogenic shock). A silent unit occurs in the absence of ventilation and perfusion due to blockages (e.g., pneumothorax, ARDS; Hinkle & Cheever, 2018).
Neurologic Control of Ventilation
Ventilation control depends on the neuronal network in the brainstem, which controls the activities of the motor neurons that innervate the respiratory muscles. The inspiratory and expiratory centers in the medulla oblongata and pons control the rate and depth of ventilation. The pneumotaxic center in the upper pons controls the respiration pattern, while the apneustic center in the lower pons stimulates the inspiratory medullary center to promote deep inspiration. In addition, various groups of receptor sites in the brain assist with respiratory control. Central chemoreceptors within the medulla respond to chemical changes in cerebrospinal fluid. An increase or decrease in pH triggers these receptors, resulting in a change in the rate and depth of respiration. Peripheral chemoreceptors from the aorta to the arch and in the carotid arteries respond to changes in PaO2, followed by changes in PaCO2 and pH. Within the lungs, various receptors—including stretch, irritant, and juxtacapillary mechanoreceptors—respond to changes in resistance by altering breathing patterns. Proprioceptors in the muscles and chest wall react to body movements such as range-of-motion (ROM) exercises, stimulating an increase in ventilation. Finally, baroreceptors in the aorta and carotid bodies respond to changes in arterial blood pressure, causing reflex hypoventilation or hyperventilation (Hinkle & Cheever, 2018).
Mechanical Ventilation
As previously discussed, healthy respiratory function works as a negative-pressure system. During inspiration, the diaphragm moves downward, creating negative pressure in the pleural cavity. This negative intrathoracic pressure decreases right atrial (RA) pressure, which creates a pulling effect on the inferior vena cava (IVC), resulting in increased venous return. Conversely, MV alters normal respiratory function by pushing air into the upper airways and alveoli, creating positive pressure in the thoracic cavity. This positive intrathoracic pressure increases RA pressure and decreases venous return, reducing preload (i.e., the force that stretches the cardiac muscle before constriction). As less blood reaches the right ventricle and subsequently the left ventricle, cardiac output decreases. With decreased cardiac output and preload, mean arterial pressure (MAP) will drop if there is no compensatory increase in systemic vascular resistance (SVR). In addition, the positive pressure generated by MV can significantly reduce a patient’s WOB. This reduction in WOB allows blood flow to be redistributed to more critical organs, limits carbon dioxide and lactate generation, and improves acidosis. Unfortunately, it also induces respiratory muscle and diaphragmatic weakness, a significant predictor of PMV (see Figure 3; King Han, 2020; Mora Carpio & Mora, 2021).

Indications for MV
A mechanical ventilator decreases the WOB until patients can resume normal breathing independently. A ventilator ensures that a patient receives adequate oxygen to be used by the tissues and carbon dioxide is removed. Many patients requiring MV can breathe spontaneously, although the effort needed to do so can be exhausting (Hinkle & Cheever, 2018). By partially or fully assisting with respiration, MV preserves a stable airway and allows for respiratory muscle relaxation while a patient recovers from an illness or injury (Cleveland Clinic, 2019). The most common indication for intubation and MV is acute respiratory failure. The lungs cannot adequately perform gas exchange in this condition, resulting in hypoxia, hypercarbia, and persistent acidosis (decreased pH). MV may also be indicated for patients requiring airway protection to reduce the risk of aspiration (e.g., neurological impairment from drugs, poisons, spinal cord injury, or myasthenia gravis). Following a traumatic injury, patients may require MV depending on the injury's severity and location (e.g., head, neck, and chest). Other indications for MV can include cardiopulmonary arrest, cardiovascular impairment (e.g., strokes, emboli, tumors), and pulmonary impairment (e.g., tumors, infections, COPD, pneumothorax; American Thoracic Society, 2020; Mora Carpio & Mora, 2021; Perry et al., n.d.).
Consequences of Mechanical Ventilation
Patients requiring MV are at high risk for complications and poor outcomes, including death. Barotrauma, pulmonary embolism (PE), sepsis, acute respiratory distress syndrome (ARDS), and ventilator-associated pneumonia (VAP) are VAEs that can affect patients receiving MV. These complications can result in PMV, prolonged ICU stays, increased healthcare costs, and high mortality. Prolonged ventilatory dependence can stem from many factors, including respiratory insufficiency, reduced ventilatory capacity, and cardiovascular insufficiency. Respiratory insufficiency occurs when there is an imbalance between respiratory pump capacity and demand. In addition, PMV can lead to diaphragmatic weakness and atrophy, especially when accompanied by passive modes of ventilation. Other factors contributing to respiratory muscle weakness include malnutrition, immobility, excessive corticosteroid use, sedative medications, and a systemic inflammatory response (SIR) associated with sepsis. Cardiovascular insufficiency (i.e., heart failure [HF]) can also increase the risk of PMV. Positive intrathoracic pressure is lost when a patient transitions from MV to spontaneous breathing. This shift in pressure raises venous return in the right ventricle, increasing preload and afterload. This greater cardiac workload can elevate myocardial oxygen demand for patients with coronary artery disease, resulting in ischemia (Fadila et al., 2021).
The following factors can increase the risk of PMV:
- history of COPD
- history of restrictive pulmonary disease (i.e., sarcoidosis, interstitial lung disease, obesity)
- neuromuscular disorders
- ICU admission due to pneumonia, ARDS, head trauma, or postoperative intracerebral hemorrhage
- abnormal arterial carbon dioxide, serum blood urea nitrogen (BUN), serum creatinine, arterial pH, white blood cell (WBC) count, or body temperature on the first ICU day
- age 85 years and older
- extended inpatient length of stay before ICU admission (Fadila et al., 2021; King Han, 2020) I
Indications for Tracheostomy Placement
A tracheostomy is a commonly performed surgical procedure for patients requiring MV for acute respiratory failure. Although the number of patients requiring a tracheostomy is small, these patients contribute to the use of significant ventilator, ICU, hospital, and post-hospital resources. Financially, the cost of caring for patients with a tracheostomy is among the highest of any diagnostic or procedure group. Patients with tracheostomies may need four different transitions of care between the ICU, acute care, long-term care, and home, contributing to high long-term healthcare costs. Due to the long-term complex care needed for patients with a tracheostomy, higher morbidity and mortality rates are experienced by this population. Efforts to optimize tracheostomy management and care can positively impact patients' quality of life (QOL) and healthcare resource expenditure (Brown, 2020; Freeman, 2016; Mussa et al., 2021).
Indications for tracheostomy placement can be emergent or elective, depending on the injury or disease process. Emergent tracheostomy placement is uncommon but may be needed for patients with an acute upper airway obstruction with failed intubation (i.e., anaphylaxis, angioedema, hematoma, submandibular space infection, retropharyngeal abscess, or mass). An emergency cricothyrotomy is preferred to emergent tracheostomy when possible because it is easier and quicker to perform, has fewer complications, and does not require cervical spine manipulation for patients with traumatic injuries. A cricothyrotomy, also known as a cricothyroidotomy, involves the placement of a tube through an incision in the cricothyroid membrane (see Figure 4; Sakles, 2021). Once the patient is stabilized, the cricothyrotomy is replaced by a tracheostomy (Mayo Clinic, 2019). Additional indications for emergent tracheostomy include fractures of the face and neck (e.g., LeFort III fracture of the midface) or penetrating laryngeal trauma. Emergent tracheostomies are most likely to be performed in prehospital or emergency department settings (Cleveland Clinic, n.d.; Hyzy & McSparron, 2021b; Raimonde & Westhoven, 2021).
The most common elective indication for tracheostomy placement is a need or anticipated need for PMV, usually due to difficulty weaning from the ventilator. Additional indications for elective tracheostomy include poor airway or secretion control due to neuromuscular disorders (e.g., amyotrophic lateral sclerosis [ALS]) resulting in recurrent aspiration or an inability to breathe independently. Prophylactic tracheostomy may be necessary for airway protection before head or neck surgery or other extensive upper airway procedures such as radiation. Finally, patients with severe obstructive sleep apnea (OSA), subglottic stenosis, and vocal cord paralysis refractory to other therapies may require elective tracheostomies. The few absolute contraindications for tracheostomy placement include cellulitis of the anterior neck, absence of a cervical trachea (i.e., due to a prior resection), and an uncorrectable bleeding condition (Hyzy & McSparron, 2021b; Mayo Clinic, 2019; Raimonde & Westhoven 2021).
Figure 4
Diagram of Cricothyrotomy
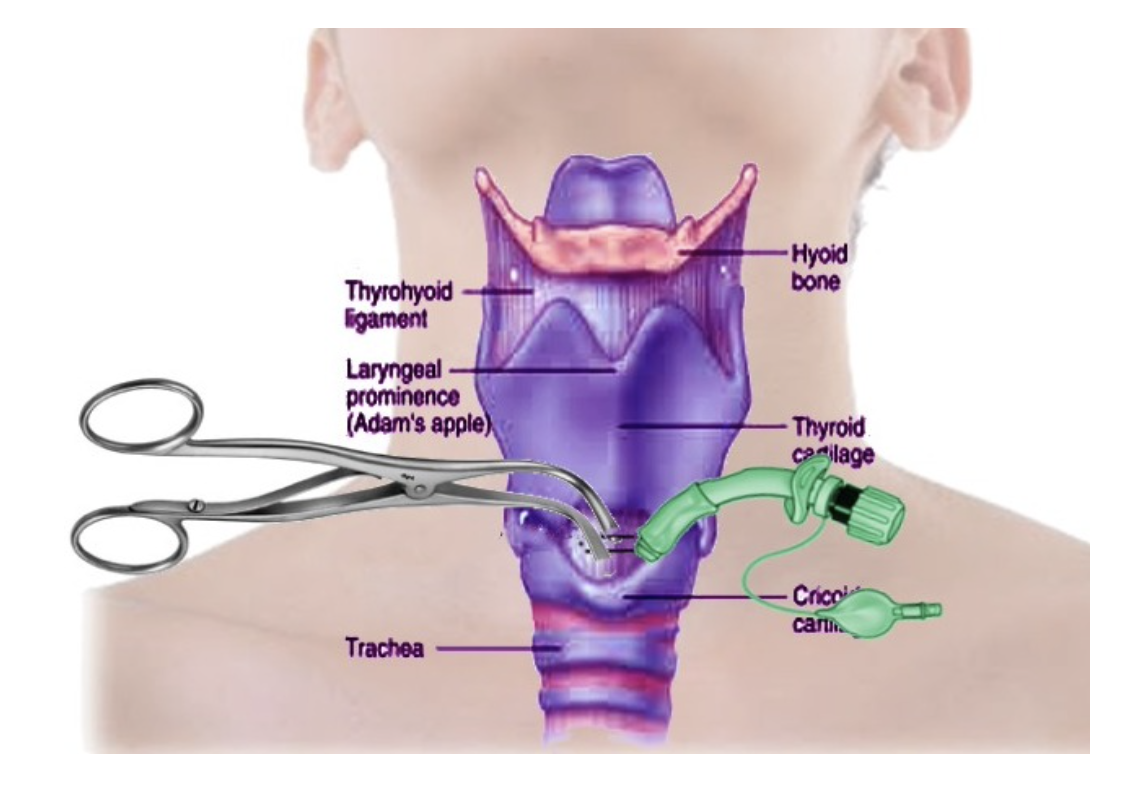 (Bhimji, 2021)
(Bhimji, 2021)
Tracheostomy Placement
The trachea is a part of the upper respiratory system that is approximately 4.5 inches long, connecting the subglottic larynx to the mainstem bronchi (see Figure 5). The trachea lies in the anterior portion of the neck and consists of incomplete cartilaginous rings (except the first ring, which is complete). The posterior wall of the trachea shares the anterior wall of the esophagus. The first ring of the trachea connects to the larynx and is called the cricoid cartilage. The trachea lies deep to the sternohyoid and sternothyroid muscles. The thyroid gland typically overlies the second to fourth tracheal rings. The recurrent laryngeal nerves and peritracheal lympho-fatty tissue lie lateral to the cervical trachea. These structures are surrounded by the middle layer of the deep cervical fascia, while the common carotid arteries lie lateral to these structures. Finally, the thymus and anterior mediastinal contents overlie the thoracic trachea, and the innominate artery crosses over the trachea as it arises from the aorta (Raimonde & Westhoven, 2021).
To understand the tracheostomy procedure, HCPs should be familiar with the following anatomic landmarks for tracheostomy (Raimonde & Westhoven, 2021):
- The thyroid notch is a palpable landmark that identifies the superior aspect of the larynx.
- The cricothyroid membrane is the location for an emergent cricothyrotomy. It is a palpable depression between the cricoid and the thyroid cartilages.
- The cricoid cartilage is a palpable landmark identifying the larynx and the trachea junction. The incision for a tracheostomy is usually placed 1 to 2 cm below the cricoid.
- The sternal notch is a palpable landmark that identifies the thoracic inlet. It is important to palpate this area before tracheostomy placement to identify a potentially high-riding innominate artery.
Figure 5
Anatomy of the Trachea
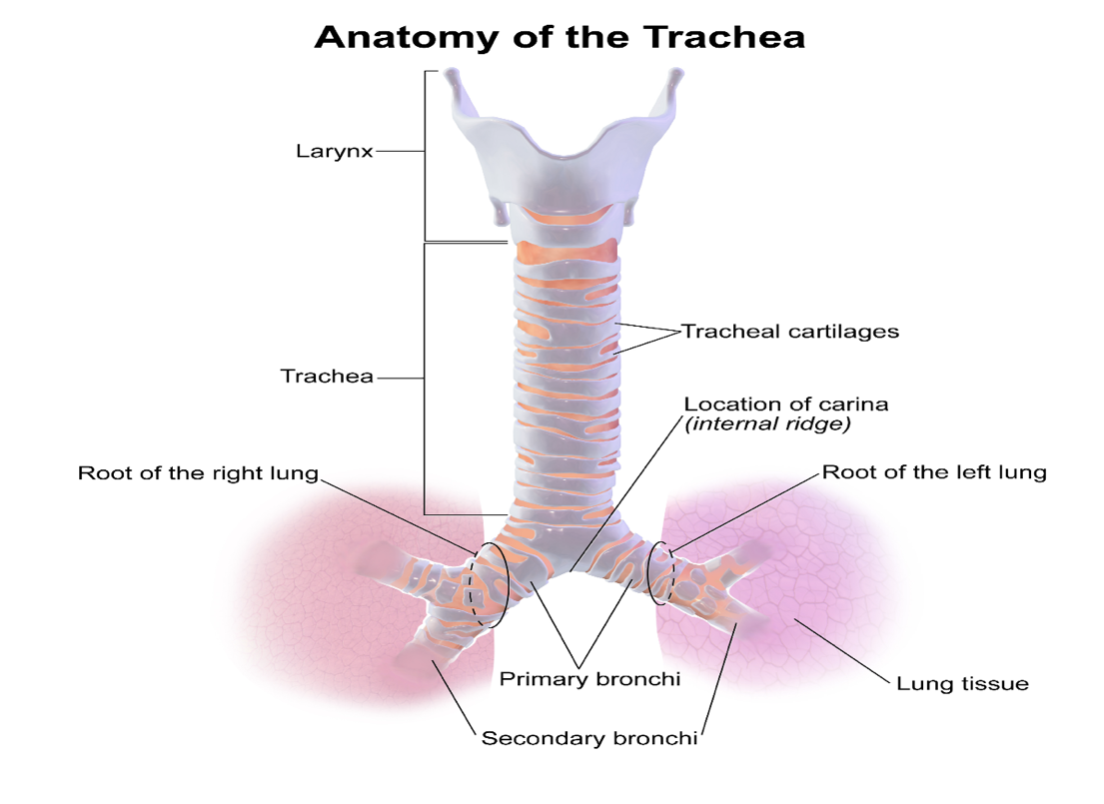 (BruceBlaus, 2013a)
(BruceBlaus, 2013a)
Tracheostomy versus ET Intubation
Although there is uncertainty about the optimal time frame for tracheostomy placement, research demonstrates clear benefits from tracheostomy placement versus prolonged endotracheal intubation. See Table 1 for the advantages and disadvantages of tracheostomy versus endotracheal intubation (American Thoracic Society, 2016b; Hyzy & McSparron, 2021b).
Table 1
Advantages and Disadvantages of Tracheostomy Versus Endotracheal Intubation (American Thoracic Society, 2016b; Hyzy & McSparron, 2021b)
(American Thoracic Society, 2016b; Hyzy & McSparron, 2021b)
In addition to the advantages described in Table 1, research has demonstrated better patient outcomes with tracheostomy placement over endotracheal intubation (American Thoracic Society, 2016b; Freeman, 2016; Hyzy & McSparron, 2021b).
- Work of breathing: For both ventilated and spontaneously breathing patients, a tracheostomy reduces the work of breathing, airway resistance, peak inspiratory pressures, and auto-PEEP. Because of these benefits, patients who are difficult to wean have shown greater improvement in weaning readiness (i.e., rapid shallow breathing index) with a tracheostomy than with endotracheal intubation. In addition, ventilator synchrony (i.e., when the ventilator provides flow and pressure as soon as the patient begins effort) and triggering may be improved. However, TV, respiratory rate, and dead space ventilation remain unchanged.
- Improved comfort: Tracheostomies are more comfortable and tolerable for patients. With the increased comfort for patients, the need for sedation is also reduced. When sedation is decreased, patients are more likely to engage in hygiene, weaning, and early mobilization.
- Facilitation of weaning: The placement of a tracheostomy tube can help to facilitate the weaning process for patients on the mechanical ventilator. The improved weaning success for patients with a tracheostomy is related to the decreased work of breathing described above. If a patient with a tracheostomy fails a weaning attempt, they can be reconnected to the ventilator without sedation and reintubation, as is necessary for intubated patients who fail a weaning attempt.
- Improved communication and swallowing: Patient communication is enhanced with a tracheostomy compared to endotracheal intubation due to communication devices and decreased sedation. In addition, the presence of a tracheostomy can promote oral hygiene and pulmonary toileting and allow for improved swallowing and oral nutrition.
- Other benefits: Patients with a tracheostomy often have improved mobility, increased secretion clearance, and decreased risk of laryngeal injury compared to endotracheal intubation. These patients can also move out of the ICU if their condition has stabilized, freeing much-needed ICU beds. Both tracheostomies and endotracheal intubation carry the risk of aspiration and pneumonia. It is unclear whether the placement of a tracheostomy decreases the risk of these complications based on conflicting data. Similarly, data vary on whether the placement of a tracheostomy decreases mortality compared to endotracheal intubation. However, tracheostomy-related deaths are more common among patients with cancer (i.e., oropharyngeal) and chronic lower respiratory tract disease. Patients who experience medical or surgical care complications and identify as African American or Hispanic American also experience more tracheostomy-related deaths (American Thoracic Society, 2016b; Freeman, 2016; Hyzy & McSparron, 2021b).
Timing of Tracheostomy Placement
The timing of elective tracheostomy placement for patients who fail to wean is debated. Therefore, daily assessment of the patient's progress and readiness to wean is essential when considering tracheostomy placement. Traditionally, tracheostomy placement was recommended between 5 and 7 days after ET intubation to minimize the risk of complications such as subglottic stenosis. However, studies have shown that early tracheostomy (i.e., before 10 days) has no proven benefit and may lead to unnecessary surgery and PMV for patients who may otherwise have been liberated from the ventilator (Freeman, 2016; Hyzy & McSparron, 2021b). For example, a meta-analysis of 14 studies found that early tracheostomy placement (i.e., within 10 days following intubation) did not reduce mortality, the duration of MV, ICU stays, or VAP compared to later tracheostomy placement. The researchers also found that early tracheostomy decreased the duration of sedation but significantly increased the number of procedures performed compared to late tracheostomies (Szakmany et al., 2014). In contrast, in another meta-analysis of 12 studies, patients who underwent early tracheostomy (i.e., within 10 days) had more ventilator-free days, shorter ICU stays, shorter duration of sedation, and reduced long-term mortality compared to later tracheostomy (i.e., after more than 10 days). However, early tracheostomy was associated with a higher overall rate of tracheostomy placement, suggesting that many patients in the later tracheostomy group avoided placement altogether (Hosokawa et al., 2015).
Although there is no optimal time for tracheostomy placement, patients should not be ventilated with an endotracheal tube for longer than 3 weeks unless they are unstable or unlikely to benefit from a tracheostomy. The time for the transition from ET tube to tracheostomy may vary among clinicians, usually occurring between 1 and 3 weeks of intubation. The decision of when to perform a tracheostomy should be collaborative, including the patient, their family, and the healthcare team. HCPs should consider the value of the tracheostomy, patient preferences, expected outcomes, and alternatives such as continued ET intubation or palliative care. If PMV is anticipated, early tracheostomy may be indicated. Later tracheostomies should be considered when successful extubation is anticipated. The need for advanced respiratory care has risen due to the COVID-19 pandemic, resulting in more endotracheal intubations. The optimal timing of tracheostomy placement in these patients is unknown. Since tracheostomy placement is an aerosol-producing procedure, the risk of COVID-19 transmission is increased. Later timing for tracheostomy placement may be justifiable in this patient population because the risk of transmission is reduced later in the course of illness. In addition, many COVID-19 patients can be safely extubated at this time, potentially avoiding the need for a tracheostomy (Freeman, 2016; Hyzy & McSparron, 2021b).
Tracheostomy Procedure
A tracheostomy generally takes about 20 to 45 minutes to perform and is done in an operating room (i.e., open surgery) or at the patient's bedside in the ICU (i.e., percutaneously; National Heart, Lung, and Blood Institute, n.d.). A surgeon, a surgical assistant, an anesthesiologist, and a scrub tech are usually needed for open tracheostomy placement in the operating room. In contrast, a percutaneous tracheostomy at the patient’s bedside requires two physicians (for performing the percutaneous incision at the neck and using a bronchoscope to visualize the passage of the wire and tube into the trachea). Finally, open tracheostomies are usually performed under general anesthesia, while percutaneous tracheostomies are typically performed with a local anesthetic to numb the neck and throat. Preoperative assessment for both open and percutaneous tracheostomies should include medical history, physical assessment, functional status, and review of imaging studies to determine the underlying pathology. In particular, HCPs should determine which positions, breathing techniques, and maneuvers improve or worsen breathing (Hyzy & De Cardenas, 2021).
Preoperative Assessment
Once a tracheostomy is indicated, HCPs must select an approach to tracheostomy placement. Surgical (open) tracheostomy was the only option in years past, with percutaneous tracheostomy procedures increasing since the late 1990s. Specific training is required to perform both tracheostomy techniques (Hyzy & De Cardenas, 2021). Factors that influence the choice between surgical versus percutaneous tracheostomy can include (Hyzy & De Cardenas, 2021):
- Institutional expertise: Most institutions will have providers trained to place surgical tracheostomies. Percutaneous tracheostomies require a different skillset and are usually performed by surgeons, interventional pulmonologists, or intensivists.
- Select patient conditions: A surgical tracheostomy may be preferred for patients with the following:
- a short neck with an inability to identify and palpate the trachea
- vascular structures (e.g., high-riding innominate or thyroid internal mammary artery)
- gross distortion of the neck (e.g., hematoma, tumor, thyromegaly, or scarring)
- severe tracheomalacia with cartilage destruction
- an inability to extend the neck safely (e.g., cervical fusion, rheumatoid arthritis, or trauma)
- prior complex tracheal surgery (e.g., tracheoplasty, tracheal resection, or reconstruction)
- children younger than 15 years
- patients requiring emergency airway
The boundaries of what is considered a contraindication for percutaneous tracheostomy have continued to evolve with improvements in technique. Percutaneous tracheostomy has been successfully performed in the following cases:
- older patients (i.e., over 80 years old)
- patients with grade 3 obesity
- patients receiving PEEP at greater than 12 cm H2O, high-frequency oscillation ventilation, or extracorporeal membrane oxygenation
- patients taking antiplatelet therapy
- Advantages and disadvantages of percutaneous tracheostomy: Advantages of percutaneous tracheostomies are that they can be performed at the bedside and are quicker and less expensive. Complications such as bleeding and infection are less likely for patients who undergo percutaneous versus surgical tracheostomy placement. However, there is an increased risk of anterior tracheal injury (i.e., ring fractures) and posterior tracheal wall perforation in percutaneous tracheostomy placement (Hyzy & De Cardenas, 2021).
Selecting a Tracheostomy Tube
Tracheostomy tubes are commercially available in various dimensions and styles and can be custom designed for patients with anatomical variants. The tubes can be made of metal (i.e., stainless steel or silver), a nonmetal (i.e., polyvinyl chloride [PVC], silicone, or polyurethane), or hybrid (i.e., combined pliable material with metal spiral wire reinforcement to enhance airway comfort and conformation). Metal tracheostomy tubes are rarely used in critical care settings as they are expensive, rigid, and do not have cuffs that allow for mechanical ventilation. However, metal tracheostomy tubes are durable, easy to clean (i.e., via repeated sterilization by autoclaving for reuse by the same patient), and are more resistant to infections than nonmetal tubes. Therefore, these tubes are suitable for life-long tracheostomy patients who do not need ventilation. Nonmetal tubes typically have an inflatable cuff and a universal adapter that attaches to ventilator tubing. These tubes are cheaper and do not require sterilization. Instead, they are replaced every 6 to 8 weeks. Nonmetal tracheostomy tubes are less rigid and will conform to the shape of the airway as PVC softens at body temperature (Hyzy & De Cardenas, 2021).
Nonmetal tracheostomy tubes have the following components (see Figure 6; Hyzy & De Cardenas, 2021):
- The connector/adapter connects the ventilator to the tracheostomy tube. For some tubes, the connector is part of the inner cannula.
- The flange is between the connector and the shaft of the tube. The flange has side holes for tracheostomy ties that secure the tracheostomy tube to the neck.
- The tube shaft leads from the connector to the distal tip, usually tapered for easy insertion. The shaft is angled or curved to accommodate its position in the neck/trachea.
- The cuff appears just above the distal tip of the shaft and can be inflated using a pilot balloon to create a one-way valve. Some tracheostomy tubes are cuffless.
- The inner solid trocar (obturator) is smooth and rounded and has a tapered distal end that aids in the placement or replacement of the tracheostomy tube. It should be removed once the tube is in place.
Figure 6
Nonmetal Tracheostomy Tube
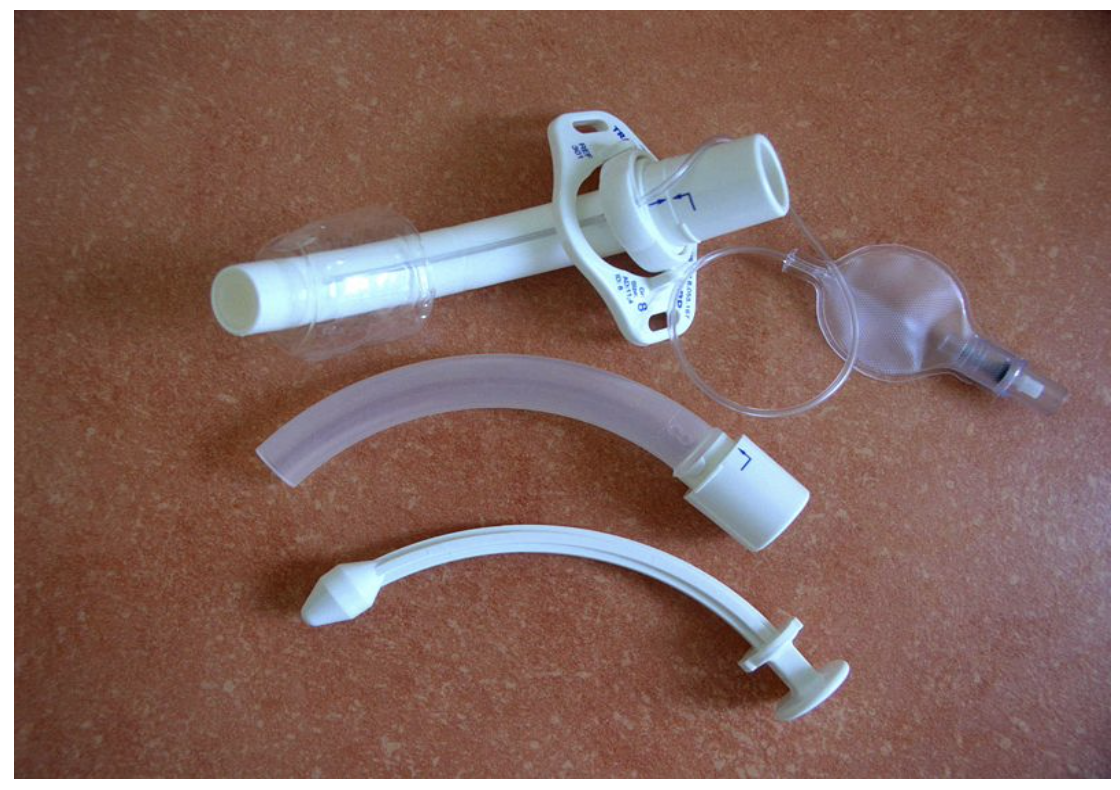
(Klaus, 2008)
Tracheostomy tubes can have a single lumen (i.e., single-cannula tube) or an inner cannula (i.e., dual-cannula tube). The inner cannula (i.e., narrow, and hollow) lies inside the main lumen of the tracheostomy tube. Therefore, the inner cannula can be easily removed and cleaned regularly without removing the tracheostomy tube from the stoma, keeping the airway intact and stable. The narrow lumen of the inner cannula can increase airway resistance; therefore, it is often removed during MV. However, some tracheostomy tubes have the ventilator adapter connected to the inner cannula, which cannot be detached. The shaft of the tracheostomy tube can be angled or curved to accommodate the tract along which the tube lies. Angled tubes generally have an acute angle and a straight shaft, whereas curved tubes have a gentle bend throughout their entire length. Angled tubes are preferred because there is less risk for a posterior wall injury than curved tubes. The dimensions of a tracheostomy tube are measured in millimeters (mm) and can be found on the packaging and the flange. However, the same tube dimensions can vary slightly among manufacturers. The length of the tracheostomy tube includes the entire length from the adapter to the distal tip and usually varies from 60 mm to 120 mm. Some tubes have an adjustable flange to lengthen or shorten the tube as needed. The inner and outer diameters of the tracheostomy tube refer to the smallest and widest dimensions. The inner diameter correlates with the diameter of the ET tube (Hyzy & De Cardenas, 2021).
Most tracheostomy tubes are nonfenestrated (i.e., do not have any holes along the shaft of the tube) and have an inflatable cuff, although variations do exist. For patients receiving MV through a tracheostomy, a nonfenestrated tracheostomy tube with an inflated cuff is required to ensure the delivery of the set TV or pressure and prevent air leaks. Fenestrated tubes are not frequently used due to the risk of granulation tissue formation from turbulent flow through the fenestration onto the tracheal wall (see Figure 7). When the cuff is inflated, a fenestrated tube allows air to flow around the tube, promoting respiration and phonation through the vocal cords. In contrast, if a nonfenestrated tube is present, the cuff must be deflated for this to occur. For fenestrated tubes, an inner cannula will block the fenestrations unless the inner cannula also has fenestrated holes. Like fenestrations in a tracheostomy tube, a deflated cuff allows air to move around the tube, facilitating phonation and respiration through the vocal cords. Most cuffs are high-volume/low-pressure and are inflated with either air or sterile water. The cuff obtains a tight seal while also limiting pressure on the tracheal wall (i.e., 20 to 30 cm H2O), thereby reducing hyperinflation. Complications associated with hyperinflation can include tracheal stenosis, necrosis with perforation of the tracheal wall, or tracheal fistulas (Hyzy & De Cardenas, 2021; Raimonde & Westhoven, 2021).
When selecting the appropriate tracheostomy tube for a patient, HCPs should consider the patient's neck and tracheal size, age, weight, and height. In addition, HCPs should consider the purpose of the tracheostomy (e.g., airway secretion clearance, phonation, weaning) and the underlying tracheal pathology (e.g., tracheal stenosis or distortion). Most patients will start with a standard, commercially available tracheostomy tube. For patients with the goal of weaning and decannulation, a size 6 to 8 nonfenestrated, cuffed tracheostomy tube is appropriate. In contrast, a nonfenestrated, cuffed, or cuffless tracheostomy tube is appropriate for patients with upper airway obstruction or who need airway secretion clearance. Next, the dimensions of the tracheostomy tube must be chosen. Initially, most patients will be fitted with a standard-dimension tracheostomy tube that can be changed later to improve comfort and fit (Hyzy & De Cardenas, 2021; Raimonde & Westhoven, 2021).
HCPs should consider the following when sizing an initial tracheostomy (Hyzy & De Cardenas, 2021):
- Diameter: Most adult patients will initially be fitted with a 6 mm to 8 mm diameter tracheostomy tube. An appropriate diameter is necessary to maintain a good seal and minimize airway resistance and work of breathing. Higher cuff pressures are needed to maintain a good seal if the inner tracheostomy tube diameter is too small. Higher cuff pressures can increase airway resistance, work of breathing, and the risk of tracheal injury or stenosis. These changes also interfere with ventilator weaning. In contrast, a fully inflated cuff is necessary if the diameter of the tracheostomy tube is too large. A fully inflated cuff can increase tracheal wall pressure, leading to stenosis. Smaller tracheostomy tubes (i.e., 4 mm to 6 mm) are generally selected for patients who are underweight, are younger, or have a small body habitus. A large tracheostomy tube (i.e., 8 mm) is generally chosen for patients who may need a bronchoscopy (e.g., for an obstructing tumor).
- Length: Most tracheostomy tubes come in a standard length (i.e., 60 mm to 65 mm targeted at 4 cm to 6 cm above the carina). Selecting the appropriate length maximizes patient comfort, fit, and positioning in the tracheal lumen. Selecting a tracheostomy tube that is too short can place the cuff in the stoma. When this occurs, patients can have air leaks, high cuff pressures, and malposition of the trachea's distal opening, leading to granulation tissue formation or obstruction of the lumen by the posterior tracheal wall. An extra-long tracheostomy tube may be appropriate for patients with a thick anterior neck (i.e., over 4.4 cm) due to anatomical structures or obesity. Other patients who may benefit from an extra-long tracheostomy tube include those who are tall or have tracheal stenosis, tracheoesophageal fistulas, or tumors in the mid-trachea (i.e., to bypass structural abnormalities).
- Dual or single cannula: A single-lumen tracheostomy tube is selected for patients with tenacious secretions. A dual-lumen tracheostomy tube can be used if the inner cannula is removed to facilitate airway clearance.
Figure 7
Fenestrated versus Nonfenestrated Tracheostomy Tubes
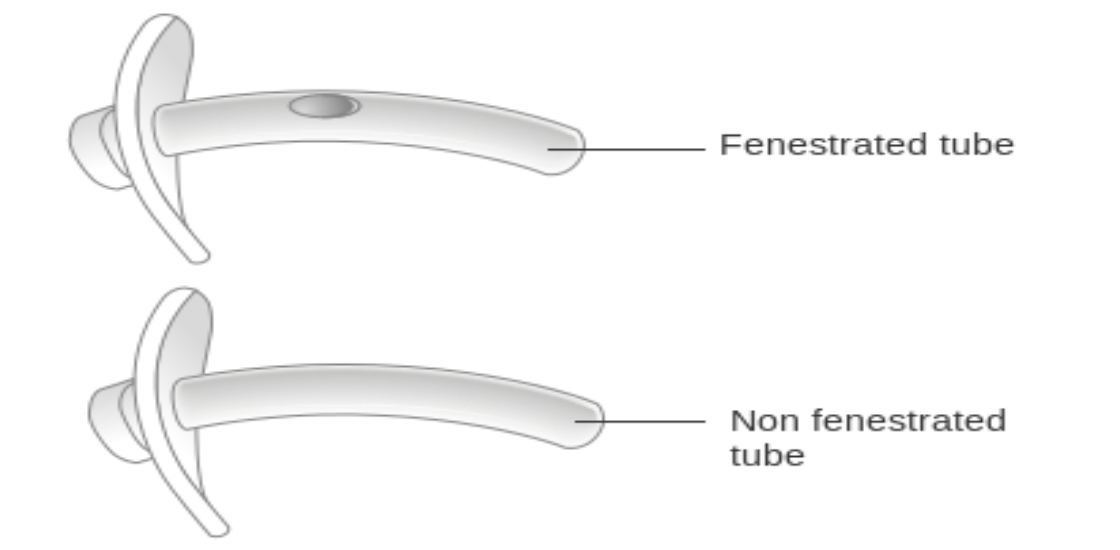
(Cancer Research UK, 2014)
A tracheostomy tube should be placed between the second and third tracheal rings whenever possible (see Figures 8 and 9). Tube placement below the third tracheal ring can increase the risk of innominate artery bleeding, while placement above the first tracheal ring can increase the risk of subglottic stenosis. A bedside ultrasound is recommended to identify vascular and other anatomical structures (i.e., thyroid, tracheal rings), particularly for patients at risk of complications and those undergoing percutaneous tracheostomy. A pre-procedure checklist should be used to ensure all necessary equipment (i.e., airway cart, tracheostomy kit) and staff are present for the intended procedure. Since tracheostomy placement is an aerosol-generating procedure, full personal protective equipment should be used for patients with potentially infectious secretions. Finally, HCPs should immediately confirm the proper inflation of a cuffed tracheostomy tube to reduce the risk of ventilatory failure or emergent airway loss (Hinkle & Cheever, 2018; Hyzy & De Cardenas, 2021; Raimonde & Westhoven, 2021).
Elective, open surgical tracheostomy placement is generally performed in the operating room with general anesthesia. However, this procedure may be performed using a local anesthetic in rare circumstances (e.g., submandibular space infections). First, the surgeon will palpate and mark the thyroid notch, cricoid cartilage, and sternal notch (paying close attention to detecting a high-riding innominate artery). During the procedure, the surgeon will make a 2 cm to 3 cm horizontal or vertical incision through the skin on the anterior part of the lower neck, midway between the sternal notch and cricoid cartilage. Next, the surrounding strap muscles (i.e., sternothyroid and sternohyoid) are pulled back, and then a small portion of the thyroid gland is cut to expose the trachea. An additional incision is made between the second and third tracheal rings. Next, a Bjork flap is created by cutting part of the tracheal cartilage, then folding and suturing the flap to maintain a patent stoma. The tracheostomy tube is then placed through the stoma. Finally, the tracheostomy tube is secured in place with a soft-transcervical tie. (Hyzy & De Cardenas, 2021; Mayo Clinic, 2019; Raimonde & Westhoven, 2021; Rashid & Islam, 2017).
Percutaneous tracheostomy placement is generally performed at the bedside in the ICU. However, it can also be done for patients at risk for complications (e.g., bleeding) in the operating room. This procedure is preferably performed under deep sedation and paralysis using a short-acting neuromuscular blocker. A local anesthetic is also injected into the anterior neck. Patients should have an airway in place to maintain adequate ventilation during the procedure. First, the surgeon will palpate and mark the thyroid notch, cricoid cartilage, and sternal notch (paying close attention to a high-riding innominate artery). A percutaneous technique is not recommended if the cricoid cartilage or tracheal rings cannot be palpated. Next, a bronchoscope is inserted through the ET tube, and an airway examination is performed. The cuff is deflated, and the ET tube is slowly retracted to the level of the cricoid cartilage. Once the tip of the ET tube is positioned at the level of the cricoid cartilage, the cuff of the ET tube is re-inflated. A 1.5 cm to 2 cm vertical incision is made in the anterior midline neck. A needle is then inserted through the tracheal rings (either first and second or second and third). Next, using the Seldinger technique, a guidewire is inserted through the needle, and the needle is removed. A straight-punch dilator is inserted over the guidewire. A second dilator (curved and tapered) is used to dilate the stoma. Once the stoma is adequately dilated, a tracheostomy tube is inserted over the guidewire and into the trachea. The bronchoscope is removed from the ET tube and then inserted into the tracheostomy tube to confirm placement. The distal end of the tube should be at least 1 cm proximal to the main carina. Finally, the ET tube is completely removed, and the tracheostomy tube is secured with velcro ties or sutures (Hyzy & De Cardenas, 2021; Mayo Clinic, 2019; Raimonde & Westhoven, 2021; Rashid & Islam, 2017).
Figure 8
Tracheostomy Tube Placement
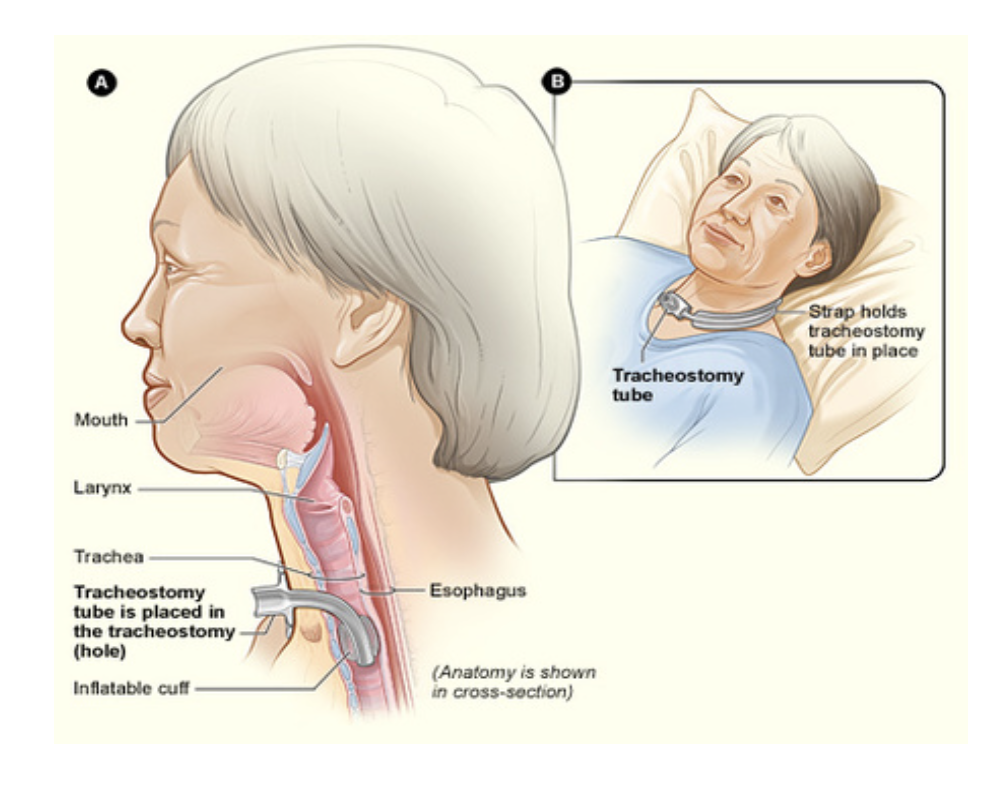
Figure 9
Tracheostomy Tube Placement

Tracheostomy Complications
Tracheostomies are routinely performed and are generally considered safe, with similar complication rates between surgical and percutaneous tracheostomies. Tracheostomy complications can be intraoperative, early, and late. Intraoperative complications can include bleeding, posterior wall perforation, tracheal ring fracture, pneumothorax/pneumomediastinum, and airway loss or fire. HCPs caring for patients during the tracheostomy placement should be aware of these complications and strategies to mitigate them (Hyzy & De Cardenas, 2021; Mayo Clinic, 2019; Raimonde & Westhoven, 2021; Rashid & Islam, 2017).
- Bleeding is the most common intraoperative complication; it can lead to death when severe. Many patients requiring tracheostomy placement are critically ill and have underlying coagulopathy. Risk factors for bleeding can include a platelet count less than 50 x 109/L, activated partial thromboplastin (APTT) longer than 50 seconds, or the presence of two or more coagulation disorders. The administration of prophylactic subcutaneous heparin for deep vein thrombosis (DVT) prevention does not increase the risk of bleeding. When comparing both tracheostomy techniques, bleeding is more common in surgical than percutaneous tracheostomy placement. A platelet transfusion can minimize intraoperative bleeding for thrombocytopenic patients. In addition, ensuring the correct anatomical location for tube placement (i.e., not below the second or third tracheal ring) can prevent innominate artery bleeding. Finally, utilizing a small incision and lidocaine with 1.5% epinephrine can decrease the risk of bleeding.
- Esophageal perforation is a rare complication that occurs when a perforation of the posterior wall of the trachea carries through to the esophagus. Directing the bevel of the introducer needle and dilators caudally (distally) during insertion can reduce the risk of perforation.
- Tracheal ring fracture occurs in 3% to 36% of tracheostomy procedures, with an increased risk in percutaneous tracheostomy placement due to the pressure on the trachea from repetitive dilation. In addition, calcified tracheal rings increase the risk of fracture. Tracheal ring fractures rarely require treatment unless they are displaced.
- Pneumothorax/pneumomediastinum occurs when the tracheostomy tube is not correctly placed into the trachea. Instead, it is placed into a false passage anterior to the trachea. Pneumothorax or pneumomediastinum can also occur during percutaneous tracheostomy procedures if the guidewire punctures the posterior or lateral wall of the trachea. A chest radiograph is used to confirm a pneumothorax or pneumomediastinum. The tracheostomy should be removed, and a chest tube should be placed if confirmed. To decrease the risk of a pneumothorax or pneumomediastinum, HCPs should pay close attention to landmarks when placing the tracheostomy tube and use capnography or fiberoptic endoscopy to confirm tube placement.
- Loss of airway refers to accidental extubation during surgical and percutaneous tracheostomy placement. This complication occurs more frequently in percutaneous approaches when the patient is not adequately paralyzed and coughs during the procedure. The loss of an airway can result in death; therefore, HCPs should ensure adequate paralysis and good communication among staff during the procedure. If the airway is lost, a new one should be established immediately by intubation or completion of the tracheostomy.
- Airway fire occurs during a surgical tracheostomy from oxygen combustion by the electrocautery unit. Although rare, airway fire is more likely to occur in patients needing high FiO2. HCPs can minimize the risk of this complication by ensuring good communication during the procedure about when the electrocautery is being used. If an airway fire occurs, the patient should be immediately disconnected from the anesthesia circuit and manually bagged with a mask until the tracheostomy is completed (Hyzy & De Cardenas, 2021; Mayo Clinic, 2019; Raimonde & Westhoven, 2021; Rashid & Islam, 2017).
Early Postoperative Complications
Many patients who undergo tracheostomy placement will experience minor complications, such as pain and minor bleeding. Although more severe complications are rare, HCPs should be aware of potential complications in the postoperative period, including strategies to mitigate these complications. Postoperative complications can be categorized as early (i.e., occurring within 7 to 10 days of placement) or late (i.e., 10 days after placement). Early postoperative complications can include bleeding, pneumothorax, infection, obstruction, and accidental decannulation (Hyzy & De Cardenas, 2021; Mayo Clinic, 2019; Raimonde & Westhoven, 2021; Rashid & Islam, 2017).
- Bleeding from the stoma site during the early postoperative period is usually minor and resolves within a few days. HCPs should routinely monitor the stoma site, assessing the frequency of dressing changes and the quality of secretions during tracheal suctioning. Minor bleeding usually resolves within a few days without treatment. Significant bleeding (i.e., frequent saturation of dressings or frank bleeding) should be reported immediately, as tracheostomy revision may be necessary. Bleeding complications are more frequent in patients who undergo surgical tracheostomy than percutaneous tracheostomy.
- Pneumothorax and subcutaneous emphysema are rare in the early postoperative period, occurring in 0.8% and 1.4% of cases, respectively. As discussed above, these complications are most likely due to creating a false tract anterior to the trachea. In addition, perforation of the posterior tracheal wall during percutaneous tracheostomy placement can also lead to pneumothorax or subcutaneous emphysema. A chest radiograph should be ordered if suspected, and chest tube placement may be indicated. If a pneumothorax is confirmed, the tracheostomy tube should be removed, and the patient should be ventilated through an ET tube.
- Infections (e.g., mild cellulitis at the stoma site or tracheitis) are generally minor complications after tracheostomy placement and are more likely to occur in surgical tracheostomy than percutaneous tracheostomy. Signs of infection can include redness, warmth, and purulent drainage. These infections can be treated with wound care, routine stoma site cleaning, tracheal suctioning, and dressing changes. Although rare, more severe infections (e.g., stomal abscess formation, osteomyelitis, mediastinitis, necrotizing fasciitis, and septic arthritis of the sternoclavicular joint) will require the administration of systemic antibiotics.
- Obstruction can occur in the early or late postoperative periods due to thickened secretions or blood obstructing the tracheostomy tube. Signs of obstruction can include acute respiratory distress, respiratory arrest, and hypoxia. Treatment for an obstructed tracheostomy includes removing and inspecting the potentially obstructed equipment (i.e., ventilator circuit, speaking valve, or inner cannula) and suctioning the tracheostomy tube. Protocolized tracheostomy care (i.e., regular suctioning, humidified oxygen, and scheduled daily cleaning or replacement of the inner cannula) can prevent obstruction from occurring.
- Tracheostomy tube dislodgement can occur accidentally during patient procedures, transport, turning, and bouts of coughing. Accidental decannulation occurs in up to 15% of tracheostomy patients, with a higher risk in those who undergo percutaneous tracheostomy than surgical tracheostomy. Other risk factors include traumatic brain injury, increased secretions, recent tube changes, increased neck thickness, and altered mental status. Accidental decannulation is a serious complication, particularly within the first week of a tracheostomy placement since the tract has not epithelialized. Management usually includes ET intubation until the tracheostomy can be replaced in a controlled setting. This involves fiberoptic guidance to avoid the creation of a false tract (Hyzy & De Cardenas, 2021; Mayo Clinic, 2019; Raimonde & Westhoven, 2021; Rashid & Islam, 2017).
Late Postoperative Complications
Mortality related to tracheostomy placement is rare (less than 1%) and is usually related to underlying cardiopulmonary disease. Late postoperative complications (i.e., after more than 10 days) include tracheal stenosis, tracheoarterial fistula, obstruction, tracheomalacia, tracheoesophageal fistula, tracheostomy tube dislodgement, aspiration, and nosocomial pneumonia, reduced phonation, and dysphagia (Hyzy & De Cardenas, 2021; Mayo Clinic, 2019; Raimonde & Westhoven, 2021; Rashid & Islam, 2017).
- Tracheal stenosis occurs in up to 16% of patients due to tracheal injury or ischemia from cuff overinflation. Tracheostomy-related tracheal stenosis tends to be thicker and more circumferential, occurring later than ET tube stenosis. Tracheal stenosis can develop along the shaft or tip of the tracheostomy tube (due to unusual tube angulation) at the cuff site. However, this complication is more likely to occur in the subglottic area with fenestrated tubes. Other risk factors can include obesity, hypotension, and surgical site infection. Approximately 3% to 12% of tracheal stenoses require treatment (i.e., endoscopic stenting, balloon dilations, laser resection); most cases are asymptomatic. A patient becomes symptomatic when the degree of stenosis is greater than 50% of the tracheal diameter, presenting with dyspnea, stridor after decannulation, or inability to tolerate a speaking valve or tracheostomy cap.
- Stoma stenosis occurs less commonly than tracheal stenosis. It is caused by trauma from tube insertion, excessive movement at the entry site, or pressure on the cricoid or tracheal cartilage from unsupported tubing. HCPs should suspect stomal stenosis if resistance is met with tracheostomy tube exchange. Treatment can include stomal dilation, cautery with silver nitrate sticks, or surgical revision.
- Tracheoarterial fistula is a rare (affecting less than 1% of patients) but often fatal complication (fewer than 14% of patients survive) due to massive hemorrhage. These fistulas occur due to erosion from the tracheostomy tube tip or cuff into the anterior wall of the trachea. Once the erosion appears, a fistula is created with the innominate artery, leading to a massive hemorrhage (usually 3 to 4 weeks after tracheostomy placement). Various factors can increase the risk of fistula formation, including placement of the tracheostomy below the third tracheal ring, a high-riding innominate artery, tracheostomy tubes that are too long or large, excessive tube movement, overinflation of the cuff, and tracheostomy infection. As many as 50% of tracheoarterial fistulas begin with a small initial bleed, followed by massive hemorrhage hours to days later; therefore, all bleeding instances should trigger fistula formation evaluation using angiography, computed tomography, or bronchoscopy. Treatment involves surgical ligation of the innominate artery.
- Obstruction in the late postoperative period can occur due to secretions or a foreign body. Treatment is like obstruction in the early postoperative period, except the tracheostomy tube can be more easily exchanged.
- Tracheomalacia can develop weeks to months after tracheostomy placement due to thinning and destruction of cartilaginous tissues from elevated cuff pressures (i.e., greater than 25 cm H2O). Signs and symptoms of tracheomalacia dyspnea, cough, and sputum retention. Treatment can include placing a longer tracheostomy tube that bypasses the affected segment. In addition, an airway stent or surgical resection may be needed for long-term tracheomalacia.
- Tracheoesophageal fistula (TEF) is less common in patients with a tracheostomy (affecting less than 5%) than prolonged ET intubation. TEF is due to the posterior orientation of the tracheostomy tube or excessive pressure on the posterior tracheal wall from an overinflated cuff. Other risk factors include pressure from a nasogastric tube, excessive movement, high airway pressures, steroid use, diabetes, poor nutritional status, and gastroesophageal reflux (GERD). TEF may present with recurrent aspiration pneumonia or hypoxemic events, acute respiratory distress, the presence of enteral feed during suctioning, and gastric distention. Spontaneous closure of a TEF is rare and surgical correction is the procedure of choice for TEF closure.
- Tracheostomy tube dislodgement is discussed in detail in the early postoperative complications above. Accidental dislodgement in the late postoperative period is usually resolved by replacing the tube. Replacement should occur quickly as the tract can undergo spontaneous closure within hours to days of decannulation.
- Aspiration occurs in 30% to 50% of patients with a tracheostomy tube due to pooling pharyngeal secretions above the airway cuff and delayed triggering of the swallowing response. Occlusion of the tracheostomy tube with a cap does not significantly increase the risk for aspiration; similarly, keeping the cuff inflated does not decrease the risk of aspiration. About 75% to 82% of aspiration cases are clinically silent, making detection difficult. Aspiration is only treated with antibiotics when bacterial superinfection occurs. Nosocomial pneumonia occurs more frequently in patients with underlying medical conditions and immunosuppression. The timing of the tracheostomy (early versus late) does not appear to impact the rate of pneumonia.
- Reduced phonation occurs after tracheostomy placement and generally improves over weeks to months. However, prolonged symptoms may require a laryngoscope evaluation and specialized speech services for some patients.
- Dysphagia can occur in patients after decannulation due to oropharyngeal weakness or skin that adheres to the trachea. Swallowing should return to normal in most patients, although some may have long-term deficits. After decannulation, a swallow evaluation is recommended to evaluate dysphagia. Patients should work with a speech and language pathologist (SLP) for dysphagia treatment (Hyzy & De Cardenas, 2021; Mayo Clinic, 2019; Raimonde & Westhoven, 2021; Rashid & Islam, 2017).
Tracheostomy Removal
Decannulation refers to removing a tracheostomy tube once the patient has been weaned from mechanical ventilation, has a strong cough, and can clear secretions. The timing of tracheostomy removal often depends on why it was placed. Once the patient can tolerate a tracheostomy mask for 24 hours or longer, MV is no longer required, and HCPs should consider decannulation. An upper airway assessment should be completed next, looking for airway patency. The patient must be able to protect their airway for decannulation to be considered. The HCP should deflate the cuff, occlude the tracheostomy tube, and ask the patient to speak a few words. Decannulation should be successful if the patient can phonate comfortably without labored breathing. Endoscopic examination of the upper airway, vocal cords, subglottic space, and trachea should be done if the patient cannot phonate or has labored breathing. Next, the HCP should assess the ability of the patient to cough and clear their secretions. A peak cough flow (i.e., with a tracheostomy adapter) should be considered for patients with respiratory muscle weakness. A peak cough flow greater than 160 L/minute is suggestive of successful decannulation. Decannulation is not recommended for patients with thick, tenacious secretions (American Thoracic Society, 2016b; Hyzy & De Cardenas, 2021; Rashid & Islam, 2017).
Techniques used to wean patients from a tracheostomy tube vary among clinicians and institutions, with no specific technique appearing superior to others. However, the 2021 guidelines from the American Association of Respiratory Care (AARC) recommend a weaning/decannulation protocol to guide the weaning and removal of the tracheostomy (Mussa, 2021).
- Progressive capping trials are a relatively common technique, particularly in long-term care. These capping trials involve deflating the cuff and placing a cap over the tracheostomy tube. The patient is observed for tolerance (i.e., no respiratory distress or secretion retention), with supplemental oxygen via a nasal cannula occasionally needed. Capping trials usually start at 2 to 4 hours, progressing to 48 hours (usually over a 1- to 2-week time frame). An upper airway assessment with bronchoscopy should be used to evaluate for tracheomalacia or stenosis if capping trials fail.
- Another technique for tracheostomy weaning involves progressively decreasing the size of the tracheostomy tube. This technique is recommended for patients with larger tubes (i.e., size 8). The HCP should reduce the tube size every 1 to 2 days until spontaneous breathing is documented with the smallest tube (i.e., size 4).
- The third technique for tracheostomy weaning is immediate decannulation, which involves an abbreviated capping trial (i.e., 12 hours) and no downsizing of the tube in patients whose airway has been visually inspected. This technique is only appropriate for a small subset of patients, and HCPs should maintain telemetry and oximetry monitoring for 24 hours after decannulation (Hyzy & De Cardenas, 2021; Rashid & Islam, 2017).
Most tracheocutaneous tracts will begin to close within the first 48 hours after decannulation. By day 7, the tract should be closed or almost closed. If the tract has not closed after 3 to 6 months, a tracheocutaneous fistula is diagnosed. A prolonged tracheostomy, corticosteroid use, malnutrition, and advanced age increase the risk of tracheocutaneous fistulas. Patients may have a weak cough and phonation, skin irritation, infection, and recurrent aspiration. Treatment includes excision, cauterization, and occasionally surgical closure. A tracheostomy plug may be needed during weaning trials, particularly for patients who have borderline secretion clearance. A tracheostomy plug is a small device that can be placed through the stoma after decannulation. Factors that determine successful decannulation are poorly defined. If decannulation fails, the reason for failure should be determined and treated before reattempting decannulation. If decannulation fails before the stoma is closed, a mini-tracheostomy can be placed for suctioning and ventilation while the tract is reopened with dilators. Orotracheal intubation is needed if the stoma has closed (Hyzy & De Cardenas, 2021; Rashid & Islam, 2017).
Tracheostomy Care
Most patients will be transferred to an ICU in the immediate postoperative period. The AARC guidelines (2021) recommend using protocolized care bundles following the placement of a tracheostomy. Tracheostomy bundles should be created and approved by a multidisciplinary team of individuals experienced in tracheostomy management. This approach has been shown to decrease tracheostomy-related complications and decannulation time and improve oral diet tolerance. The guidelines also recommend creating a multidisciplinary tracheostomy team for adult patients in an acute care setting. The composition of the multidisciplinary team can vary among institutions but usually includes physicians, advanced practice providers (APPs), nurses, respiratory therapists, speech and language pathologists, dietitians, and social workers. A multidisciplinary tracheostomy team has been shown to decrease LOS, tracheostomy-related complications, and decannulation time (Mussa et al., 2021). Guidelines for postoperative tracheostomy care can be differentiated into the immediate postoperative period, days 0 to 4, and subsequent care (see Table 2; Hyzy & De Cardenas, 2021).
Table 2
Guidelines for Tracheostomy Care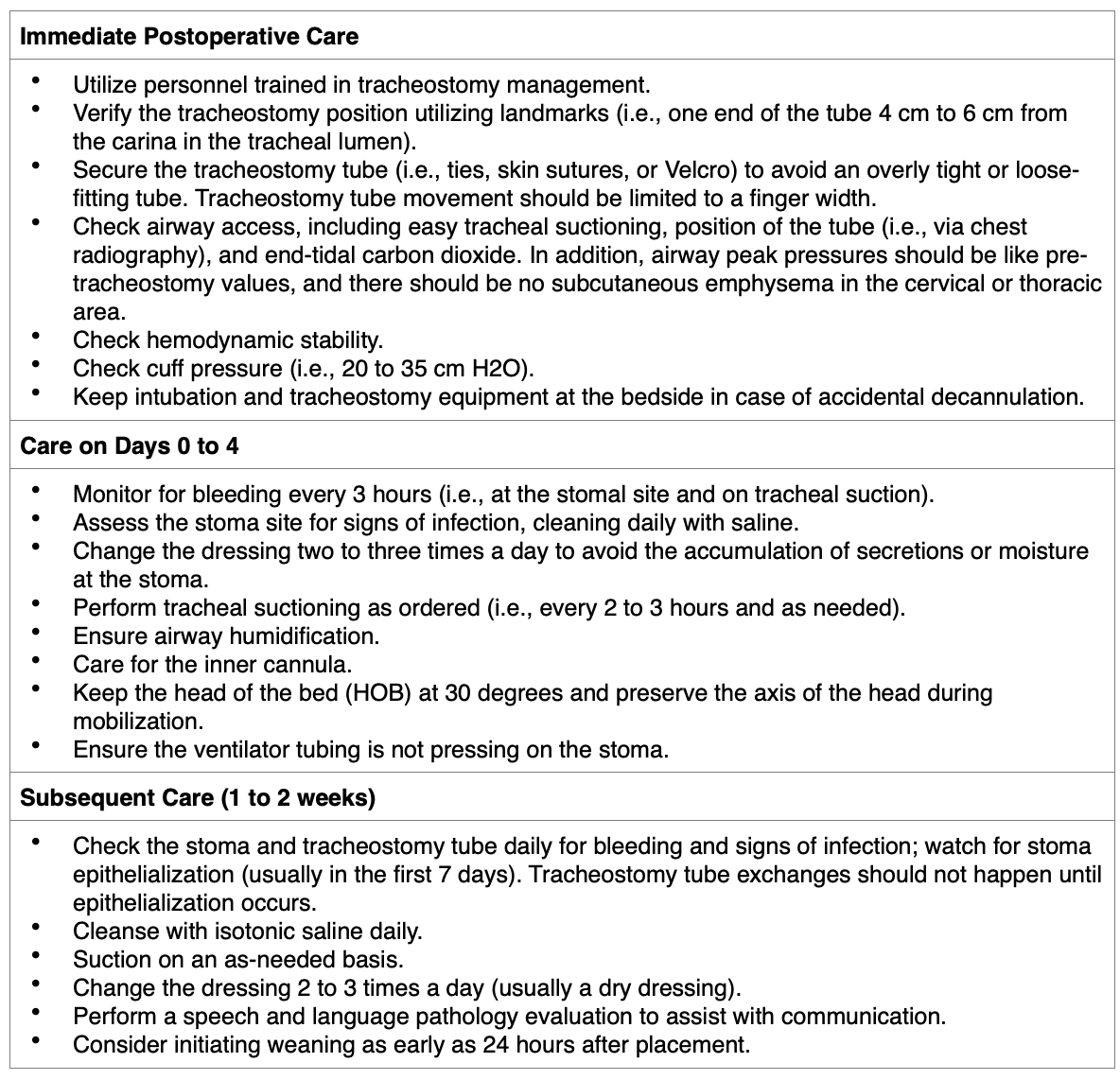
(Hyzy & De Cardenas, 2021)
Tracheostomy Cleaning
Much of tracheostomy care includes daily tracheostomy cleaning. An HCP will clean the tracheostomy site and tube for patients in a hospital setting. The patient or a caregiver will assume responsibility for tracheostomy care in a home setting. Tracheostomy tubes with an inner cannula must be cleaned regularly to prevent the buildup of dried mucus. In addition, the skin around the stoma should be cleaned 2 to 3 times per day to remove dried mucus and prevent excessive moisture that can lead to a rash. HCPs (i.e., nurses or respiratory therapists) can perform tracheostomy care. To prepare for tracheostomy care, HCPs should assess the patient's respiratory status (i.e., rate, depth, rhythm, ease of breathing, lung sounds, and oxygen saturation), heart rate, and ability to understand and perform tracheostomy care independently. In addition, peristomal and intratracheal secretions (i.e., features and amount), the presence of drainage from the dressing or tracheostomy ties, and the appearance of the incision (i.e., redness, swelling, or odor) should be assessed. Finally, the HCP should determine the type of tracheostomy tube (i.e., whether an inner cannula is present, the tube is cuffed, and the cuff is inflated). Next, the HCP should collect the necessary supplies, including a sterile/disposable tracheostomy cleaning kit (i.e., sterile containers, sterile applicators, sterile nylon brush, gauze squares), saline, hydrogen peroxide, gloves, towels, a suction catheter kit, a moisture-proof bag, a tracheostomy dressing or sterile gauze, cotton ties, and sterilized scissors (American Thoracic Society, 2016a; Craven et al., 2021; Unitek College, 2021).
HCPs should perform tracheostomy cleaning using the following steps (Craven et al., 2021; Oregon Health & Science University, n.d.; Unitek College, 2021):
- Verify the provider order.
- Perform hand hygiene and don gloves to reduce the transmission of microbes.
- Introduce yourself, verify the patient's identity, and explain the procedure to the patient.
- Ensure privacy by closing the door or curtains.
- Place the patient in a semi- or high-Fowler's position.
- Suction the tracheostomy if needed to ensure a patent airway for cleaning.
- Replace oxygen or humidification and encourage the patient to take deep breaths.
- Prepare the cleaning supplies by opening the sterile tracheostomy kit. The HCP pours normal saline into a sterile container and hydrogen peroxide into another. The HCP dons sterile gloves and opens several cotton-tipped sterile applicators and a sterile pre-cut tracheostomy dressing, placing them onto the sterile field. If the kit does not contain these pieces, these items should be opened before donning sterile gloves. In addition, if the kit does not contain tracheostomy ties, twill tape should be cut into two 15-inch pieces and set them aside.
- The oxygen source should be removed with one hand (now no longer sterile).
- If an inner cannula is present, the cannula is unlocked by turning counterclockwise and then removing it. The inner cannula should be placed into the hydrogen peroxide to loosen secretions.
- Replace the oxygen source over the outer cannula.
- Clean the sides and lumen of the inner cannula using a pipe cleaner or sterile brush.
- Rinse the inner cannula in the sterile saline container.
- Remove oxygen and replace the inner cannula. Lock the cannula into place by turning it clockwise until both blue dots align. Then, replace the oxygen or humification.
- For tracheostomies without an inner cannula, remove the tracheostomy dressing from under the faceplate or flange of the tracheostomy.
- Clean under the faceplate with saline-soaked cotton applicators using a circular motion. In addition, clean any dried secretions from all exposed outer cannula surfaces.
- Use a 40-inch by 40-inch gauze to dry any moist areas. Then, place a sterile, dry, pre-cut tracheostomy dressing under the faceplate.
- If the tracheostomy ties need to be changed, have an additional HCP don sterile gloves to hold the tracheostomy tube in place.
- Cut a half-inch slit approximately 1 inch from an end of both clean ties. Next, remove the soiled tracheostomy ties. Thread the end of the tie through the slit in the tie and pull it tight. Repeat these steps with the second tie. Finally, bring both ties together on one side of the patient's neck. Ensure the ties are tight enough (i.e., only allowing a finger between the tie and the neck). Use 2 square knots to secure the ties and then cut the excess.
- Use a second HCP to hold the tracheostomy tube in place for a tracheostomy collar. Then, gently pull the Velcro tab and remove the collar on one side. Next, insert a new collar into the opening and secure the Velcro tab. Repeat the same steps on the other side. Finally, remove and discard the old collar.
- Remove gloves and assist the patient into a comfortable position.
- Some patients will perform tracheostomy care at home independently. These patients should follow similar steps as described above. They should sit in a chair in front of a mirror. Home cleaning is usually done with clean and not sterile gloves.
Tracheostomy Suctioning
The upper airways of the respiratory system warm, clean, and moisten the air. Tracheostomies tubes bypass these mechanisms, instilling cooler, dryer, and not as clean air. In response to these changes in air quality, the body increases mucus production. Secretions that remain in the airway can lead to a blockage and difficulty breathing. In addition, secretion stasis can increase the risk of pneumonia. Tracheostomy suctioning can clear these secretions and ensure adequate breathing and oxygenation. The frequency of suctioning needed will vary from patient to patient. However, suctioning too frequently can lead to increased secretion production and buildup. Tracheal suctioning is almost always necessary for patients with a tracheostomy tube due to a decreased effectiveness of the cough mechanism. Suctioning should be performed when the patient cannot cough up secretions independently, a change in breathing pattern is noted, or the frequency of coughing increases. For mechanically ventilated patients, an in-line suction catheter can be used to allow rapid suction and minimize the risk of contamination by airborne pathogens. In addition, the in-line suction does not require the patient to be disconnected from the ventilator, decreasing hypoxemia and sustaining PEEP (Craven et al., 2021; Johns Hopkins Medicine, n.d.; Oregon Health & Science University, n.d.).
HCPs must be familiar with the procedure for suctioning a tracheostomy without in-line suctioning. To prepare for tracheal suctioning, the HCP should assess respiratory status (i.e., rate, depth, rhythm, ease of breathing, lung sounds, and oxygen saturation), heart rate, and blood pressure. In addition, assess the level of consciousness and ability to protect the airway. Finally, the HCP should assess the patient's ability to cough, noting the amount and character of the sputum. Next, gather the necessary equipment, including a portable or wall suction with appropriate tubing, sterile suction kit (i.e., gloves, a container for saline, catheter lubricant, and appropriate size catheter [8 to 10 French for adults], and sterile saline). Then, the HCP should perform tracheostomy suctioning using the following steps (Craven et al., 2021; Johns Hopkins Medicine, n.d.; Oregon Health & Science University, n.d.):
- Verify the provider order.
- Perform hand hygiene and don gloves to reduce the transmission of microbes.
- Introduce yourself, verify the patient's identity, and explain the procedure to the patient.
- Ensure privacy by closing the door or curtains.
- Place the patient in the semi-Fowler's position if they are conscious to prevent aspiration. Place the patient in a side-lying position facing the HCP if unconscious.
- Turn on the suction device and adjust the pressure to 100 to 120 mm Hg for adults. Excessive suction pressure can damage the mucosa and induce hypoxia.
- Open the sterile suction catheter kit. Prepare the sterile field and pour sterile saline into the sterile container or cup from the kit.
- Preoxygenate the patient with 100% oxygen to prevent hypoxia.
- Don sterile gloves. If the kit only contains one glove, place it on the dominant hand, which will remain sterile during suctioning. A clean glove can be placed on the non-dominant hand.
- Pick up the sterile suction catheter with the dominant hand. Use the non-dominant hand to pick up the connecting suction tubing (clean hand). Attach the suction catheter to the suction tubing without contaminating the sterile (dominant) hand.
- Test the suction equipment by placing the catheter into the sterile saline and covering the open port with the non-dominant thumb.
- Apply a nonpetroleum-based lubricant on the end of the suction catheter if suctioning nasally.
- Insert the suction catheter into the trachea (i.e., through the tracheostomy or nasally) during inspiration and without suction. During inspiration, the epiglottis opens, allowing the catheter into the trachea.
- Advance the catheter until resistance is met. Then, retract the catheter 1 cm before applying suction to prevent mucosal damage. The patient will likely cough when the catheter moves into the trachea.
- Apply suction by placing the non-dominant thumb over the open port. Then, rotate the catheter with the dominant hand while withdrawing the catheter. Withdraw the catheter slowly over 5 to 10 seconds (doing so for more than 10 seconds can induce hypoxia).
- Hyperoxygenate or hyperinflate with a manual resuscitation bag for 1 minute between suction passes to prevent hypoxia.
- Rinse the suction catheter with saline to clear secretions.
- Repeat suctioning until the airway is clear but not to exceed three passes.
- After tracheal suctioning, the catheter can be placed into the oropharynx to clear secretions. This area is done last since the oropharynx is less clean than the trachea. Apply suction for 5 to 10 seconds as the catheter is rotated and withdrawn.
- Allow 1 to 2 minutes between passes in the oropharynx to ventilate.
- Remove gloves by holding the catheter in the dominant hand and pulling the glove off over the catheter.
- Turn off the suction and position the patient in a comfortable position. Replace oxygen if needed.
- Perform hand hygiene.
Changing a Tracheostomy Tube
A tracheostomy tube exchange should be part of routine long-term tracheostomy maintenance to prevent infection. These routine changes occur between 7 and 30 days after the initial insertion, then subsequently every 30 to 90 days. In addition, a tracheostomy tube exchange may occur emergently for accidental decannulation or obstruction. Other indications for tracheostomy tube exchange include patient discomfort (e.g., size or manufacturer change) or malpositioning (e.g., cuff leak or patient-ventilator asynchrony). The personnel and location needed for a tracheostomy tube change will vary among institutions. Therefore, each institution should create a tracheostomy tube exchange protocol. A surgeon usually performs the initial tracheostomy tube exchange. Trained staff can perform subsequent exchanges, including a respiratory therapist, an anesthesiologist, an APP, or a nurse (Hyzy & De Cardenas, 2021; Ng & Hamrang-Yousefi, 2021).
A tracheostomy tube exchange does not generally require sedation, and the steps include the following (Hyzy & De Cardenas, 2021; Ng & Hamrang-Yousefi, 2021):
- Verify the provider order.
- Perform hand hygiene and don gloves to reduce the transmission of microbes.
- Introduce yourself, verify the patient's identity, and explain the procedure to the patient.
- Ensure privacy by closing the door or curtains.
- Ensure emergency equipment (i.e., bag valve mask, oxygen) is available at the beside before beginning the procedure.
- Check the tracheostomy dimensions of the flange to ensure the replacement tube is equivalent in size.
- Remove the tracheostomy tube from the package. Inflate the cuff to test for air leaks by placing it in sterile water, then deflate the cuff.
- The inner trocar should be inserted, and the device's tip should be covered with lubricant.
- Place the patient in a semi-recumbent position and gently extend their neck to expose the stoma.
- Preoxygenation may be required for high-risk patients.
- Suction the patient.
- Loosen and remove the tracheostomy ties from the flange and then clean around the stoma site with saline.
- Deflate the cuff entirely and remove the tracheostomy swiftly and smoothly using an out-then-down semi-circular maneuver. If significant resistance is met, do not proceed with the removal.
- Once the tracheostomy tube is removed, inspect the stoma and clean it again if needed.
- Insert the new tracheostomy tube by starting horizontally, using a 45-degree caudal rotational maneuver during insertion; however, do not advance the tube further if resistance is met.
- Once the tracheostomy tube is in place, remove the trocar and inflate the cuff. Next, secure the flange to the neck with clean tracheostomy ties, leaving a finger width between the neck and the tracheostomy collar.
- Once the tube is secured, the tracheostomy can be suctioned, and the inner cannula can be replaced.
- Confirm intraluminal airway placement by auscultating lung sounds and watching for bilateral chest rising.
Living with a Tracheostomy
HCPs in various clinical care settings may care for patients with a tracheostomy. Many critically ill patients in the ICU require MV for respiratory support. As their condition stabilizes, the healthcare team will attempt to wean the patient from the ventilator. Unfortunately, various factors, including illness severity and underlying medical conditions, will prevent some patients from weaning successfully. If PMV is anticipated or weaning trials have failed, the patient will likely require the placement of a tracheostomy. Once the patient's condition has stabilized, they may be transferred to an acute care floor or long-term care (LTC) setting, depending on their needs. Some patients with a tracheostomy may require MV continuously, while others can successfully wean from the ventilator (entirely or for parts of the day). Whether a patient with a tracheostomy can be discharged home will depend on whether (a) they require ventilatory support and (b) the patient or caregiver can assume tracheostomy care. Before discharge from a hospital or LTC setting, the HCPs must provide education and hands-on training for tracheostomy cleaning and suctioning. See Table 3 for education topics that HCPs should review with patients and caregivers regarding the care of their tracheostomy (American Thoracic Society, 2016a).
Table 3
Living with a Tracheostomy: Patient Education
(American Thoracic Society, 2016a; Oregon Health & Science University, n.d.)
References
American Thoracic Society. (2016a). Living with a tracheostomy. https://www.thoracic.org/patients/patient-resources/resources/tracheostomy-in-adults-2.pdf
American Thoracic Society. (2016b). Tracheostomy in adults. https://www.thoracic.org/patients/patient-resources/resources/tracheostomy-in-adults-1.pdf
American Thoracic Society. (2020). Mechanical ventilation. https://www.thoracic.org/patients/patient-resources/resources/mechanical-ventilation.pdf
Andriolo, B. N. G., Andriolo, R. B., Saconato, H., Atallah, A. N., & Valente, O. (2015). Early versus late tracheostomy for critically ill patients. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.CD007271.pub3
Bhimji, S. (2021). Diagram of cricothyrotomy [Image]. https://commons.wikimedia.org/wiki/File:Cricothyrotomystat.jpg
Brown, J. (2020). Tracheostomy to noninvasive ventilation. Sleep Medicine Clinics, 15(4), 593-598. https://doi.org/10.1016/j.jsmc.2020.08.003
BruceBlaus. (2013a). Anatomy of the trachea [Image]. https://commons.wikimedia.org/wiki/File:Blausen_0865_TracheaAnatomy.png
BruceBlaus. (2013b). Respiratory system [Image]. https://commons.wikimedia.org/wiki/File:Blausen_0770_RespiratorySystem_02.png
Cancer Research UK. (2014). Diagram showing a fenestrated and a nonfenestrated tracheostomy tube [Image]. https://commons.wikimedia.org/wiki/File:Diagram_showing_a_fenestrated_and_a_non_fenestrated_tracheostomy_tube_CRUK_066.svg
Centers for Disease Control and Prevention. (2021). Deaths and mortality. https://www.cdc.gov/nchs/fastats/deaths.htm
Cleveland Clinic. (n.d.). Tracheostomy care. Retrieved December 10, 2021, from https://my.clevelandclinic.org/health/treatments/17568-tracheostomy-care
Cleveland Clinic. (2019). Mechanical ventilation. https://my.clevelandclinic.org/health/articles/15368-mechanical-ventilation
Craven, R. F., Hirnle, C. J., & Henshaw, C. M. (2021). Fundamentals of nursing: Concepts and competencies for practice (9th ed.). Wolters Kluwer.
Dirkes, S. M., & Kozlowski, C. (2019). Early mobility in the intensive care unit: Evidence, barriers, and future directions. Critical Care Nurse, 39(3), 33-43. https://doi.org/10.4037/ccn2019654
Fadila, M., Rajasurya, V., & Regunath, H. (2021). Ventilator weaning. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK430712/
Freeman, B. D. (2016). Tracheostomy update: When and how. Critical Care Clinics, 33(2), 311-322. http://dx.doi.org/10.1016/j.ccc.2016.12.007
Hinkle, J. L., & Cheever, K. H. (2018). Textbook of medical-surgical nursing (14th ed.). Wolters Kluwer.
Hosokawa, K., Nishimura, M., Egi, M., & Vincent, J. L. (2015). Timing of tracheostomy in ICU patients: A systematic review of randomized controlled trials. Critical Care, 19, 424. https://doi.org/10.1186/s13054-015-1138-8
Hyzy, R. C., & De Cardenas, J. L. (2021). Tracheostomy in adults: Techniques and intraoperative complications. UpToDate. Retrieved December 10, 2021, from https://www.uptodate.com/contents/tracheostomy-in-adults-techniques-and-intraoperative-complications
Hyzy, R. C., & McSparron, J. I. (2021a). Overview of initiating invasive mechanical ventilation in adults in the intensive care unit. UpToDate. Retrieved December 10, 2021, from https://www.uptodate.com/contents/overview-of-initiating-invasive-mechanical-ventilation-in-adults-in-the-intensive-care-unit
Hyzy, R. C., & McSparron, J. I. (2021b). Tracheostomy: Rationale, indications, and contraindications. UpToDate. Retrieved December 10, 2021, from https://www.uptodate.com/contents/tracheostomy-rationale-indications-and-contraindications
Jarrett, N. M., Palmer, L., Lewis, R., Le, T., Free, L., Sabouri, S., Bernacet, A., & Eng, T. (2016). Ventilator weaning (liberation) quality measures pilot test for long-term care hospitals: A summary of findings. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/LTCH-Quality-Reporting/Downloads/Ventilator-Weaning-QM-Pilot-Summary-Report-2016.pdf
Johns Hopkins Medicine. (n.d.). Tracheostomy service: Suctioning. https://www.hopkinsmedicine.org/tracheostomy/living/suctioning.html
King Han, M. (2020). Management and prognosis of patients requiring prolonged mechanical ventilation. UpToDate. Retrieved December 10, 2021, from https://www.uptodate.com/contents/management-and-prognosis-of-patients-requiring-prolonged-mechanical-ventilation
Klaus, P. D. (2008). Tracheostomy tubes [Image]. https://commons.wikimedia.org/wiki/File:Tracheostomy_tube.jpg
Lutz, E. (2020). Biology of ventilation [Image]. https://commons.wikimedia.org/wiki/File:Biology_of_ventilation.gif
Mayo Clinic. (2019). Tracheostomy. https://www.mayoclinic.org/tests-procedures/tracheostomy/about/pac-20384673
Medic Tests. (2021). Human gas exchange [Image]. https://medictests.com/units/the-mechanics-of-respiration
Mora Carpio, A. L., & Mora, J. I. (2021). Ventilator management. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK448186/
Mussa, C. C., Gomaa, D., Rowley, D. D., Schmidt, U., Ginier, E., & Strickland, S. L. (2021). AARC clinical practice guideline: Management of adult patients with tracheostomy in the acute care setting. Respiratory Care, 66(1), 156-169. https://doi.org/10.4187/respcare.08206
National Center for Chronic Disease Prevention and Health Promotion (2021). About chronic diseases. Centers for Disease Control and Prevention. https://www.cdc.gov/chronicdisease/about/index.htm
National Heart, Lung, and Blood Institute. (n.d.). Tracheostomy. US Department of Health and Human Services. Retrieved December 10, 2021, from https://www.nhlbi.nih.gov/health/tracheostomy
Ng, J., & Hamrang-Yousefi, S. (2021). Tracheostomy tube change. StatPearls [Internet]. https://www.statpearls.com/articlelibrary/viewarticle/30436/
Oregon Health & Science University. (n.d.). Trach care guide: For patients and families. https://www.ohsu.edu/sites/default/files/2019-03/OHSU-Trach-Care-Guide.pdf
Perry, W., Lanham, J., Martin, L., Sheriff, S., & Smith, J. (n.d.). Mechanical ventilation. Retrieved December 10, 2021, from http://hsc.ghs.org/wp-content/uploads/2013/10/MECHANICAL_VENTILATION.pdf
Raimonde, A., & Westhoven, N. (2021). Tracheostomy. StatPearls [Internet]. https://www.statpearls.com/articlelibrary/viewarticle/30433/
Rashid, A. O., & Islam, S. (2017). Percutaneous tracheostomy: A comprehensive review. Journal of Thoracic Disease, 9(Suppl 10), s1128-s1138. https://doi.org/10.21037/jtd.2017.09.33
Respiratory Therapy Zone. (2021). Respiratory therapy normal values: Reference guide for board exams. https://www.respiratorytherapyzone.com/respiratory-therapy-normal-values/
Sakles, J. C. (2021). Emergency cricothyrotomy (cricothyroidotomy). UpToDate. Retrieved December 10, 2021, from https://www.uptodate.com/contents/emergency-cricothyrotomy-cricothyroidotomy
Society of Critical Care Medicine. (n.d.). Critical care statistics. Retrieved December 10, 2021, from https://www.sccm.org/Communications/Critical-Care-Statistics
Szakmany, T., Russell, P., Wilkes, A. R., & Hall, J. E. (2014). Effect of early tracheostomy on resource utilization and clinical outcomes in critically ill patients: Meta-analysis of randomized controlled trials. British Journal of Anaesthesia, 114(3), 396-405. https://doi.org/10.1093/bja/aeu440
Unitek College. (2021). A step-by-step guide to tracheostomy care. https://www.unitekcollege.edu/blog/a-step-by-step-guide-to-tracheostomy-care/
Villalba, D., Rossetti, G. G., Scrigna, M., Collins, J., Rocco, A., Matesa, A., Areas, L., Golfarini, N., Pini, P., Hannun, M., Boni, S., Grimaldi, S., Pedace, P., Diaz-Ballve, L., Andreu, M., Bunirigo, P., Noval, D., & Planells, F. (2019). Prevalence of and risk factors for mechanical ventilation reinstitution in patients weaned from prolonged mechanical ventilation. Respiratory Care, 65(2), 210-216. https://doi.org/10.4187/respcare.06807
Williams, T., & McGrath, B. A. (2021). Tracheostomy for COVID-19: Evolving best practice. Critical Care, 25, 316. https://doi.org/10.1186/s13054-021-03674-7
Wunsch, H. (2020). Mechanical ventilation in COVID-19: Interpreting the current epidemiology. American Journal of Respiratory and Critical Care Medicine, 202(1), 1-21. http://doi.org/10.1164/rccm.202004-1385ED