About this course:
This module will review cervical precancer and cancer, risk factors, signs, symptoms, diagnosis, management, and side effects of common treatments. It will also discuss the core components of cervical cancer prevention and early detection to inform nursing practice and improve patient outcomes.
Course preview
Cervical Cancer for Nurses
This module will review cervical precancer and cancer, risk factors, signs, symptoms, diagnosis, management, and side effects of common treatments. It will also discuss the core components of cervical cancer prevention and early detection to inform nursing practice and improve patient outcomes.
By the completion of this learning activity, the nurse should be able to:
- discuss the epidemiology of cervical cancer in the US and risk factors for the development of the disease
- review the anatomy of the cervix and the pathophysiology leading to the development of cervical precancer and cancer
- summarize cervical cancer screening and early detection guidelines, including the human papillomavirus (HPV) vaccine and immunization schedules
- identify the signs and symptoms of cervical cancer
- describe the management of cervical precancer and invasive cervical cancer, including an overview of treatment risks, side effects, and patient education
Cervical cancer is the fourth most common cause of cancer incidence and mortality in women worldwide (Cibula et al., 2023). According to the American Cancer Society (ACS, 2023a), approximately 13,960 women in the US will be diagnosed with invasive cervical cancer in 2023, and about 4,310 will die from the disease. Previously cited as one of the most common causes of cancer-related deaths, cervical cancer mortality rates have declined dramatically over recent decades with the widespread utilization of the Papanicolaou (Pap) smear, followed by the introduction of the human papillomavirus (HPV) co-test. The advent of the HPV vaccination ignited an era in cervical cancer prevention, substantially reducing invasive cervical cancer. When diagnosed early and managed effectively, cervical cancer is highly treatable and potentially curable. Nurses practicing in primary care and women’s health settings must remain informed on evidence-based guidelines regarding the prevention, early detection, and management of precancerous lesions and invasive cervical cancers to facilitate an early diagnosis and reduce the morbidity and mortality associated with the disease (ACS, 2023a, 2021c; Bruni et al., 2023).
Epidemiology in the US
Approximately 0.7% of females (1 in 159) will be diagnosed with cervical cancer during their lifetime. According to the National Cancer Institute (NCI, 2023a), cervical cancer's annual age-adjusted incidence rate is 7.7 per 100,000 women. Racial disparities in cervical cancer are large. Hispanic women have the highest incidence (9.9 per 100,000), followed by non-Hispanic Black (NHB; 8.8 per 100,000) and non-Hispanic American Indian/Alaska Native women (AI/AN; 8.8 per 100,000). Non-Hispanic Asian/Pacific Islanders (API) have the lowest incidence (6.1 per 100,000). NHB women are 30% more likely to develop and 60% more likely to die from the disease than non-Hispanic White women (NHW; ACS, 2022, 2023a; Spencer et al., 2023). The median age at diagnosis is 50, and the disease occurs most frequently in women aged 35 to 44 (24.1%), followed by those aged 45 to 54 (21.6%). While 20% of cases occur in women over 65, cervical cancer rarely develops in those who have maintained compliance with recommended screenings (ACS, 2021c, 2023a, 2023b).
The 5-year survival rate of localized cervical cancer (i.e., cancer that has not spread outside of the cervix or uterus) is 92%, declining to 17% for those with distant metastases (i.e., cancer spread to distant organs or body parts). NHB women are more likely to be diagnosed at advanced stages of the disease, reducing their 5-year survival rate to 56% compared to 67% in NHW women (ACS, 2022, 2023a, 2023c). While survival has improved since the 1970s for most cancer types, it has remained relatively stagnant for cervical cancers; this is attributed to the lack of significant treatment advances for patients with recurrent and metastatic disease. Furthermore, nearly 90% of women who die from cervical cancer have inadequate access to prevention, screening, and treatment (World Health Organization [WHO], 2022).
Risk Factors
Risk factors for cervical cancer include HPV infection, tobacco use, early onset of sexual activity, multiple sexual partners, and HIV infection. Predominantly transmitted through sexual contact, HPV is the chief risk factor for cervical cancer, leading to nearly 99% of diagnoses. Tobacco use is the only nonsexual behavior associated with cervical dysplasia (i.e., abnormal cell growth on the cervix) and cancer. Smokers are at least twice as likely as non-smokers to be diagnosed with cervical cancer. Research has demonstrated that among women with HPV infections, those who smoke have a significantly higher viral load on the cervix, heightening the risk of cancer development (Fang et al., 2018; Truth Initiative, 2019). Early sexual activity and multiple sexual partners (especially those with numerous partners) increase the risk of HPV exposure. Immunocompromised people—such as those with HIV, transplant recipients, cancer patients, and those receiving immunosuppressive medications—are at higher risk for acquiring HPV (ACS, 2023a, 2023d).
Pathophysiology
The cervix is the fibromuscular lower portion of the uterus (i.e., the hollow, pear-shaped organ that carries a fetus) connecting the uterine cavity to the vagina (see Figure 1). It measures 4 cm in length and 3 cm in diameter in an adult woman of childbearing age and undergoes progressive involution following menopause. The cervix is substantially larger in parous women (i.e., those who have given birth) than in nulliparous women (i.e., those who have never carried a pregnancy to term; McCance & Heuther, 2019; Mehta & Sachdeva, 2017).
Figure 1
Female Reproductive System
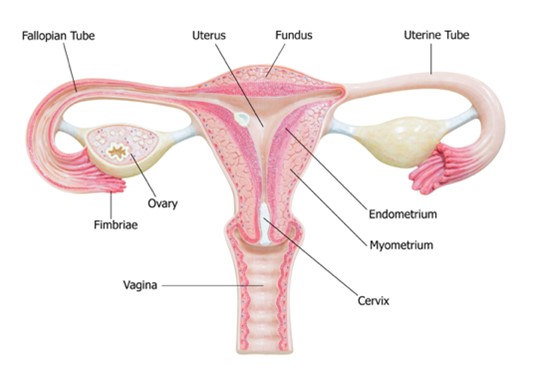
(iStock Photo ID: 538949875)
Two sections comprise the cervix: the ectocervix and the endocervix. The ectocervix extends into the vagina and is lined with stratified squamous non-keratinized epithelium. This squamous epithelium contains four layers: basal, parabasal, intermediate, and superficial. The cervix opens into the vagina through the external os (external orifice), the readily visible portion of the cervix. The external os marks the transition point to the endocervix (endocervical canal), which is the inner part of the cervix. The endocervix is rarely visible on an internal pelvic examination, but it is lined with mucus-secreting columnar epithelium or glandular cells. It forms a passageway between the external and internal os (internal orifice or isthmus). The internal os is a narrow opening that marks the transition from the endocervix to the endometrium, which is the lining of the uterine cavity. The cervix enables the passage of sperm into the uterine cavity through dilation of the external and internal os. The uterus maintains a sterile environment through frequent shedding of the endometrium, thick cervical mucus production, and a narrow external os to protect against bacterial invasion (McCance & Heuther, 2019; Mehta & Sachdeva, 2017).
The area between the endocervix and the ectocervix is called the squamocolumnar junction (SCJ), where cervical dysplasia occurs most often. At birth, the SCJ lies close to the external os as described above but moves internally with advancing age, parity, and menopause. The SCJ is no longer visible in a postmenopausal woman as it has receded into the endocervix. The columnar epithelium of the endocervix expands outward onto the ectocervix, exposing the tissue to estrogen and irritants from the vagina’s aci
...purchase below to continue the course
HPV
HPV is a ubiquitous group of tiny viruses that carry deoxyribonucleic acid (DNA), can adapt to their host, and evade the immune system. According to the Centers for Disease Control and Prevention [CDC, 2022b], more than 9 out of 10 cervical cancers are caused by HPV, and approximately 11,000 new cases of HPV-associated cervical cancers are diagnosed in the US annually. A growing body of evidence links HPV to other anogenital cancers (anal, vulvar, vaginal, penile) and head and neck cancers. Of the 200 identified strains of HPV, approximately 40 have a particular affinity for the genital region. While most of these strains are low-risk and non-oncogenic (i.e., wart-causing), about 14 are high-risk and oncogenic (i.e., cancer-causing). High-risk HPV subtypes can cause several types of cancer, but HPV-16 and HPV-18 are responsible for at least 70% of cervical cancers. While nearly all sexually active males and females will be infected with HPV during their lifetime, most of these infections remain clinically silent and dormant. The immune system habitually mediates the spontaneous clearance of HPV from the cervix within two years of acquiring the infection. However, about 10% of women infected with a high-risk HPV subtype will develop a persistent infection (Bruni et al., 2023; CDC, 2022a, 2022b; NCI, 2023b; Mello & Sundstrom, 2022; Stankiewicz & Spach, 2021; Wang et al., 2018; WHO, 2022).
The Role of HPV in Cervical Carcinogenesis
Chronic high-risk HPV infections can lead to cervical precancers and cervical intraepithelial neoplasia (CIN). CIN is a term used to describe abnormal changes in the epithelial cells lining the surface of the cervix. CIN is a noninvasive, precancerous condition in which the abnormal cells are confined to the epithelium and relates directly to the integration of an HPV infection. Left untreated, CIN can invade local tissues and develop into invasive cancer. It takes 10 to 20 years for HPV-infected cells to develop into cervical cancer. HPV infection leads to CIN and invasive cancer by altering oncogenes and tumor suppressor genes. An oncogene is a mutation associated with cancer development; it can be inherited or caused by exposure to environmental carcinogenic substances, such as ultraviolet (UV) radiation and free radicals. In their normal and non-mutated state, oncogenes are called proto-oncogenes, regulating healthy cell growth and division. When a proto-oncogene mutates into an oncogene, it becomes permanently activated (i.e., turned on). Oncogenes permit unregulated cell growth, leading to cancer growth. In their healthy state, tumor suppressor genes regulate normal cell growth and division, repair DNA errors, and induce apoptosis (i.e., programmed cell death). When tumor suppressor genes are mutated or inactivated (i.e., turned off), these processes become unregulated, allowing cancer to grow (CancerQuest, n.d.; Pal & Kundu, 2020; Stankiewicz & Spach, 2021; Wang et al., 2018).
Pap Smear and HPV Testing
Since CIN does not cause symptoms, cellular abnormalities are identified on a Pap smear. A Pap smear is a noninvasive test for cervical abnormalities, precancerous lesions, and invasive cancer. It can also diagnose benign conditions such as infection and inflammation, but it does not diagnose HPV. HPV can be detected about a year after the cervix is infected, and HPV testing is a critical component of cervical cancer screening. US cervical cancer screening guidelines support primary HPV testing alone or HPV co-testing. Primary HPV testing is a relatively novel screening modality performed alone, whereas HPV co-testing is done simultaneously with a Pap smear. The ACS (2020f) recommends the primary HPV test for cervical cancer screening in women aged 25 to 65. In contrast, the American Society for Colposcopy and Cervical Pathology (ASCCP) supports using either test, endorsing any type of HPV–based testing as the basis for risk estimation (Perkins et al., 2020). The US Preventive Services Task Force (USPSTF) last updated their cervical cancer screening guidelines in March 2022, although an update for this topic is currently in progress as of September 2023. Table 1 compares the ACS (2021c) guidelines and the USPSTF (2022) guidelines for the prevention and early detection of cervical cancer.
Table 1
ACS & USPSTF Cervical Cancer Screening Guidelines
|
|
|
|
|
|
|
|
|
|
(ACS, 2021c; 2020e; USPSTF, 2022)
The US Food & Drug Administration (FDA) has approved specific primary HPV tests, including target- and signal-amplification assays of HPV DNA, as well as HPV mRNA. Most FDA-approved primary HPV assays have demonstrated similar performance metrics regarding efficacy across clinical trials; however, they are not yet widely available or adopted across practice settings. Therefore, HPV co-testing remains the most common screening test among US women aged 30 to 65 (Perkins et al., 2020). Regardless of the modality, all HPV tests explicitly evaluate the cells’ internal environment for signs of infection with high-risk viral subtypes. Viral nucleic acid (DNA or messenger ribonucleic acid [mRNA]) must be detected within the cervical cells to form a definitive diagnosis. To perform a Pap smear, the clinician positions a vaginal speculum to visualize the cervix, as shown in Figure 2. Then, the cervix is scraped using a small, cone-shaped brush and plastic spatula to collect cells. Next, the brush and spatula are rinsed in a liquid-filled vial and sent to the laboratory for processing. Most women tolerate the test without complications or pain. Slight vaginal spotting may occur immediately following the Pap smear, typically resolving within 24 hours (NCI, 2021).
Figure 2
Pap Smear
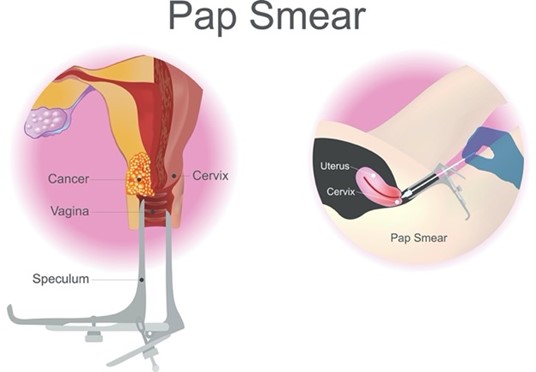
(iStock illustration ID:698717224)
Pap Smear Results
The Pap smear results indicate if cervical cells are normal or abnormal, but some samples may return as unsatisfactory (see below). All Pap smears must include cells within the SCJ to produce a satisfactory result (ACS, 2020e; Mello & Sundstrom, 2022; Perkins et al., 2020).
Normal Results
Normal (negative) findings signify no abnormal cells were identified, and patients do not require further workup or management. According to evidence-based cervical cancer screening guidelines, patients are usually advised to undergo routine screening every 3 to 5 years, as outlined in Table 1 (ACS, 2020e; Perkins et al., 2020).
Unsatisfactory Results
Unsatisfactory Pap smear results can indicate insufficient cells to evaluate the specimen or the cells were obscured by blood or mucus. Patients with unsatisfactory results typically require repeat testing (Perkins et al., 2020).
Abnormal Results
Abnormal (positive) results indicate cellular changes were seen in the sample. A pathologist classifies the severity of the abnormalities based on their risk of progressing to invasive cancer. Any grade of dysplasia warrants further workup. There are several abnormal Pap results; the most common are outlined in Table 2 (ACS, 2020e; Perkins et al., 2020).
Table 2
Abnormal Pap Smear Results
Result | Description |
Atypical squamous cells of undetermined significance (ASCUS) | ASCUS is the most common abnormal Pap result and confirms the presence of abnormal cells. ASCUS is an inconclusive finding and does not determine the origin of the abnormality or whether the changes are secondary to an HPV infection. Several benign causes of atypical-appearing cells include yeast infection, irritation, polyps, cysts, and hormonal changes during pregnancy or menopause. Management of ASCUS typically includes HPV co-testing or a repeat Pap smear in 1 year. |
Atypical glandular cells (AGC)
| AGC is rare, only found in about 1% of cervical cytology specimens. AGC signifies that some glandular cells have abnormal cytologic features that are too pronounced to be called inflammatory or reactive yet cannot be classified as malignant. AGC can include inflammatory conditions but can also indicate dysplasia or malignancy in up to 10% of cases. Management is based on the type of AGC but generally requires additional testing such as colposcopy and biopsy. About 50% of patients with AGC will have negative findings on colposcopy and endometrial sampling. |
Squamous intraepithelial lesion (SIL)
| SIL refers to dysplastic changes in the cervix and is categorized into low-grade (LSIL) or high-grade (HSIL). LSIL denotes early cellular changes (mild dysplasia), meaning cells have only a few abnormal characteristics and still resemble healthy cells. HSIL indicates moderate or severe dysplasia; cells appear highly abnormal under the microscope but are confined to the cervix’s surface. While HSIL does not indicate invasion into deeper parts of the cervix, it can progress to cervical cancer if not treated. |
Carcinoma in situ (CIS) | CIS denotes early-stage noninvasive cancer, where the cancerous cells arise from and are confined to the squamous epithelium. CIS has not penetrated more deeply into the tissues. |
Adenocarcinoma in situ (AIS) | AIS indicates a noninvasive lesion of the glandular tissue and is much less common than CIS. AIS has not penetrated more deeply into the tissues. |
Invasive cervical cancer | Less commonly, Pap smears can detect malignant cells (squamous or adenocarcinoma). However, invasive cervical cancer is rarely identified on a Pap smear in women who have undergone screening at regular intervals, as cervical abnormalities typically manifest as precancerous lesions. |
(ACS, 2020e, 2020f, 2022; Arshi & Farci, 2022; Mello & Sundstrom, 2022; NCI, 2023c; Perkins et al., 2020)
HPV Test Results
HPV tests are simpler to interpret than Pap smear results as the result is either positive (high-risk HPV was found) or negative (high-risk HPV was not found; ACS, 2020f; Perkins et al., 2020).
Follow-Up of Abnormal Results
Colposcopy
As demonstrated in Figure 3, colposcopy is a noninvasive procedure typically performed in follow-up to abnormalities detected on Pap or HPV testing. A colposcopy detects precancerous changes and benign conditions, such as polyps, cervicitis (i.e., cervix inflammation), or genital warts. During the procedure, a weak acetic acid solution (i.e., diluted vinegar) is applied to the cervix. The vinegar turns abnormal areas white, allowing for direct visualization of cervical changes under a stereomicroscope (ACS, 2020e; Burness et al., 2020).
Figure 3
Colposcopy
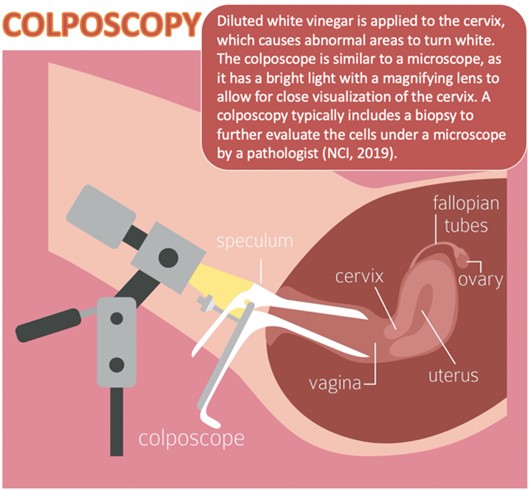
(iStock illustration ID: 1226029410)
A biopsy of the abnormal area is performed, as tissue sampling and histological analysis are the only way to diagnose precancer or cancer definitively. There are three major types of cervical biopsies, as described in Table 3. A colposcopy is a safe procedure that carries minimal risks. The potential harms include pain, psychological distress, infection, and bleeding (ACS, 2020e; Burness et al., 2020). While there is a consensus that analgesia should be administered before colposcopy and biopsy procedures, the most optimal pain management remains controversial. A 2016 Cochrane review of 19 randomized controlled trials (RCTs) explored varying pain relief interventions in outpatient settings. Findings demonstrated that oral analgesia, topical eutectic mixture of lidocaine 2.5% and prilocaine 2.5% (EMLA) cream, transcutaneous electrical nerve stimulation (TENS), lidocaine spray, or benzocaine gel did not provide any benefit in pain relief during cervical colposcopy treatment. Spraying the cervix with cocaine spray (i.e., 3 to 4 mL of a cocaine 10% solution preserved in nipasept [a mixture of the methyl, ethyl, and propyl esters of p‐hydroxybenzoic acid]) before treatment led to superior pain relief during the procedure and reduced postprocedural bleeding. Further, applying a local anesthetic combined with a vasoconstrictor resulted in better pain control than placebo and was also associated with reduced bleeding. Finally, injecting the cervix with a local anesthetic with a vasoconstrictor agent reduced pain scores during the procedure and is recommended for all cervical colposcopy treatments. However, the optimal number and location of cervical injections are unclear. Additional high‐quality RCTs are needed to determine the optimal route of administration and dose of local anesthetics (Gajjar et al., 2016).
Table 3
Cervical Biopsy
Biopsy | Description | Risks/Benefits |
Punch biopsy (see Figure 4) |
|
|
Endocervical curettage (ECC) |
|
|
Cold knife cone (CKC, conization; see Figure 5) |
|
|
(ACS; 2020e; Burness et al., 2020; Mello & Sundstrom, 2022; Zebitay et al., 2017)
Figure 4
Cervical Punch Biopsy

(Assessment Technologies Institute [ATI], 2019a)
Figure 5
Cold Cone Biopsy

(ATI, 2019b)
CIN
CIN is classified into three grades based on the extent of dysplasia and how deeply the abnormal cells invade the tissue. CIN grade is determined by biopsy based on the appearance of cells under the microscope. As the CIN grade advances, the risk for progression to invasive cancer heightens, and natural regression is uncommon. The classification of CIN is further outlined in Table 4; Mello & Sundstrom, 2022).
Table 4
CIN Classification
Grade | Thickness of Abnormal Epithelium | Classification |
CIN-1 |
|
|
CIN-2 |
|
|
CIN-3 (CIS) |
|
|
(Mello & Sundstrom, 2022; Wright et al., 2023)
CIN Management
CIN treatment aims to eradicate the precancerous lesions to prevent them from progressing to invasive cancer. Treatment selection depends on the extent and severity of CIN, the patient’s age, medical history, cost-effectiveness, availability, and each method's potential risks and benefits. Procedures to treat CIN can affect future childbearing potential, so shared decision-making is crucial to ensure patients are fully informed of the potential risks before deciding on a therapy option. Most low-grade CINs resolve without intervention; only about 1% of cases will progress to cervical cancer. According to the ASCCP, most patients with CIN-1 can be adequately managed with close observation using Pap smears, HPV testing, and regular colposcopies. In contrast, patients with high-grade CIN typically require intervention. Treatment options include ablative and excisional techniques. Ablative techniques (i.e., cryotherapy) destroy abnormal tissues by freezing them, whereas excisional methods (i.e., loop electrosurgical excision procedure [LEEP] and CKC) remove the abnormal tissue surgically. The WHO and ASCCP strongly recommend excisional treatment over cryotherapy in all settings where a LEEP procedure is available and accessible. LEEP and CKC are the two most common and effective excisional procedures for CIN-2, CIN-3, and CIS (Perkins et al., 2020; Wright et al., 2023).
LEEP
LEEP is a common technique for high-grade cervical dysplasia following colposcopy. During the procedure, a thin wire loop that functions as a scalpel is used to excise abnormal cells. An electric current is passed through the device to remove a thin layer of the cervix. Patients may feel mild discomfort during the procedure, so local anesthesia is typically used for pain control. The procedure is commonly performed in an office or outpatient setting and takes about 15 minutes. At the end of the procedure, a reddish-brown paste called ferric subsulfate (Monsel’s solution) may be applied to the cervix to control bleeding; alternatively, the blood vessels may be cauterized (i.e., heated with a device to control bleeding). If ferric subsulfate (Monsel’s solution) is used, patients should be counseled to expect a harmless brownish vaginal discharge for up to 48 hours after the procedure. The most common side effects include mild discomfort and slight bleeding for 2 to 3 weeks after the procedure. To allow the cervix to heal fully, patients should avoid placing anything inside the vagina (including tampons and douching) and abstain from sexual intercourse for 4 to 6 weeks. Patients may shower as usual but should avoid baths and swimming for 2 to 3 weeks. A LEEP is associated with a small increased risk for future pregnancy complications, including premature birth and low birth weight. In rare cases, the cervix may narrow after the procedure, causing menstrual irregularities (ACS, 2020e; American College of Obstetricians and Gynecologists [ACOG], 2023; Perkins et al., 2020).
Cryotherapy
Cryotherapy, called cryosurgery or laser ablation, involves applying a cooled metal disc (i.e., cryoprobe) to the cervix to freeze precancerous cells. The cryoprobe is cooled using compressed carbon dioxide (CO2) or nitrous oxide (NO) gas. Cryotherapy is a simple, fast outpatient procedure performed without anesthesia. It typically takes about 15 minutes and may cause mild discomfort. Following cryotherapy, the frozen area regenerates with normal epithelium in about one month, and some patients can experience watery vaginal discharge during this time. Patients should avoid sexual intercourse or placing anything in the vagina during the healing process for 4 to 6 weeks (Mello & Sundstrom, 2022; Perkins et al., 2020).
According to the ASCCP guidelines, cryotherapy should not be used in any of the following clinical circumstances:
- for lesions that extend into the canal
- for lesions covering more than 75% of the surface area of the ectocervix
- if the SCJ or upper limit of any lesion is not fully visualized
- if the CIN cannot be graded or is CIN-2 or higher
- after prior treatment for CIN-2 or higher, or
- in the setting of inadequate cervix biopsies to confirm a histologic diagnosis (Perkins et al., 2020, p. 118)
Surveillance Following CIN Treatment
The ASCCP supports continued surveillance with HPV testing or co-testing at 3-year intervals for at least 25 years after treatment for HSIL, CIN-2, CIN-3, or CIS. Extending surveillance beyond 25 years is acceptable if the patient’s life expectancy and screening capabilities are not compromised by concomitant health conditions (Perkins et al., 2020).
AIS Management
Although AIS frequently coexists with CIS, it is less common and requires higher management. The incidence of AIS has increased in recent decades among women aged 30 to 40. The average interval between the diagnosis of AIS and progression to early invasive cancer is about five years. While AIS may be detected on a Pap smear, a definitive diagnosis requires tissue sampling. AIS typically develops in the TZ and extends proximally to the endocervical canal. When diagnosed with a cervical biopsy, about 15% of patients have an invasive component (Teoh et al., 2020). The gold standard treatment for AIS is hysterectomy (uterus removal) unless future fertility is desired. If fertility is desired, excisional procedures (e.g., LEEP or CKC) are acceptable if the patient is willing to adhere to recommendations for increased surveillance with HPV co-testing and endocervical sampling every six months for at least three years, then annually for at least two years (or until hysterectomy). A hysterectomy should be performed after childbearing is complete (Perkins et al., 2020).
Cervical Cancer Subtypes
When precancerous cells invade the basement membrane, invasive cervical cancer is diagnosed. Cervical cancer has two major subtypes: squamous cell carcinoma (SCC) and adenocarcinoma. Most cervical cancers (>80%) are SCC, originate in the squamous epithelium in the TZ, and closely correlate with chronic HPV infection, as described earlier. Cervical adenocarcinoma begins in the glandular cells of the endocervix or the TZ and causes about 20% of cervical cancers. Adenocarcinomas are distinct from SCC in that HPV does not uniformly cause them (Bruni et al., 2023; Hodgson & Park, 2019). Neuroendocrine carcinoma of the cervix (NECC) is a third, rare, and more aggressive type that accounts for only 1.5% of all cervical cancers. There are four subtypes of NECC tumors: small cell neuroendocrine carcinoma (SCNEC), large cell neuroendocrine carcinoma (LCNEC), typical carcinoid tumor, and atypical carcinoid tumor. SCNEC is the most common and aggressive type and carries the poorest prognosis. In contrast, carcinoid tumors typically have a more indolent disease trajectory and a more favorable prognosis. The role of HPV in mediating the development of NECC tumors is not clearly understood. Unlike SCC and adenocarcinoma, NECC is not universally preceded by preinvasive disease. Therefore, early detection via Pap smear screening is less common. The management of NECC is more complex and less precise due to the condition's rarity but typically includes multiple chemotherapy agents (Tempfer et al., 2018).
Signs and Symptoms
Women with early-stage cervical cancers typically do not have symptoms, as symptoms usually do not develop until the cancer advances and invade nearby tissue. The most common symptoms of advanced disease include the following:
- abnormal vaginal bleeding, such as spotting between periods, bleeding after intercourse, menstrual periods that are longer or heavier than usual, and postmenopausal bleeding
- dyspareunia (pain during or immediately following intercourse) or pain in the pelvis not related to intercourse
- abnormal vaginal discharge
- swelling of the legs due to impaired lymphatics caused by tumor compression
- urinary symptoms such as frequency, dysuria, inability to void, or hematuria
- constipation (ACS, 2020c)
Principles of Pathologic Review
The biopsy sample undergoes a series of tests to determine the cancer’s pathologic features, evaluate its behavior, and select the best treatment options. Tumor grade assesses how different the cancer cells look from healthy cells under the microscope. The grading determines the likelihood of cancer growth and spread and is defined as follows:
- Grade 1 is well-differentiated, appears similar to healthy cells, and is the least aggressive.
- Grade 2 is moderately differentiated, appears less like healthy cells, and is an intermediate grade (i.e., more aggressive than grade 1).
- Grade 3 is poorly differentiated or undifferentiated, does not resemble healthy cells, is high-grade, and is the most aggressive. Grade 3 cancer grows and spreads more quickly (NCI, 2022; Yarbro et al., 2018).
Gene and Molecular Biomarker Analyses
Tumor protein p53 (TP53)
Under physiologic conditions, TP53 functions as a tumor suppressor gene, regulating cellular growth and division by preventing cells from growing and dividing uncontrollably. TP53 keeps cells with mutated or damaged DNA from dividing, thereby preventing the development of tumors. Mutations in TP53 impair its ability to control cell proliferation, as it cannot trigger apoptosis in cells with mutated or damaged DNA. Consequently, DNA damage accumulates in cells, which divide uncontrollably, leading to tumor growth. In cervical cancer, TP53 is inactivated by the HPV oncoprotein E6, thereby driving carcinogenesis (Nakamura et al., 2019; US National Library of Medicine, 2020).
p16 Immunohistochemistry
P16 is a tumor suppressor protein critical in regulating the normal cell cycle. Nearly all HPV-positive cervical precancers and cancers are accompanied by p16 overexpression. P16 interacts with HPV, inducing cell cycle dysfunction, interfering with apoptosis, facilitating cell invasion, and inducing angiogenesis. P16 overexpression paradoxically intensifies with increasing aggressiveness of the cancer. P16-negative tumors are associated with poorer outcomes and reduced survival compared to their p16-positive tumors. Despite the widespread prevalence of p16 overexpression in cervical cancer, its clinical significance remains unclear. A lack of consensus or guidelines persists regarding how p16 overexpression should guide treatment (Nicolas et al., 2020).
Mismatch Repair (MMR)/Microsatellite Instability (MSI)
Advancements in modern technology have led to novel treatment strategies utilizing the immune system to attack cancer. Specialized tests called microsatellite instability-high (MSI-H) testing and mismatch repair deficiency (dMMR) determine whether cancer is susceptible to the antitumor efforts of immune-based therapies. MSI-H or dMMR act as inhibitors to the anti-programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) pathway, which serves essential functions in regulating the immune system. The National Comprehensive Cancer Network (NCCN) recommends that all patients with recurrent, progressive, or metastatic cervical cancer undergo MSI-H or dMMR testing. MMR deficiency may be reported as MSI-H or dMMR, but they share the same meaning (NCCN, 2023).
Programmed cell death-1 (PD-1)/PD-ligand 1 (PD-L1)
PD-1 is a cell-surface receptor expressed on circulating lymphocytes (i.e., T-cells, B-cells), natural killer cells, dendritic cells, and monocytes. PD-1 binds to PD-L1, an inhibitory ligand expressed in some normal and cancer cells. PD-1 acts as an “off switch” to keep the immune cells from attacking other cells in the body and maintain an appropriate immune response. When PD-1 attaches to PD-L1, it acts as an “off switch” to keep them from attacking other cells in the body (i.e., preventing autoimmune conditions). The binding of PD-1 to PD-L1 signals T-cells to leave neighboring cells alone, including cancer cells. Some cancer cells have large amounts of PD-L1, which allows them to evade immune attacks. Drugs that target PD-1 or PD-L1 block this binding and boost the immune response, dislocating the immune system's brakes so T-cells are freed to recognize and attack cancer cells. Thus, determining the presence of high levels of PD-L1 overexpression can serve as a road map for personalizing treatment. A combined positive score (CPS) of 1 or higher in cervical cancer indicates positive-PD-L1 expression. This scoring method evaluates the number of PD-L1–staining cells (i.e., tumor cells, lymphocytes, macrophages) relative to all viable tumor cells. A minimum of 100 viable tumor cells in the PD-L1–stained sample is required for the specimen to be considered adequate for evaluation (Cibula et al., 2023; Huang et al., 2022; Merck & Co., 2023b; Sasikumar & Ramachandra, 2018).
Cancer Staging
The International Federation of Gynecology and Obstetrics (FIGO) staging system guides gynecological cancer staging worldwide. The cervical cancer guidelines are integrated within the NCCN guidelines. The four stages of cervical cancer are displayed in Figure 6. Since many cases require specialized surgical care, it is recommended that all patients with suspected cervical cancers be referred to a gynecologic oncologist (ACS, 2023b; Bhatla et al., 2018, 2021; NCCN, 2023).
Figure 6
Cervical Cancer Staging
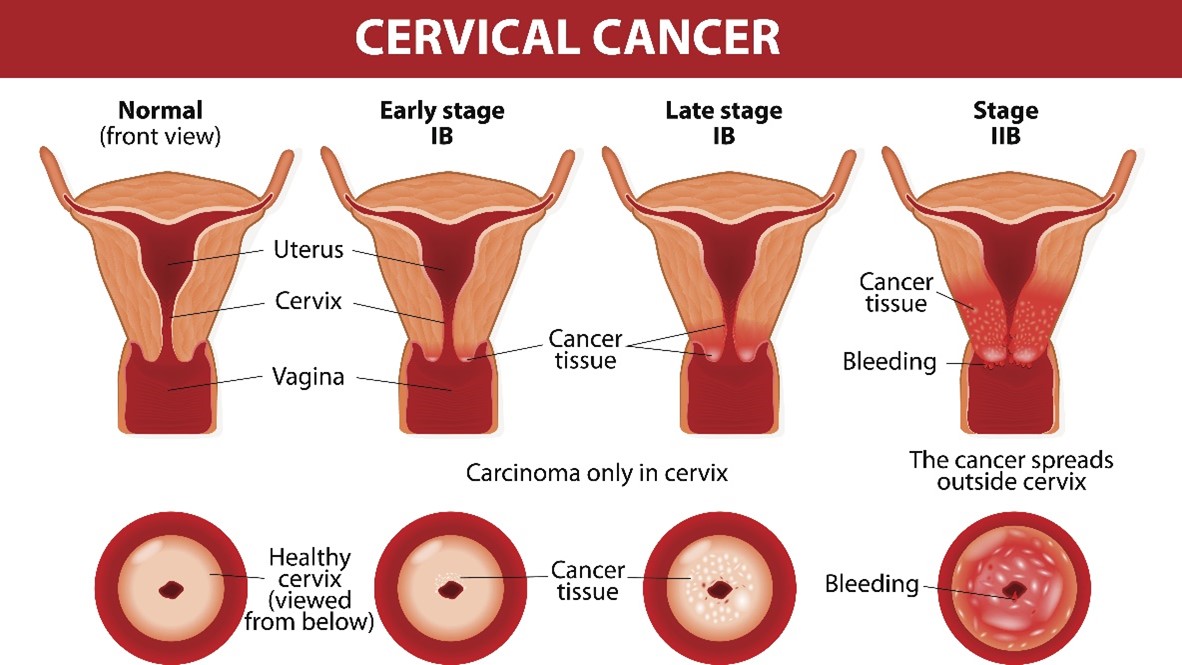
(iStock Illustration ID: 499566179)
Cervical Cancer Treatment
The optimal cervical cancer treatment depends on various factors, including pathologic features, cancer stage, plans for fertility, patient preference, age, and medical history. Treatment is often multimodal, with several therapies combined and administered simultaneously (concurrently) or sequentially. This section will provide a synopsis of the most common evidence-based treatment strategies (ACS, 2021d; NCCN, 2023).
Surgery
Surgical intervention for cervical cancer can be fertility-sparing or non-fertility-sparing. Most early-stage cervical cancers (stage IA1) can be effectively managed with LEEP or CKC, which are fertility-sparing treatments. Management may include a radical trachelectomy with lymphadenectomy in patients with stage IA2 or stage IB1 cervical cancer. A radical trachelectomy, also called a cervicectomy, involves surgically removing the cervix, upper vagina, and supporting ligaments, but the uterine corpus is preserved. The surrounding pelvic lymph nodes are also removed. Concerning its impact on future pregnancies, a radical trachelectomy is associated with a 10% likelihood of second-trimester loss, but more than 70% of women carry gestation to 37 weeks or more (ACS, 2020d; NCCN, 2023).
When fertility preservation is not desired or irrelevant, early-stage cervical cancers are managed with radical hysterectomy. The standard of care for stage IA2, IB1, IB2, IB3, and IIA1 cervical cancers is a radical hysterectomy with bilateral pelvic lymphadenectomy or pelvic lymph node dissection (PLND). A radical hysterectomy removes the uterus, cervix, a portion of the vagina, and the parametrium (the connective tissue surrounding the cervix). PLND involves the removal of the lymph nodes from the pelvis; the ovaries and fallopian tubes remain intact (ACS, 2020d; Cibula et al., 2023; NCCN, 2023).
A complete parametrectomy/upper vaginectomy is a surgical procedure for select patients with residual disease following a hysterectomy. It involves surgical resection of the parametrium and removal of the upper portion of the vagina. It carries significant morbidity, including risk for bowel and bladder injury, incontinence, and sexual dysfunction (NCCN, 2023).
Patients with persistent disease (i.e., continued evidence of cancer following definitive therapy) or localized cancer recurrence (i.e., cancer that returns in only the pelvis following a disease-free interval) may be cured with a surgical procedure called pelvic exenteration. A pelvic exenteration is a drastic surgical procedure in which many, if not all, organs are removed from the pelvis. The extent of surgery varies but typically involves removing the uterus, fallopian tubes, ovaries, vagina, bladder, urethra, rectum, and anal sphincter. The patient will have a permanent colostomy and a urinary diversion following the procedure. A colostomy is formed by bringing the remaining intestine to the abdominal wall’s surface to provide a stool outlet (i.e., stoma) to evacuate the bowel. With a urinary diversion, the kidneys and ureters are reconnected to a surgically created opening (i.e., urostomy) to drain the urine (ACS, 2020d; Cibula et al., 2023; NCCN, 2023; Yarbro et al., 2018).
Surgical Risks and Side Effects
Surgical risks and side effects depend on the size and degree of cancer invasion, the extent of surgery, and the structures removed. All surgeries and invasive procedures for cervical cancer are accompanied by risks, such as adverse reactions (ARs) to anesthesia, bleeding, blood clots, fistula formation (i.e., an abnormal connection between two hollow spaces within the body), bowel and bladder injury, infection, sexual dysfunction, and life-threatening sepsis. Fertility loss can negatively impact interpersonal relationships, quality of life, psychological health, and emotional well-being. Patients who undergo pelvic exenteration have difficulty adapting to the sweeping life alterations from surgery and struggle to care for colostomy and urostomy devices. Similarly, patients may be affected by the physical and psychological aspects of body image distortion. Nurses are vital in helping patients acclimate to these life-altering changes by facilitating healthy coping, addressing concerns, and referring patients to appropriate support groups or therapists (ACS, 2020d; NCCN, 2023; Yarbro et al., 2018).
Radiation Therapy
Conventional radiation therapy delivers a precisely measured amount of high-energy, focused ionizing radiation beams to the tumor, causing biological changes in cellular DNA and preventing cancer from reproducing or spreading. All healthy and cancer cells are vulnerable to the effects of radiation and may be injured or destroyed; however, healthy cells can repair themselves and remain functional. The total radiation dose is hyper-fractionated, which means it is delivered to the tumor in small, divided doses (i.e., fractions) rather than all at once. Hyper-fractionation allows healthy cells a chance to recover between treatments, thereby reducing side effects. The total number of fractions (i.e., doses) administered depends on the cancer type, size, location, reason for treatment (i.e., curative intent or palliation), overall health, performance status, and concurrent therapies. Radiation therapy is central in treating cervical cancer and can be delivered externally or internally; some patients may receive both. The most common types of radiation for cervical cancer include external beam radiation therapy (EBRT) and brachytherapy (ACS, 2020b; Cibula et al., 2023; Nettina, 2019).
EBRT
EBRT delivers radiation from outside the body and is the most common technique for cervical cancer. Traditionally, radiation beams could only match the tumor’s height and width, exposing more healthy tissue to the consequences of radiation. Further advancements in imaging technology have led to more precise treatment mechanisms. Intensity-modulated radiation therapy (IMRT) is a newer, highly conformal form of radiation that modulates the radiation beam’s intensity, delivering a higher radiation dose to a precise location, reducing unintended exposure to healthy tissues, enhancing clinical outcomes, and limiting side effects. IMRT helps minimize radiation exposure to the bowel and other critical structures, especially following a hysterectomy. EBRT is typically given concurrently with platinum-based chemotherapy (i.e., chemoradiation) to enhance the therapeutic response. Chemoradiation will be discussed in the next section (ACS, 2020b; NCCN, 2023; Nettina, 2019).
Brachytherapy
Brachytherapy is a critical component of cervical cancer treatment, particularly for patients who are not surgical candidates. It is also frequently used following EBRT to deliver an added radiation boost to a specified area or to palliate symptoms. Brachytherapy involves the internal implantation of an intrauterine device. The radioactive source is inserted directly into or near the tumor (ACS, 2020b).
Intraoperative Radiation Therapy (IORT)
IORT is a specialized technique that delivers a single dose of highly focused radiation to the tumor bed during surgery. IORT is particularly useful for patients with recurrent cancer within a previously irradiated field. During IORT, the overlying normal tissues and structures (e.g., bowel and surrounding organs) are manually displaced outside the radiation field to minimize exposure. IORT is delivered with electrons using preformed applicators matched to the surgically defined area of risk (Biete & Oses, 2018; NCCN, 2023).
Radiation Side Effects
Side effects depend on the specific area of the body exposed and the radiation dose. Superficial skin irritation at the site of the EBRT beams is likely and may include redness, blistering, and changes that look like a sunburn. The urinary system, bowel, and genitalia may be affected if they are in the radiation field. Bladder dysfunction can manifest as dysuria, hematuria, acute kidney injury, hydronephrosis, and incontinence. Gastrointestinal (GI) issues are common due to the cervix’s proximity to the bowel. Patients may experience nausea, vomiting, diarrhea, constipation, blood in stools, pain with defecation, and incontinence due to a loss of anal sphincter control. Sexual dysfunction is likely, particularly dyspareunia, atrophic vaginitis (i.e., inflammation and dryness of the vaginal tissue), vaginal agglutination (i.e., fusion and fibrosis of the vaginal walls), and recurrent yeast infections. If the ovaries are within the radiation field, patients may experience a permanent loss of ovarian function and develop premature menopause. Systemic effects can include fatigue, weakness, and dehydration (ACS, 2020b; Nettina, 2019; Yarbro et al., 2018).
Chemotherapy
Chemotherapy includes a group of high-risk, hazardous medications given to destroy cancer cells throughout the body. Chemotherapy interferes with the normal cell cycle, impairing DNA synthesis and cell replication to prevent cancer cells from dividing and multiplying (Yarbro et al., 2018). Chemotherapy serves a prominent role in treating many types of cervical cancers. Chemoradiation, which includes the use of platinum-based chemotherapy (e.g., cisplatin [Platinol] or carboplatin [Paraplatin]) administered alongside radiation, is the standard of care for curable cervical cancer not amenable to surgery. Cisplatin (Platinol) and carboplatin (Paraplatin) act as radiosensitizers, rendering cancer cells more vulnerable to radiation’s toxic effects. According to the NCCN (2023), the best outcomes occur when chemoradiation is completed within eight weeks. Adjuvant chemotherapy (i.e., administered after surgery) aims to prevent cancer recurrence, reduce micro-metastases, and eradicate any remaining cancer cells. Chemotherapy is commonly used to manage recurrent cervical cancer and for patients with metastatic disease. In these instances, chemotherapy is considered palliative to enhance comfort, reduce symptom burden, improve quality of life, and extend survival. As listed in Table 5, systemic therapy regimens for recurrent or metastatic disease typically include intravenous formulations with at least two or three medications. While bevacizumab (Avastin) and pembrolizumab (Keytruda) are listed as common components of the listed regimens, they are not chemotherapy agents; their unique mechanisms will be discussed in the next section (ACS, 2020a; Olsen et al., 2023; NCCN, 2023).
Table 5
Cervical Cancer Systemic Therapies
Curative Therapy | Recurrent or Metastatic Disease | |
Chemoradiation | First-Line Therapy | Second-Line Therapy |
| Preferred regimens:
| Preferred regimen:
|
(NCCN, 2023, p. CERV-F/1 of 3)
Chemotherapy Side Effects
The side effects of chemotherapy vary based on the drug type, dosage, treatment duration, underlying medical comorbidities, and overall performance status. Since cancer cells divide rapidly, chemotherapy is primed to target rapidly dividing cells, impacting normal cells that divide quickly, like those in the GI tract, skin/hair cells, and bone marrow. As a group, the most common side effects include pancytopenia (i.e., lowering of the blood counts), including anemia, thrombocytopenia, neutropenia, fatigue, anorexia, alopecia, nausea/vomiting, diarrhea, constipation, skin changes, and peripheral neuropathy (i.e., damage to the sensory nerves). Chemotherapy-induced hair loss generally begins with hair thinning, which occurs about 7-15 days following the first dose of chemotherapy. Chemotherapy damages dividing hair matrix cells, which causes the hair shaft to break at the follicular orifice or bulb. While the degree of hair loss depends on the chemotherapy agent, dose, and administration schedule, paclitaxel (Taxol) and docetaxel (Taxotere) typically cause significant alopecia in most patients. Nurses should reassure women that their hair will begin to regrow within a few weeks following the cessation of chemotherapy, as permanent alopecia following chemotherapy is rare (ACS, 2020a; Olsen et al., 2023).
Cisplatin (Platinol) is a moderate-to-highly emetogenic agent that induces acute and delayed nausea. Poorly controlled chemotherapy-induced nausea and vomiting (CINV) are associated with unfavorable treatment compliance, impairing survival. Aprepitant (Emend) is approved to reduce CINV associated with cisplatin (Platinol) therapy. Available in oral and intravenous preparations, aprepitant (Emend) is a neurokinin-1 (NK-1) receptor antagonist that blocks substance P/neurokinin 1 in the brain. It is used in combination with a 5-hydroxytryptamine type 3 (5HT3) receptor antagonist (e.g., ondansetron [Zofran] or palonosetron [Aloxi]) and a corticosteroid (e.g., dexamethasone [Decadron]) for therapy (Burns et al., 2020). Patients require aggressive hydration before and after administering cisplatin (Platinol) to manage associated nephrotoxicity and protect the renal system from injury (Brown et al., 2019; Olsen et al., 2023).
Chemotherapy-induced peripheral neuropathy (CIPN) is a frequent side effect of cisplatin (Platinol), carboplatin (Paraplatin), paclitaxel (Taxol), and docetaxel (Taxotere). It is the most common dose-limiting toxicity (DLT) of these agents. DLTs are severe, often debilitating toxicities that warrant a dose reduction, interruption, or sometimes treatment discontinuation. CIPN results from demyelination of the sensory and motor axons. Patients experience reduced nerve conduction velocity, leading to the loss of deep tendon reflexes, paresthesia (numbness and tingling), weakness, and burning pain. CIPN initially affects the body’s most distal points, such as the fingertips and toes, and moves proximally toward the midline as the damage progresses. In severe cases, patients may lose all sensation in the fingers, hands, toes, and feet; this can cause significant disability, such as the inability to grasp or hold items and gait disturbance, leading to imbalance and falls. The etiology of CIPN is complex, as no single pathophysiologic process has been identified. CIPN is dose-dependent and progressive during treatment but also can have a cascading effect after treatment ends. During this cascading phenomenon, symptoms become more prominent after discontinuing the offending agent. Pain, sensory changes, and weakness that manifest during treatment generally lead to chemotherapy dose reductions, changes in treatment protocols, or termination of the therapeutic agent entirely. The morbidity associated with CIPN can profoundly impact quality of life and activities of daily living (ACS, 2020a; Brown et al., 2019).
Currently, no medications or supplements are effective in preventing CIPN. Exercising regularly, reducing alcohol use, and treating preexisting medical conditions (vitamin B12 deficiency) may reduce the risk of CIPN. Management for CIPN is complex, and effective treatment options are limited. Pharmacologic treatment focuses on symptom relief, although many agents are not highly effective. Over-the-counter pain medications, menthol creams, capsaicin creams, or lidocaine patches may be used for comfort. Some patients may be prescribed medications such as gabapentin (Neurontin), an anticonvulsant/anti-epileptic agent that also can treat neuropathic pain. Some patients may find relief from selective serotonin-norepinephrine reuptake inhibitors (SNRIs) like duloxetine (Cymbalta). The American Society of Clinical Oncology (ASCO, 2020) recommends duloxetine (Cymbalta) as the only agent with appropriate evidence to support its use in patients with established painful CIPN. However, the degree of benefit is limited (Loprinzi et al., 2020). Patients with CIPN must be counseled on ways to avoid injury through wearing supportive shoes and paying attention to home safety, such as using handrails on stairs and removing throw rugs. Patients must also be mindful of water temperatures, as they may become less sensitive to hot water, increasing their risk of burns when bathing or washing dishes. Improvement in function and resolution of symptoms often occur over time, but nerve damage may be permanent (Brown et al., 2019).
Hypersensitivity Reactions to Chemotherapy
A hypersensitivity reaction (HSR) occurs when a foreign substance overstimulates the immune system and creates antibodies, causing an immune response. HSRs are commonly associated with several chemotherapy agents that are used widely in cervical cancer treatment—most prominently paclitaxel (Taxol), docetaxel (Taxotere), and carboplatin (Paraplatin). HSR risk can be reduced by pre-medicating patients with corticosteroids (e.g., dexamethasone [Decadron]), antihistamines (e.g., diphenhydramine [Benadryl], famotidine [Pepcid]), and/or acetaminophen (Tylenol). HSRs can occur during the initial chemotherapy infusion or after subsequent administrations of the same agent. Paclitaxel (Taxol) is well-known for its risk of nearly immediate acute HSR, whereas carboplatin (Paraplatin) more commonly induces an HSR after several repeated doses. Most HSRs occur during the first 15 minutes of the infusion. Initial signs and symptoms can include hives, urticaria, pruritis, swelling, back pain, facial flushing, rhinitis, abdominal cramping, chills, hypotension, and anxiety. Patients may require supplemental oxygen, fluid resuscitation, and other emergency medications as indicated. For life-threatening symptoms like bronchospasm, angioedema (swelling of the oral cavity, lips, or tongue), or anaphylaxis, epinephrine 0.1-0.5 mg (1:10,000 solution for adult patients) has been required (Nettina, 2019).
Targeted Therapy
Bevacizumab (Avastin) is a humanized monoclonal antibody that binds to and inhibits the activity of human vascular endothelial growth factor (VEGF) to its receptors, thereby blocking the proliferation and formation of new blood vessels that supply tumor cells. VEGF is a signaling protein that stimulates angiogenesis (i.e., the formation of new blood vessels) in healthy and cancerous cells. Blood vessels carry oxygen and nutrients, supporting growth and survival. Thus, tumors need blood vessels to grow and spread. Anti-angiogenesis inhibits the formation of new blood vessels by blocking the VEGF receptors. Angiogenesis inhibitors (i.e., VEGF inhibitors) sever the blood supply to cancer cells by interfering with the VEGF receptor, so tumors stay small and starve. Bevacizumab (Avastin) is commonly used as a combination therapy to treat recurrent and metastatic cervical cancers, as shown in Table 7. While it is generally well-tolerated, potential side effects include bleeding events (i.e., hemorrhage), headaches, hypertension, and proteinuria (i.e., protein spilling in the urine due to increased pressure in the kidneys). Patients may require concurrent treatment with antihypertensives to optimize blood pressure. Bevacizumab (Avastin) is contraindicated within 28 days of major or elective surgery (preoperatively or postoperatively) due to an increased risk for wound healing complications, hemorrhage, and fistula formation. The drug also carries a black box warning for GI perforation (i.e., a hole in the intestines) and fistula formation. Patients should be counseled to promptly report any sudden onset of severe and diffuse abdominal pain, bloating, firm abdomen, or acute bleeding (i.e., hemoptysis, rectal bleeding; ACS, 2021b; NCCN, 2023; Olsen et al., 2023).
Immunotherapy
As the name suggests, immunotherapy stimulates the immune system to recognize and destroy cancer cells. Immunotherapy strives to produce antitumor effects by modifying the actions of the body’s natural host defense mechanisms, priming it to become more sensitive to recognizing and attacking cancer cells. Immune-based treatments work differently than chemotherapy because they are highly specialized and targeted in their activity. Immune checkpoint inhibitors block the receptors that cancer cells use to inactivate immune cells (i.e., T-cells). When this signal is blocked, T-cells can better differentiate between healthy and cancer cells, thereby augmenting the cancer cells’ immune response. The role of immunotherapy in the treatment of cervical cancers has expanded significantly over recent years. Pembrolizumab (Keytruda) is a humanized monoclonal antibody that binds with high affinity to PD-1, preventing its interaction with PD-L1 and PD-L2. In the phase II KEYNOTE-158 clinical trial, Pembrolizumab (Keytruda) demonstrated promising and durable antitumor activity in patients with PD-L1-positive cervical cancer, offering a clinically meaningful and viable treatment strategy. Based on these results, the FDA granted accelerated approval of pembrolizumab (Keytryuda) for patients with advanced PD-L1–positive cervical cancer and disease progression or recurrent disease after chemotherapy. Recently, at the 2023 ASCO Annual Meeting, the overall survival analysis of the KEYNOTE-826 trial was presented, leading to a pivotal change in the standard of care for patients with persistent, recurrent, or metastatic cervical cancer. KEYNOTE-826 is a randomized, double-blind, phase 3 study of pembrolizumab (Keytruda) with chemotherapy compared to placebo with chemotherapy. After a median follow-up of 39.1 months, findings demonstrated that the addition of pembrolizumab (Keytruda) to chemotherapy with or without bevacizumab (Avastin) continued to show clinically meaningful improvements in overall survival and progression-free survival with no new safety signals (Columbo et al., 2021; Tanzola, 2023).
The European Society of Gynecological Oncology (ESGO), the European Society for Radiotherapy and Oncology (ESTRO), and the European Society of Pathology (ESP) released updated joint guidelines for the management of patients with cervical cancer in May 2023. They recommend the addition of pembrolizumab (Keytruda) to platinum-based chemotherapy (with or without bevacizumab [Avastin]) in all patients with PD-L1 positive tumors, denoted by a CPS score of 1 or higher (Cibula et al., 2023). Pembrolizumab (Keytruda) is generally tolerated well; the most common side effects include fatigue, nausea, anorexia, coughing, diarrhea, skin rash, and itching. However, patients may experience severe and possibly fatal autoimmune-related ARs. Although any organ system can be affected, the most commonly observed reactions include colitis, hepatitis, endocrinopathies (thyroid and adrenals), pneumonitis, and rash progression to Stevens-Johnson syndrome (SJS; ACS, 2021a; Chung et al., 2019; Feng et al., 2018; Sasikumar & Ramachandra, 2018).
Antibody Drug Conjugates (ADCs)
ADCs are an emerging class of targeted agents that deliver chemotherapy directly to cancer cells via a linker attached to a monoclonal antibody (i.e., a protein) that binds to a specific target on the cancer cell. Once bound, the ADC releases a powerful cytotoxic drug into the cancer cell. ADCs aim to improve efficacy while minimizing systemic toxicity to normal tissue using a targeted delivery mechanism (Pettinato, 2021). In 2021, tisotumab vedotin (Tivdak) received accelerated FDA approval for recurrent or metastatic cervical cancer based on the Phase 2 innovaTV 204 clinical trial, which revealed clinically meaningful and durable antitumor activity and a 24% objective response rate; nearly 1 in 4 patients responded to treatment in the trial (Coleman et al., 2021). Tisotumab vedotin (Tivdak) is directed against tissue factor (TF), a core component of the coagulation cascade. TF also affects the clinical progression of certain types of cancers by impacting the proliferation, infiltration, and metastases of malignant cells. TF is highly prevalent in cervical cancer cells, serving as a prime therapeutic target (Zhao et al., 2018).
While tisotumab vedotin (Tivdak) has demonstrated a manageable and tolerable safety profile, it does have unique ARs that require close monitoring. The most common ARs include anemia, lymphopenia, diarrhea, fatigue, nausea, diarrhea, peripheral neuropathy, alopecia, and skin rash. It can also cause bleeding events such as epistaxis and hemorrhage due to increased prothrombin international normalized ratio (PT/INR) and prolonged activated partial thromboplastin time (aPTT). Severe skin reactions, including fatal or life-threatening SJS, have been reported in select patients. Most notably, tisotumab vedotin (Tivdak) carries a boxed warning for ocular toxicity, possibly leading to treatment discontinuation in severe cases. Ocular ARs occurred in 60% of patients across clinical trials; most were conjunctival ARs (40%), dry eye (29%), corneal irritation (21%), and blepharitis (8%). Grade 3 ocular ARs occurred in 3.8% of patients, including severe ulcerative keratitis in 3.2% of patients. One patient experienced ulcerative keratitis with perforation requiring corneal transplantation. In the innovaTV 204 clinical trial, 4% of patients experienced visual acuity changes to 20/50 or worse, and 75% resolved (Coleman et al., 2021; FDA, 2023).
Given the potential for ocular ARs, all patients must be referred to eye care providers (i.e., ophthalmologist or optometrist) for an ophthalmic exam before each dose. To reduce the risk of ocular ARs, the FDA (2023) strongly advises adherence to the following recommendations:
- An ophthalmic exam, including a visual acuity test and slit lamp exam, should be performed at baseline, before each dose, and as clinically indicated.
- Patients should be started on prophylactic topical corticosteroid eye drops (one drop in each eye) before each infusion. Patients should continue administering eye drops in each eye as prescribed for 72 hours following each infusion.
- Immediately before each infusion, administer premedication with topical ocular vasoconstrictor drops in each eye.
- Apply cooling eye pads to both eyes during the infusion.
- Patients should be instructed to administer topical lubricating eye drops into both eyes daily for the duration of therapy and 30 days following the last dose.
- Patients should avoid wearing contact lenses (unless advised by their eye care provider) for the duration of therapy.
When the above recommendations are adhered to, ocular ARs are significantly reduced. Tisotumab vedotin (Tivdak) remains the only FDA-approved ADC for cervical cancer but is currently under investigation in combination with checkpoint inhibitors and carboplatin (Paraplatin; FDA, 2023; Podwika & Duska, 2023).
HPV Vaccination
According to the WHO (n.d.), effective cervical cancer primary prevention (i.e., HPV vaccination) combined with secondary prevention strategies (i.e., screening tests and early treatment of precancerous lesions) will thwart most cervical cancers. Cervical cancer is highly preventable and one of the only two cancers that can be mitigated through screening; colorectal cancer is the other. Among females of all races, cervical cancer screening is lowest in those without health insurance and lower education levels. The FDA approved three HPV vaccines to protect against high-risk HPV subtypes that are linked to cancer: nine-valent (9vHPV, Gardasil 9), quadrivalent (4vHPV, Gardasil), and bivalent (2vHPV, Cervarix). Each offers protection against the high-risk oncogenic subtypes HPV-16 and HPV-18. Gardasil also protects against subtypes 6 and 11, while Gardasil 9 protects against types 31, 33, 45, 52, and 58. Gardasil was initially a quadrivalent vaccine offering protection against four different types of HPV; it was approved for use in 2006 for females aged 9 through 26. It was first recommended as a series of three injections for females aged 11 through 26. In 2018, the FDA expanded the use of Gardasil 9—a 9-valent vaccine with increased coverage of additional high-risk HPV types—in those ages 9-45 years (CDC, 2021a, 2021b, 2022b; Meites et al., 2019; NCI, 2021). Gardasil 9 protects against 9 types of HPV: cervical, vaginal, and vulvar cancers in females, anal cancer, certain head and neck cancers (i.e., the back of the mouth and throat), and genital warts in both males and females (Merck & Co., 2023a).
Since the introduction of the vaccine, HPV infections and cervical precancers have dropped significantly. Over the past decade, females aged 12-19 have experienced a 71% decrease in high-risk HPV infections associated with cancer and anogenital warts. During the same timeframe, there has been a 61% decrease in high-risk HPV infections in females aged 30-45. Precancerous cervical lesions caused by HPV types 16 and 18 have dropped 40% among vaccinated women. Clinical data demonstrate that vaccination protection remains high after ten years, with no decrease in protection when measuring antibodies in the blood. While the HPV vaccination could prevent more than 90% of cancer caused by HPV from ever developing, nearly one-half (50%) of adolescent girls in the US have not been vaccinated (CDC, 2021a, 2021b, 2022b; Meites et al., 2019; NCI, 2021).
Side Effects
According to the CDC (2023), more than 15 years of research have demonstrated that the HPV vaccines are safe and effective. More than 135 million doses of HPV vaccines have been administered. Some of the most commonly reported side effects include the following:
- pain, redness, or edema near the injection site
- fever
- dizziness or fainting immediately after the injection
- nausea
- headaches
- tiredness
- joint or muscle pain (CDC, 2021a, 2021b, 2023; NCI, 2021)
Schedule and Dosing
The CDC’s Advisory Committee on Immunization Practices (ACIP) develops recommendations regarding all vaccination schedules and regimens in the US. The current ACIP recommendations for HPV vaccination are as follows (CDC, 2021a; Meites et al., 2019):
Routine and Catch-up Vaccination
- HPV vaccination is routinely recommended at ages 11-12 but may begin as early as 9 years.
- HPV vaccination is recommended for all individuals up to 26 years who were not adequately vaccinated.
- The 2- or 3-dose series depends on the patient’s age at initial vaccination:
- Age 9 through 14 years at initial vaccination: follow the 2-dose series and administer injections at 0 and 6-12 months
- the minimum interval between vaccinations is 5 months
- repeat dose if administered too soon
- Age 15 years or older at initial vaccination: following the 3-dose series and administer injections at 0, 1-2 months, and 6 months
- the minimum interval between vaccinations is as follows:
- dose 1 to dose 2: 4 weeks
- dose 2 to dose 3: 12 weeks
- dose 1 to dose 3: 5 months
- repeat dose if administered too soon
- the minimum interval between vaccinations is as follows:
- Age 27 through 45 years: Vaccination is not recommended for everyone older than 26 years. The ACIP recommends shared clinical decision-making for patients aged 27 through 45 years who have not previously been vaccinated to discuss the risk for new HPV infections and the possible benefits of vaccination with their healthcare provider. HPV vaccination in this age range provides less benefit because more people have already been exposed to the virus, but it has still demonstrated efficacy. Adults who were not vaccinated against HPV should discuss the risk for new HPV infections and the possible benefits of vaccination with their healthcare provider to determine if vaccination is still advised.
- Age 9 through 14 years at initial vaccination: follow the 2-dose series and administer injections at 0 and 6-12 months
Special Circumstances
- Immunocompromised patients, including those with HIV infection: administer the 3-dose series outlined above
- Children with a history of sexual abuse or assault: initiate at age 9 years
- Pregnancy: delay HPV vaccination until after pregnancy, but pregnancy testing is not required before vaccination. There is no evidence that vaccination will affect pregnancy or harm a fetus (CDC, 2021a, 2021b; Meites et al., 2019)
To learn more about HPV and vaccination, review the HPV NursingCE course and earn 1 ANCC contact hour.
References
American Cancer Society. (2020a). Chemotherapy for cervical cancer. https://www.cancer.org/cancer/types/cervical-cancer/treating/chemotherapy.html
American Cancer Society. (2020b). Radiation therapy for cervical cancer. https://www.cancer.org/cancer/types/cervical-cancer/treating/radiation.html
American Cancer Society. (2020c). Signs and symptoms of cervical cancer. https://www.cancer.org/cancer/types/cervical-cancer/detection-diagnosis-staging/signs-symptoms.html
American Cancer Society. (2020d). Surgery for cervical cancer. https://www.cancer.org/cancer/types/cervical-cancer/treating/surgery.html
American Cancer Society. (2020e). Tests for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/how-diagnosed.html
American Cancer Society. (2020f). The HPV test. https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/screening-tests/hpv-test.html
American Cancer Society. (2021a). Immunotherapy for cervical cancer. https://www.cancer.org/cancer/types/cervical-cancer/treating/immunotherapy.html
American Cancer Society. (2021b). Targeted drug therapy for cervical cancer. https://www.cancer.org/cancer/types/cervical-cancer/treating/targeted-therapy.html
American Cancer Society. (2021c). The American Cancer Society guidelines for the prevention and early detection of cervical cancer. https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/cervical-cancer-screening-guidelines.html
American Cancer Society. (2021d). Treatment options for cervical cancer, by stage. https://www.cancer.org/cancer/types/cervical-cancer/treating/by-stage.html
American Cancer Society. (2022). Cancer facts & figures for African American/Black People 2022-2024. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-facts-and-figures-for-african-americans/2022-2024-cff-aa.pdf
American Cancer Society. (2023a). Cancer facts & figures 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
American Cancer Society. (2023b). Key statistics for cervical cancer. https://www.cancer.org/cancer/types/cervical-cancer/about/key-statistics.html
American Cancer Society. (2023c). Survival rates for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/survival.html
American Cancer Society. (2023d). What is cervical cancer? https://www.cancer.org/cancer/cervical-cancer/about/what-is-cervical-cancer.html
American College of Obstetricians and Gynecologists. (2023). Loop electrosurgical excision procedure [LEEP]. https://www.acog.org/en/Womens%20Health/FAQs/Loop%20Electrosurgical%20Excision%20Procedure
Arshi, J., & Farci, F. (2022). Atypical glandular cells (AGS). StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK564318/
Assessment Technologies Institute. (2019a). Cervical punch biopsy [image]. https://cms.ascendlearning.com/share/page/site/digital-asset-librarytest/document-details?nodeRef=workspace://SpacesStore/6bf58a2a-81a5-4a91-91a8-d2c29121fa53
Assessment Technologies Institute. (2019b). Cold cone biopsy [image]. https://cms.ascendlearning.com/share/page/site/digital-asset-librarytest/document-details?nodeRef=workspace://SpacesStore/0621296e-a7c8-4fe6-8ed4-eaa55484d00d
Bhatla, N., Aoki, D., Sharma, D. N., & Sankaranarayanan, R. (2018). Cancer of cervix uteri. International Journal of Gynecology & Obstetrics, 143(Suppl. 2), 22-36. https://doi.org/10.1002/ijgo.12611
Bhatla, N., Aoki, D., Sharma, D. N., & Sankaranarayanan, R. (2021). Cancer of cervix uteri: 2021 update. International Journal of Gynecology & Obstetrics,155(Suppl. 1), 28-44. https://doi.org/10.1002/ijgo.13865
Biete, A., & Oses, G. (2018). Intraoperative radiation therapy in uterine cervical cancer: A review. Reports of Practical Oncology & Radiotherapy, 23(6), 589-594. https://doi.org/10.1016/j.rpor.2018.07.005
Brown, T. J., Sedhom, R., & Gupta, A. (2019). Chemotherapy-induced peripheral neuropathy. JAMA Oncology, 5(5),750. https://doi.org/10.1001/jamaoncol.2018.6771
Bruni, L., Albero, G., Serrano, B., Mena, M., Collado, J. J., Gomez, D., Munoz, J., Bosch, F. X., & de Sanjose, S. (2023). Human papillomavirus and related diseases report. ICO/IARC Information Centre on HPV and Cancer, 1-158. https://hpvcentre.net/statistics/reports/XMX.pdf
Burness, J. V., Schroeder, J. M., & Warren, J. B. (2020). Cervical colposcopy: Indications and risk assessment. American Family Physician, 102(1), 39-48. https://www.aafp.org/afp/2020/0701/p39.html
Burns, D., Kula, J., Marshall, S., Ashworth, E., & Ornelas, O. (2020). Best practice approach to successful conversion of fosaprepitant to aprepitant IV in a large multisite community oncology infusion center: A retrospective analysis. Advances in Therapy, 37, 3265-3277. https://doi.org/10.1007/s12325-020-01377-z
CancerQuest. (n.d.). Cancer genes. Retrieved September 2, 2023, from https://cancerquest.org/cancer-biology/cancer-genes
Centers for Disease Control and Prevention. (2021a). HPV vaccine schedule and dosing. https://www.cdc.gov/hpv/hcp/schedules-recommendations.html
Centers for Disease Control and Prevention. (2021b). Human papillomavirus (HPV): For healthcare professionals. https://www.cdc.gov/hpv/hcp/index.html
Centers for Disease Control and Prevention. (2022a). Basic information about HPV and cancer. https://www.cdc.gov/cancer/hpv/basic_info/index.htm
Centers for Disease Control and Prevention. (2022b). Cancers caused by HPV. https://www.cdc.gov/hpv/parents/cancer.html
Centers for Disease Control and Prevention. (2023). HPV vaccine. https://www.cdc.gov/hpv/parents/vaccine-for-hpv.html
Chung, H. C., Ros, W., Delord, J., Perets, R., Italiano, A., Shapiro-Frommer, R., Manzuk, L., Piha-Paul, S. A., Xu, L., Zeigenfuss, S., Pruitt, S. K., & Leary, A. (2019). Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: Results from the phase II KEYNOTE-158 study. Journal of Clinical Oncology, 37(17), 1470-1478. https://doi.org/10.1200/JCO.18.01265
Cibula, D., Raspollini, M. R., Planchamp, F., Centeno, C., Chargari, C., Felix, A., Fischerová, D., Jahnn-Kuch, D., Joly, F., Kohler, C., Lax, S., Lorusso, D., Mahantshetty, U., Mathevet, P., Naik, R., Nout, R. A., Oaknin, A., Peccatori, F., Persson, J., Querleu, D., Bernabé, S. R., Schmid, M. P., Stepanyan, A., Svintsitskyi, V., Tamussino, K., Zapardiel, I., & Lindegaard, J. (2023). ESGO/ESTRO/ESP guidelines for the management of patients with cervical cancer – Update 2023*. International Journal of Gynecological Cancer, 33, 649-666. https://doi.org/10.1136/ijgc-2023-004429
Coleman, R. L., Lorusso, D., Gennigens, C., Gonzalez-Martin, A., Randall, L., Cibula, D., Lund, B., Woelber, L., Pignata, S., Forget, F., Redondo, A., Vindelov, S. D., Chen, M., Harris, J. R., Smith, M., Nicacio, L. V., Teng, M. S., Laenen, A., Rangwala, R., Manso, L., Mirza, M., Monk, B. J., & Vergote, I. (2021). Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncology, 22(5), 609-619. https://doi.org/10.1016/S1470-2045(21)00056-5
Columbo, N., Dubot, C., Lorusso, D., Caceres, V., Hasegawa, K., Shapira-Frommer, R., Tewari, K. S., Salman, P., Usta, E. H., Yanez, E., Gumus, M., Hurtado de Mendoza, M. O., Samouelian, V., Castonguay, V., Arkipov, A., Toker, S., Li, K., Keefe, S. M., & Monk, B. J. (2021). Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. NEJM, 385, 1856-1867. https://doi.org/10.1056/NEJMoa2112435
Fang, J., Yu, X., Zhang, S., & Yang, Y. (2018). Effect of smoking on high-grade cervical cancer in women on the basis of human papillomavirus infection studies. Journal of Cancer Research and Therapeutics, 14(8), S184-S189. https://doi.org/10.4103/0973-1482.179190
Feng, Y., Ji, W., Yue, N., Huang, Y., & Ma, X. (2018). The relationship between PD-1/PD-L1 pathway and DNA mismatch repair in cervical cancer and its clinical significance. Cancer Management and Research, 10, 105-113. https://doi.org/ 10.2147/CMAR.S152232
Gajjar, K., Martin-Hirsch, P. P., Bryant, A., Owens, G. L. (2016). Pain relief for women with cervical intraepithelial neoplasia undergoing colposcopy treatment. Cochrane Database Syst Rev, 18(7), CD006120. https://doi.org/10.1002/14651858.CD006120.pub4
Hodgson, A., & Park, K. J. (2019). Cervical adenocarcinomas: A heterogeneous group of tumors with variable etiologies and clinical outcomes. Archives of Pathology & Laboratory Medicine, 143(1), 34-46. https://doi.org/10.5858/arpa.2018-0259-RA
Huang, W., Liu, J., Xu, K., Chen, H., & Bian, C. (2022). PD-1/PD-L1 inhibitors for advanced or metastatic cervical cancer: From bench to bed. Frontiers in Oncology, 12(849352). https://doi.org/10.3389/fonc.2022.849352
Loprinzi, C. L., Lacchetti, C., Bleeker, J., Cavaletti, G., Chauhan, C., Hertz, D. L., Kelley, M. R., Lavino, A., Lustberg, M. B., Paice, J. A., Schneider, B. P., Smith, E. M. L., Smith, M. L., Smith, T. J., Wagner-Johnston, N., & Hershman, D. L. (2020). Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. Journal of Clinical Oncology, 38(28), 3325-3348. https://doi.org/10.1200/JCO.20.01399
McCance, K. L., & Heuther, S. E. (2019). Pathophysiology: The biologic basis for disease in adults and children. (8th ed.). Elsevier.
Mehta, S., & Sachdeva, P. (2017). Colposcopy of female genital tract. Springer.
Meites, E., Szilagyi, P. G., Chesson, H. W., Unger, E. R., Romero, J. R., & Markowitz, L. E. (2019). Human papillomavirus vaccination for adults: Updated recommendations of the advisory committee on immunization practices. Morbidity and Mortality Weekly Report (MMWR), 68(32), 698-702. http://dx.doi.org/10.15585/mmwr.mm6832a3
Mello, V., & Sundstrom, R. K. (2022). Cervical intraepithelial neoplasia. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK544371/
Merck & Co. (2023a). Tell me about the HPV vaccine. https://www.gardasil9.com/
Merck & Co. (2023b). Understanding PD-L1 testing & scoring. https://www.keytrudahcp.com/biomarker-testing/pd-l1/
Nakamura, M., Obata, T., Daikoku, T., & Fujiwara, H. (2019). The association and significance of p53 in gynecologic cancers: The potential of targeted therapy. International Journal of Molecular Sciences, 20(5482), 1-16. https://doi.org/10.3390/ijms20215482
National Cancer Institute. (2021). Human papillomavirus (HPV) vaccines. https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-vaccine-fact-sheet
National Cancer Institute. (2022). Tumor grade. https://www.cancer.gov/about-cancer/diagnosis-staging/diagnosis/tumor-grade
National Cancer Institute. (2023a). Cancer stat facts: Cervical cancer. https://seer.cancer.gov/statfacts/html/cervix.html
National Cancer Institute. (2023b). HPV and cancer. https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer
National Cancer Institute. (2023c). HPV and pap test results: Next steps after an abnormal cervical cancer screening test. https://www.cancer.gov/types/cervical/screening/abnormal-hpv-pap-test-results#2
National Comprehensive Cancer Network. (2023). NCCN clinical practice guidelines in oncology (NCCN guidelines®): Cervical cancer version 1.2023 – April 28, 2023. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
Nettina, S. M. (2019). Lippincott manual of nursing practice (11th ed.). Wolters Kluwer.
Nicolas, I., Saco, A., Barnadas, E., Marimon, L., Rakislova, N., Fuste, P., Rovirosa, A., Gaba, L., Bunesch, L., Gil-Ibanez, B., Pahisa, J., Dias-Feijoo, B., Torne, A., Ordi, J., & del Pino, M. (2020). Prognostic implications of genotyping and p16 immunostaining in HPV-positive tumors of the uterine cervix. Modern Pathology, 33, 128-137. https://doi.org/10.1038/s41379-019-0360-3
Olsen, M., LeFebvre, K., Walker, S., & Dunphy, E. P. (2023). Chemotherapy and immunotherapy guidelines and recommendations for practice (2nd ed.). Oncology Nursing Society.
Pal, A., & Kundu, R. (2020). Human papillomavirus E6 and E7: The cervical cancer hallmarks and targets for therapy. Frontiers in Microbiology, 10(3116). https://doi.org/10.3389/fmicb.2019.03116
Perkins, R. B., Guido, R. S., Castle, P. E., Chelmow, D., Einstein, M. H., Garcia, F., Huh, W. K., Kim, J. J., Moscicki, A., Nayar, R., Saraiya, M., Sawaya, G. F., Wentzensen, N., & Schiffman, M. (2020). 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. Journal of Lower Genitourinary Tract Disease, 24(2), 102-131. https://doi.org/10.1097/LGT.0000000000000525
Pettinato, M. C. (2021). Introduction to antibody-drug conjugates. Antibodies (Basel), 10(4), 42. https://doi.org/10.3390/antib10040042
Podwika, S. E., & Duska, L. R. (2023). Top advances of the year: Cervical cancer. Cancer, 129(5), 657-663. https://doi.org/10.1002/cncr.34617
Sasikumar, P. G., & Ramachandra, M. (2018). Small-molecule immune checkpoint inhibitors targeting PD-1/PDL1 and other emerging checkpoint pathways. BioDrugs, 35(5), 481-497. https://doi.org/10.1007/s40259-018-0303-4.
Spencer, J. C., Kim, J. J., Tiro, J. A., Feldman, S. J., Kobrin, S. C., Skinner, C. S., Wang, L., McCarthy, A. M., Atlas, S. J., Pruitt, S. L., Silver, M. I., & Haas, J. S. (2023). Racial and ethnic disparities in cervical cancer screening from three US healthcare settings. American Journal of Preventative Medicine, 000(000), 1-11. https://doi.org/10.1016/j.amepre.2023.04.016
Stankiewicz, H. C., & Spach, D. H. (2021). Human papillomavirus infection. https://www.std.uw.edu/go/comprehensive-study/hpv/core-concept/all
Tanzola, M. (2023). KEYNOTE-826 update confirms survival benefit with addition of pembrolizumab to first-line chemotherapy in cervical cancer. ASCO Daily News. https://dailynews.ascopubs.org/do/keynote-826-update-confirms-survival-benefit-addition-pembrolizumab-first-line
Tempfer, C. B., Tischoff, I., Dogan, A., Hilal, Z., Schultheis, B., Kern, P., & Reziczek, G. A. (2018). Neuroendocrine carcinoma of the cervix: A systematic review of the literature. BMC Cancer, 18(530), 1-16. https://doi.org/10.1186/s12885-018-4447-x
Teoh, D., Musa, F., Salani, R., Huh, W., & Jimenez, E. (2020). Diagnosis and management of adenocarcinoma in situ: A society of gynecologic oncology evidence-based review and recommendations. Obstetrics & Gynecology, 135(4), 869-878. https://doi.org/10.1097/AOG.0000000000003761
Truth Initiative. (2019). Women & tobacco. https://truthinitiative.org/sites/default/files/media/files/2019/03/Truth_Women%20and%20Tobacco_FactSheet_final.pdf
US Food & Drug Administration. (2023). Highlights of prescribing information: TIVDAK (tisotumab vedotin-tftv) for injection. https://docs.seagen.com/Tivdak_Full_Ltr_Master.pdf
US National Library of Medicine. (2020). TP53 gene: Tumor protein p53. https://medlineplus.gov/genetics/gene/tp53/#conditions
US Preventive Services Task Force. (2022). Cervical cancer: Screening. https://www.uspreventiveservicestaskforce.org/uspstf/draft-update-summary/cervical-cancer-screening-adults-adolescents
Wang, X., Huang, X., & Zhang, Y. (2018). Involvement of human papillomaviruses in cervical cancer. Frontiers in Microbiology, 9 (2896), 1-14. https://doi.org/10.3389/fmicb.2018.02896
World Health Organization. (n.d.). Cervical cancer. Retrieved July 16, 2023, from https://www.who.int/health-topics/cervical-cancer#tab=tab_1
World Health Organization. (2022). Cervical cancer. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
Wright, J. D., Goff, B., & Chakrabarti, A. (2023). Cervical intraepithelial neoplasia: Management. UpToDate. https://www.uptodate.com/contents/cervical-intraepithelial-neoplasia-management
Yarbro, C. H., Wujcik, D., & Gobel, B. H. (Eds.). (2018). Cancer nursing: Principles and practice (8th ed.). Jones & Bartlett Learning.
Zebitay, A. G., Gungor, E. S., IIhan, G., Cetin, O., Dane, C., Furtuna, C., Atmaca, F. F., & Tuna, M. (2017). Cervical conization and the risk of premature birth: A population-based multicentric trial of Turkish cohort. Journal of Clinical & Diagnostic Research, 11(3), QC21-QC24. https://doi.org/10.7860/JCDR/2017/22996.9495
Zhao, X., Cheng, C., Gou, J., Yi, T., Qian, Y., Du, X., & Zhao, X. (2018). Expression of tissue factor in human cervical carcinoma tissue. Experimental and Therapeutic Medicine, 16(5), 4075-4081. https://doi.org/10.3892/etm.2018.6723