About this course:
This course reviews the epidemiology, transmission, signs and symptoms, diagnosis, treatment, and immunization guidelines for influenza.
Course preview
Influenza for RNs and LPNs
This course reviews the epidemiology, transmission, signs and symptoms, diagnosis, treatment, and immunization guidelines for influenza.
By the conclusion of this activity, learners should be able to complete the following.
- Review the background and epidemiology of influenza and identify high-risk populations.
- Discuss subtypes, transmission, and clinical manifestations of influenza
- identify the similarities and differences between influenza and the novel SARS-CoV-2 (COVID-19) virus.
- Discuss diagnostic testing and treatment protocols for influenza, including prescribing indications, medication side effects, and patient education.
- Discuss evidence-based influenza control and prevention strategies, including influenza immunization (vaccine types, indications, contraindications).
Influenza ("the flu") is an acute viral respiratory infection of the nose, throat, and lungs that causes fevers, headache, cough, and malaise. Illness can vary from mild to severe, depending on the strain of the virus and the infected person's overall health and immune system. While influenza viruses are detected year-round in the US, the exact timing and duration of flu seasons vary. The flu season typically starts in the fall and ends in early spring (October to March), but in recent years, flu activity has been detected through May. The peak activity (the highest percentage of specimens testing positive for the flu) is noted between December and February (Boktor & Hafner, 2023; Centers for Disease Control and Prevention [CDC], 2024b; Katz, 2024).
Key Definitions
Healthcare providers (HCPs) should be aware of a few key definitions that are important to understanding the transmission and prevention of viral illnesses, such as influenza (National Institute for Occupational Safety and Health, 2022; Palmore, 2024; Rogers, 2022):
- An epidemic is a widespread occurrence of an infectious disease in a community at a particular time.
- A pandemic is a sweeping outbreak of an infectious disease over a whole country or the world.
- A microorganism (also known as a pathogen) causes diseases (parasites, bacteria, viruses, fungi).
- A carrier is an asymptomatic individual who harbors the infectious agent without demonstrating signs of active illness.
- A susceptible host is a person (or animal) that does not possess sufficient resistance to an infectious agent (such as influenza) to prevent them from contracting the illness when exposed.
- The incubation period is the time between exposure to an infection and the emergence of symptoms.
- Virulence is the ability of a microorganism to cause disease (the degree of communicability).
- A reservoir/source is an environment where a pathogen can live and multiply.
- Modes of transport (indirect [food, water] or direct [airborne, contact]) are how pathogens move from the reservoir to the susceptible host.
- A portal of entry is an opening where a pathogen can enter (mouth, eyes, incision).
- A portal of exit refers to how the pathogen exits the reservoir (blood, mucous membranes, fecal).
- Inoculation is the introduction of a pathogen or antigen into a living organism to stimulate antibody production.
Epidemiology
On average, approximately 8% of the US population is infected with the flu each year (ranging from 3% to 11%; CDC, 2024f; Tokars et al., 2018). According to the World Health Organization (WHO, 2023b), the seasonal flu causes an estimated 3 to 5 million cases of severe illness and up to 650,000 deaths worldwide each year. For children under the age of 5, 99% of influenza-related deaths occur in developing countries. Although the impact of influenza fluctuates each season, it consistently places a substantial burden on public health (CDC, 2024f; Tokars et al., 2018). The CDC tracks and estimates the burden of the flu across the US each year. The influenza disease burden is affected by several factors, such as:
- the characteristics of the circulating viruses
- the population's immunity to the circulating viruses
- the timing of the season
- how well the vaccine is working to protect against illness
- the percentage of the population immunized against the flu (CDC, 2024b; Dolin, 2024a)
Since 2010, the flu has resulted in an estimated 9.3 to 41 million illnesses, 100,000 to 710,000 hospitalizations, and 4,900 to 51,000 deaths annually in the US. During the COVID-19 pandemic (October 2020 to May 2021), influenza activity was lower than in any previous season, with only 0.5% of the 1 million collected specimens testing positive for influenza. As the prevalence of COVID-19 decreased, influenza activity subsequently increased. During the 2023–2024 influenza season, the US saw 33 to 61 million illnesses, resulting in 15 to 28 million medical visits, 370,000 to 770,000 hospitalizations, and 24,000 to 67,000 deaths (CDC, 2024a; Dolin, 2024a). Figure 1 demonstrates influenza disease burden trends by season between 2010 and 2023 (CDC, 2024b).
Figure 1
Disease Burden of Influenza
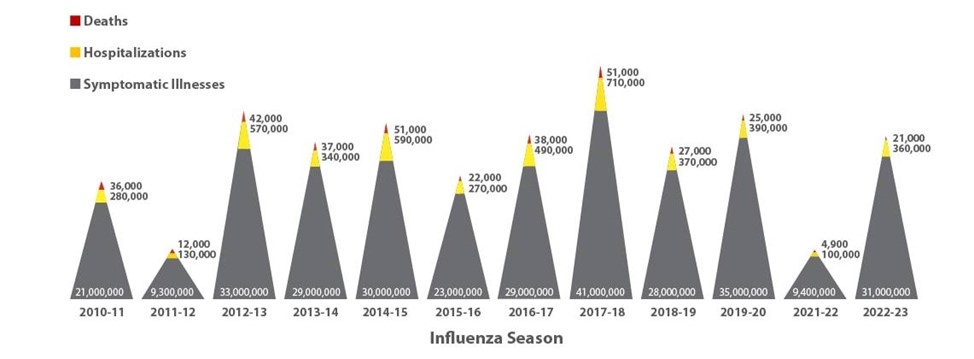
(CDC, 2024b)
While the overall mortality related to influenza is difficult to track, an innovative classification system for flu severity was adopted in 2017. This system utilizes data such as the percentage of visits to outpatient clinics with influenza-like illness, the rates of influenza hospitalizations, and the percentage of deaths resulting from pneumonia or influenza during that season (see Table 1). The 2019–2020 season was atypical, complicated by the emergence of the COVID-19 pandemic in early 2020. Flu activity rose in October 2019 and continued through mid-March 2020 but subsequently declined abruptly, likely attributable to the community prevention measures to mitigate the pandemic (social distancing, masks, handwashing). The CDC estimates that flu testing rates during the 2019–2020 season were higher than average, which is also considered a byproduct of the COVID-19 pandemic prevention strategies. However, flu activity levels were so low in 2020–2021 that disease burden estimates were not calculated (American Academy of Pediatrics [AAP], 2020; CDC, 2023b, 2024b, 2024d, 2024f).
Table 1
US Influenza Season Severity Classifications
Season | Child | Adults | Older adults | Overall |
2010–2011 | Moderate | Moderate | Moderate | Moderate |
|
...purchase below to continue the course | Low | Low | Low | Low |
2012–2013 | Moderate | Moderate | High | Moderate |
2013–2014 | Moderate | Moderate | Moderate | Moderate |
2014–2015 | Moderate | Moderate | High | High |
2015–2016 | Low | Moderate | Low | Moderate |
2016–2017 | Moderate | Moderate | Moderate | Moderate |
2017–2018 | High | High | High | High |
2018–2019 | Moderate | Moderate | Moderate | Moderate |
2019–2020 | High | High | Moderate | Moderate |
2020–2021 | Burden estimates were not calculated due to low flu activity levels. | |||
2021–2022 | Low | Low | Low | Low |
2022–2023 | High | Moderate | Moderate | Moderate |
(CDC, 2024d, 2024f, 2024h; Merced-Morales et al., 2022)
The CDC estimates that flu-associated pediatric deaths are underreported each year, as not all children whose deaths were related to influenza may have been tested for the flu. During the 2019–2020 influenza season, pediatric influenza deaths reached a new high. It was estimated that 52,000 hospitalizations occurred in patients under 18 years old and accounted for 199 deaths. Among the 199 deaths, 44% occurred in children under 5 years, 56% in children aged 5 to 17 years, and 43% in children who had a known preexisting medical condition. Only 6% of deaths were in children too young to be immunized (less than 6 months old), thereby reinforcing the critical importance of immunizing children against the flu. Estimates of disease burden for 2020–2021 were not calculated because of the limited influenza activity during the COVID-19 pandemic. Table 1 shows that during the 2021–2022 season, the disease burden remained low across all age groups. However, in the 2022–2023 season, the disease burden for children was high again. Preliminary estimates for the 2023–2024 season show that influenza activity remained high in children (CDC, 2021, 2024a, 2024f, 2024h).
Influenza Subtypes
Influenza viruses are enveloped viruses of the Orthomyxoviridae family. Initially discovered during the 1930s, the four types of seasonal flu viruses include influenza A, B, C, and D. Influenza A and B are responsible for the seasonal flu epidemics affecting humans in the US and worldwide each year. Influenza A is more contagious, responsible for moderate to severe illness in humans, birds, and swine, and is associated with high rates of mutations. Influenza A viruses have caused every known flu pandemic when new variants emerge and efficiently spread between people. Further, influenza A viruses are classified into subtypes according to the combinations of two proteins on the virus's surface, called hemagglutinin (H) and neuraminidase (N). They can be further broken down into different genetic clades (groups) and sub-clades (sub-groups), as demonstrated in Figure 2. There are 18 different H subtypes (H1 through H18) and 11 N subtypes (N1 through N11). The current influenza A viruses circulating in humans include A(H1N1) and A(H3N2). The A(H1N1) was responsible for the flu pandemic in 2009, and A(H3N2) was the most predominant strain that emerged in 2017. Influenza B causes milder disease, mutates (or changes) more slowly than influenza A, and is more commonly seen in children, on college campuses, and in long-term care facilities. Influenza B viruses are not classified into subtypes but are instead divided into lineages (B/Yamagata or B/Victoria). Influenza C is the most benign; it causes mild symptoms, often goes undetected, and does not cause flu epidemics in humans. Influenza B and C only infect humans, whereas influenza D primarily affects cattle and is unknown to infect or cause illness in humans (Boktor & Hafner, 2023; CDC, 2023g; Flerlage et al., 2021; WHO, 2023b).
Wild birds (geese, swans, ducks, gulls) are reservoirs for avian influenza A viruses. Avian influenza A viruses have evolved into distinct lineages based on geographical locations where they were first detected. The A(H5N1) avian influenza is currently widespread in birds worldwide, with outbreaks in poultry and dairy cows throughout the US. Since March of 2024, at least 550 people have been monitored for 10 days after exposure to influenza A(H5N1), with 45 people tested. Three people in 2024 tested positive for influenza A(H5N1) in Texas and Michigan, and one additional human case was reported in 2022. The CDC has determined that the public health risk is low, but continued surveillance to exposures will occur (CDC, 2024c, 2024e).
Figure 2
Influenza A and B Viruses
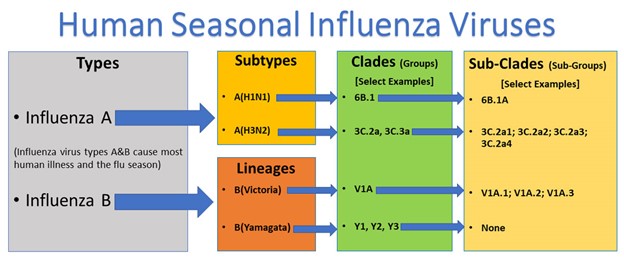
(CDC, 2023g)
Variant Influenza Viruses
Pigs can become infected with a different type of influenza called swine flu that is distinct from human flu viruses. When an influenza virus that normally circulates in pigs is detected in humans, it is called a variant. These viruses are denoted by adding the letter "v" to the end of their name. Human infections with H1N1v, H3N2v, and H1N2v have been detected in the US. For example, influenza A(H3N2) variant viruses (H3N2v) were initially identified in pigs in 2010. In 2011, there were 12 human infections with H3N2v, and in 2012, there were 309 reported cases. Sporadic cases of H3N2v have continued to be detected since that time, and most human infections have been associated with prolonged exposure to pigs at agricultural fairs. The majority of human infections with a variant virus do not result in person-to-person spread. Recurring infections and localized outbreaks may continue to occur in humans in the future, and each should be investigated to ensure that the virus does not spread in an efficient way (CDC, 2016, 2023d).
Pathogenesis of Influenza
In humans, influenza affects the respiratory tract directly or through damage caused by the immune system response. The virus preferentially targets and binds to receptors throughout the airway epithelium, from the nasopharynx to the alveoli. It primarily replicates in the upper and lower respiratory epithelium, the only sites where H is successfully cleaved. For influenza virulence, the two surface proteins H and N are necessary. H adheres to the epithelial cells in the respiratory tract, allowing the process to begin. N cleaves the bond that holds the virus together and enables its spread (see Figure 3). Viral replication combines with the immune response, causing local airway inflammation, leading to the destruction and loss of the epithelial cells throughout the respiratory tract, rendering the host more vulnerable to illness. This destruction can progress to alveolitis (inflammation of the alveoli), airway hyperreactivity and narrowing, an exacerbation of underlying conditions (asthma, COPD), and various other complications. The production and release of inflammatory mediators such as cytokines ignite the systemic symptoms of illness (Benam et al., 2019; Boktor & Hafner, 2023; Flerlage et al., 2021; Kalil & Thomas, 2019).
Figure 3
Influenza Pathogenesis
(Shutterstock/VectorMine)
Transmission
The flu primarily spreads from person to person via contact with infected droplets. Transmission most commonly occurs when an infected individual sneezes, coughs, or speaks, and flu droplets are dispersed up to 6 feet away. They can be deposited on the oral, nasal, or conjunctival membranes or inhaled into the lungs of those in the immediate vicinity. Less commonly, the flu may be contracted through contact with contaminated objects or surfaces, which are then inadvertently transferred to the eyes, nose, or mouth via touching, scratching, or rubbing. Studies have demonstrated that flu viruses can live and potentially infect a person for up to 48 hours on certain surfaces (steel, plastic) and 8 to 12 hours on cloth and facial tissues. The incubation period is 1 to 4 days, with symptoms most frequently appearing on day 2. Although infected persons are most contagious during the first 3 to 4 days after their illness begins, some may be able to infect others as early as 24 hours before—and up to 5 to 7 days after—the onset of symptoms. Studies suggest that young children and immunocompromised individuals may transmit the flu beyond 7 days. Further, some may be asymptomatic carriers, and although they feel well, they can still spread the virus to others. The high transmissibility of COVID-19 by asymptomatic carriers served a prominent role in fueling the COVID-19 pandemic, as these individuals were unknowingly spreading the virus to others (Boktor & Hafner, 2023; CDC, 2024b, 2024f).
High-Risk Populations
The CDC defines high-risk populations as persons with risk factors that increase the risk of developing serious flu-related complications, including death (CDC, 2023e). High-risk populations include:
- Age-related factors:
- older adults
- children younger than 2 years old (with the highest hospitalization and death rates among infants younger than 6 months old)
- those younger than 19 years on long-term aspirin- or salicylate-containing medications for Reye's syndrome
- Health-related factors:
- chronic pulmonary diseases (asthma, COPD, cystic fibrosis)
- neurologic and neurodevelopment conditions (cerebral palsy, epilepsy/seizure disorders, stroke, intellectual disabilities/moderate to severe developmental delay, muscular dystrophy, or spinal cord injury)
- blood disorders (sickle cell disease, hematologic cancers)
- cardiovascular disease (congenital heart disease, congestive heart failure, coronary artery disease, stroke)
- metabolic disorders (diabetes mellitus)
- renal diseases (chronic kidney disease, renal failure)
- liver disorders/conditions (hepatic impairment, hepatitis, cirrhosis)
- BMI greater than or equal to 40
- weakened immune system (either due to diseases such as HIV/AIDS or cancer or medications such as chemotherapy and chronic corticosteroids)
- women who are pregnant or postpartum (within 2 weeks after delivery)
- Other factors:
- tobacco use
- residents of skilled nursing facilities or other long-term care facilities certain racial and ethnic groups, including non-Hispanic Black, Hispanic, Latino, and Indigenous or Alaska Native persons (CDC, 2023e; Katz, 2024; Tokars et al., 2018)
Signs and Symptoms
The flu often presents with an abrupt onset of constitutional and respiratory symptoms, such as fever, chills, cough, myalgias, headache, and fatigue. In addition, some patients may describe sore throat, rhinitis, and nasal congestion. The acute symptoms typically persist for 7 to 10 days, and in most healthy individuals, symptoms are self-limited. Differentiating the flu from other types of viral respiratory infections can be difficult. The fever, usually between 101° and 102° F (38.3° and 38.9° C), can help distinguish the flu from the common cold. In pediatric populations, GI symptoms of nausea, vomiting, and diarrhea are more common than in adults; otitis media may also be seen. In healthy adolescents and adults under 65, a simple clinical definition (acute fever and cough) carries a predictive value of laboratory-confirmed influenza between 79% and 88%. In children and older adults, diagnostic testing may be necessary or helpful due to anomalous presentations in these age groups (CDC, 2022a).
The recent emergence of the COVID-19 pandemic has added another layer of complexity to the efficient and accurate diagnosis of the flu, as these illnesses can have overlapping symptoms. COVID-19 is a respiratory virus related to severe acute respiratory syndrome (SARS-CoV) and the Middle East respiratory syndrome coronaviruses. While COVID-19 continues to evolve, it can cause more severe disease in some. Symptoms of COVID-19 can take longer to develop than the flu, extending the period of contagiousness. Further, as the virus mutates, its contagiousness and infectivity seem to rise due to a higher viral load. While the signs and symptoms of COVID-19 can vary widely, the most common presenting symptoms include the following:
- fever (83% to 99% of patients)
- dry cough (59% to 82% of patients)
- fatigue (44% to 70% of patients)
- anorexia (40% to 84% of patients)
- shortness of breath (31% to 40% of patients)
- myalgias (11% to 35% of patients; CDC, 2024j; National Institute of Health, 2022; WHO, 2023a)
Loss of taste (ageusia) or smell (anosmia) preceding the onset of respiratory symptoms has also been reported with COVID-19 infections (WHO, 2023a; Wiersinga et al., 2020). In a systematic review and meta-analysis by Tong and colleagues (2020) analyzing 10 studies, there was a 43.93% prevalence of ageusia and a 52.73% prevalence of anosmia in COVID-19 patients, which typically presented early in the clinical course. Research also demonstrates that the presenting symptoms of COVID-19 vary in special populations. For example, among adults older than 75 years or immunocompromised persons, unexpected symptoms such as fatigue, reduced mobility, anorexia, reduced alertness, delirium, and agitation are more common in the absence of a fever. Therefore, HCPs practicing across specialties throughout the country must develop a keen awareness and clinical expertise in differentiating between the flu and COVID-19. Table 2 compares the cold, flu, and COVID-19 (CDC, 2024j; National Institute of Health, 2022; WHO, 2023a). Refer to the NursingCE course COVID-19 for more detailed information on COVID-19.
Table 2
Comparison of Cold, Flu, and COVID-19
| Cold | Flu | COVID-19 |
Incubation period | 1 to 3 days | 1 to 4 days | 2 to 14 days |
Symptom onset | Gradual | Abrupt | Gradual |
Fever | Rare | Usual | Common |
Body aches | Sometimes | Usual | Sometimes |
Shortness of breath | Rare | Rare | Common |
Headache | Rare | Common | Sometimes |
Fatigue | Sometimes | Usual | Common |
Extreme exhaustion | Rare | Usual | Sometimes |
Sneezing | Common | Sometimes | Sometimes |
Cough | Sometimes | Common | Common |
Rhinitis/nasal congestion | Common | Sometimes | Sometimes |
Sore throat | Common | Sometimes | Sometimes |
Vomiting/diarrhea | Rare | Sometimes (common in children) | Rare |
Unexpected sense of taste or smell | Rare | Rare | Common |
(CDC, 2022a, 2024f; WHO, 2023a)
Complications
Although most people will recover from the flu within a few days to 2 weeks, some may develop complications ranging from mild to severe. High-risk populations (as described above) have the most significant risk for complications. Some of the most frequent complications of the flu include worsening of underlying medical comorbidities (asthma, COPD, congestive heart failure) or the development of bacterial co-infection (sinus or ear infections). More serious complications include myocarditis, myositis, rhabdomyolysis, encephalopathy, and bacterial pneumonia. About 30% to 40% of hospitalized patients with the flu are also diagnosed with acute pneumonia. In both adult and pediatric populations, 30% to 50% of patients who test positive for the flu present with bacterial pneumonia co-infection (Benam et al., 2019; Dolin, 2024a; Kalil & Thomas, 2019). A worsening cough, bloody sputum, dyspnea, rales, and persistent fever are suggestive of pneumonia. In addition, the flu can trigger a severe, systemic inflammatory response throughout the body, leading to sepsis, septic shock, multi-organ failure, acute respiratory distress syndrome (ARDS), respiratory failure, and death (CDC, 2021, 2023e; Rogers, 2022). Influenza A is the primary cause of ARDS in adults and the most significant cause of respiratory viral deaths worldwide (Benam et al., 2019). According to Kalil and Thomas (2019), risk factors independently associated with influenza-induced ARDS include age between 36 and 55, pregnancy, and BMI greater than 30. In pediatrics, an increased risk of morbidity is seen in children born prematurely or with chronic illnesses such as asthma, cystic fibrosis, metabolic diseases, and sickle cell disease (CDC, 2023e; Rogers, 2022).
Diagnosis
When to Test
According to the CDC (2023c), not all patients with suspected influenza need to be tested. Testing is most beneficial when it is expected to produce clinically useful results that will impact the management plan (antiviral or antimicrobial therapy, further diagnostic evaluation, prophylaxis for high-risk contacts). The CDC guides clinicians regarding when to test patients for influenza viruses circulating in the community. The Infectious Diseases Society of America (IDSA) 2018 clinical practice guidelines encourage testing as close to the onset of symptoms as possible, ideally within 3 to 4 days. Timely diagnosis can reduce unnecessary laboratory testing, antibiotic use, and the spread of illness. Further, early treatment with antiviral medications can lessen the clinical course, duration of symptoms, and risk of hospitalization; it may also reduce mortality in high-risk populations (Dolin, 2024b; Uyeki et al., 2019).
How to Test
There are several testing options, including molecular assays (reverse transcription-polymerase chain reaction [RT-PCR], rapid molecular assays, nucleic acid amplification tests) and antigen detection tests (rapid influenza diagnostic tests [RIDTs], immunofluorescence [IF] assays). Older and less commonly used tests include viral cultures and serological antibody titers. Testing is most commonly performed by collecting upper respiratory tract specimens via nasopharyngeal swab, nasal swab, nasal wash, throat swab, or aspirate, depending on which kind of test is used. Nasopharyngeal specimens are preferred over nasal or throat swab specimens due to their higher accuracy. The sensitivity (the test's ability to correctly identify those with disease) and specificity (the test's ability to correctly identify those without disease) of each test varies based on the laboratory that performs the test, the kind of test used, the time from illness onset to specimen collection, and the type of specimen. Further, molecular assays can detect viral RNA for more extended periods after illness onset than antigen detection assays (CDC, 2020b, 2023c; Dolin, 2024b).
The RT-PCR is considered the gold standard test. It has a sensitivity of 86% to 100%. However, the processing is usually performed in special laboratories, can take up to 8 hours, and is generally more expensive than other options. Rapid molecular assays detect the virus's genetic material, are more accurate than RIDTs, and can be performed in clinics, offices, or emergency departments. They carry a high sensitivity (up to 100%) and specificity, and results are available in 15 to 20 minutes. A recent immunization with a live attenuated vaccine (LAIV) within the last 3 to 10 days may produce a false positive on these tests; thus, the patient's immunization history should be obtained. RIDTs are immunoassays that detect viral nucleoprotein antigens and are among the most commonly used tests. They provide results in 10 to 15 minutes. However, they produce a higher rate of false negatives. Most RIDTs performed in offices and clinics are 50% to 70% sensitive and more than 90% specific. Direct and indirect IF assays typically produce results in 2 to 4 hours with moderate sensitivity and high , but require a fluorescent microscope. Viral cultures can take 3 to 10 days to result and, therefore, are of less clinical utility in terms of informing management. Viral cultures are often used during outbreaks to help identify the subtype and strain of the virus circulating (CDC, 2020b, 2023c; Dolin, 2024b; Uyeki et al., 2019).
The IDSA recommends using rapid molecular assays (including RT-PCRs) over RIDTs to detect influenza viruses in outpatients. The IDSA and CDC collectively recommend using an RT-PCR (or another molecular assay test) for hospitalized patients with suspected influenza. In critically ill patients on invasive mechanical ventilation who test negative for flu on an upper respiratory tract specimen, a standard molecular assay test should be repeated using a lower respiratory tract specimen (bronchoalveolar lavage fluid or endotracheal aspirate). Viral shedding in the lower respiratory tract may be detectable for more extended periods than in the upper respiratory tract (CDC, 2020b, 2023c; Dolin, 2024b; Uyeki et al., 2019).
Interpreting Results
Interpreting the results of most rapid antigen detection tests can be tricky. During flu season, the positive predictive value of a test result is high (the chance that a positive result indicates that the patient is infected with the flu is high, implying an actual infection). The negative predictive value of a test result is low (the chance that a negative result indicates that the patient is not infected with the flu [consider the potential for false negatives]). False negatives occur more commonly with tests that have suboptimal sensitivity (an RIDT or IF assay; CDC, 2020b, 2023c). A positive result on an RIDT, IF assay, or molecular assay likely indicates that the patient has (or recently had) an infection with the influenza virus. However, it does not always mean that the patient is still infectious. The only test that can definitively confirm if infectious flu is present is a viral culture. Therefore, the CDC developed an algorithm to help interpret test results and guide clinical-decision making (CDC, 2020a).
Management/Treatment
Antiviral medications typically shorten the duration of influenza illnesses by 1 or 2 days and, most importantly, reduce the rate of hospitalization. There are currently four US Food & Drug Administration (FDA)–approved antiviral drugs recommended by the CDC (2024k) to treat influenza A and B:
- oseltamivir phosphate (Tamiflu)
- zanamivir (Relenza)
- peramivir (Rapivab)
- baloxavir marboxil (Xofluza)
These medications can be expensive, require a prescription, cause adverse effects, and have specific prescribing approvals and indications. Oseltamivir phosphate (Tamiflu), zanamivir (Relenza), and peramivir (Rapivab) are neuraminidase inhibitors (NAIs) that block the function of the viral N protein. By impeding the release of the flu virus from infected host cells, NAIs reduce the spread of the infection throughout the respiratory tract. Since influenza is characterized by its abrupt onset associated with high viral replication, NAIs require a narrow time window for optimal therapeutic effects. Further, when prescribed following contact with someone with influenza (known as post-exposure prophylaxis), NAIs must be taken for a longer duration (Parra-Rojas et al., 2018; Zachary, 2024a, 2024b).
Oseltamivir phosphate (Tamiflu) is the most common influenza treatment prescribed throughout the US. Available in an oral capsule or suspension form, oseltamivir phosphate (Tamiflu) is approved to treat acute, uncomplicated influenza in patients 2 weeks and older who have been symptomatic for no more than 48 hours. It is the preferred drug for pregnant women and is approved for post-exposure prophylaxis in patients 1 year and older (FDA, 2019). According to the AAP (2020), oseltamivir phosphate (Tamiflu) is the drug of choice for infants with influenza. Clinical trials have demonstrated that when given within 48 hours of symptom onset, oseltamivir phosphate (Tamiflu) effectively reduces viral shedding and shortens the time to symptom alleviation by up to 1.5 days. The most common adverse effects of nausea and vomiting can be mitigated by taking the medication with food (AAP, 2020; FDA, 2019; Parra-Rojas et al., 2018; Zachary, 2024b).
Zanamivir (Relenza) is an inhaled powder indicated for acute, uncomplicated influenza in patients aged 7 and older who have been symptomatic for no more than 2 days. It is also approved for post-exposure prophylaxis in patients aged 5 years and older. Zanamivir (Relenza) is not recommended for persons with underlying airway disease, such as asthma or COPD. Serious cases of bronchospasm, including fatalities, have been reported in patients with and without underlying airway disease. The medication should be permanently discontinued in patients who develop bronchospasm or a decline in baseline respiratory function (FDA, 2018). Peramivir (Rapivab) is a single-dose IV therapy for acute, uncomplicated influenza in patients 6 months and older who have been symptomatic for no more than 2 days. It is not approved for post-exposure prophylaxis. Peramivir (Rapivab) is not widely utilized due to its higher cost and the need for IV access; it is typically reserved for hospitalized patients (FDA, 2022). See Table 3 for additional information on the prescribing indications, dosing, adverse effects, warnings, and precautions associated with NAI medications (Parra-Rojas et al., 2018; Zachary, 2024a, 2024b).
Table 3
NAIs: Adverse Effects, Warnings, and Precautions
| Oseltamivir phosphate (Tamiflu) | Zanamivir (Relenza) | Peramivir (Rapivab) |
Common adverse reactions | Nausea, vomiting, headache | Sinusitis, dizziness, arthralgia, fever/ chills | Diarrhea |
Drug interactions | Avoid administration of LAIV within 2 weeks before or 48 hours after use. | ||
Warnings and precautions | Serious skin/hypersensitivity reactions (HSRs) such as Stevens-Johnson syndrome, toxic epidermal necrolysis, and erythema multiforme have been reported. | Serious, sometimes fatal bronchospasm and allergic reactions have been reported. | Cases of anaphylaxis and severe skin/HSRs such as Stevens-Johnson syndrome and erythema multiforme have been reported. |
(FDA, 2018, 2019, 2022)
Baloxavir marboxil (Xofluza) is a newer FDA-approved influenza antiviral treatment that works differently than NAIs. Baloxavir marboxil (Xofluza) is a prodrug that exerts its anti-influenza activity by blocking the polymerase acidic protein when converted to its active form. This influenza virus-specific enzyme serves a critical role in initiating viral replication. By impeding the action of polymerase acidic protein, viral replication is obstructed. Baloxavir marboxil (Xofluza) is indicated for acute, uncomplicated influenza in patients 5 years of age and older who have been symptomatic for no more than 48 hours. Baloxavir marboxil (Xofluza) is not recommended for patients less than 5 years of age due to the increased incidence of treatment-emergent resistance. In 2020, baloxavir marboxil (Xofluza) became the first single-dose influenza medicine approved for post-exposure prophylaxis. See Table 4 for the prescribing indications, dosing, adverse effects, warnings, and precautions associated with baloxavir marboxil (Xofluza; CDC, 2024k; FDA, 2024; Ng, 2019).
Table 4
Baloxavir Marboxil (Xofluza) Adverse Effects and Drug Interactions
| Baloxavir marboxil (Xofluza) |
Common adverse reactions | Diarrhea, bronchitis, nausea, sinusitis, headache |
Drug interactions | Avoid co-administration with dairy products, calcium-fortified beverages, polyvalent cation-containing laxatives or antacids, or oral supplements (calcium, iron, magnesium, selenium, zinc). Antivirals may affect LAIV. |
(FDA, 2024)
Antiviral Treatment Guidelines
The CDC and IDSA recommend priority antiviral treatment as soon as possible for patients with suspected or confirmed influenza who meet any of the following criteria:
- patients of any age who are hospitalized
- patients of any age with severe, complicated, or progressive illness (regardless of illness duration) who are seen for an outpatient visit
- patients at high risk for complications from influenza (as described above) who are seen for an outpatient visit (CDC, 2023e, 2024k; Uyeki et al., 2019; Zachary, 2024b)
For patients who have known or suspected influenza (regardless of illness duration) and are exposed to individuals at increased risk for influenza complications, antiviral treatment should be initiated. As noted in the specific prescribing guidelines outlined above, antiviral therapy should ideally be started within 48 hours of symptom onset to lessen symptom severity and shorten illness duration. Therefore, empiric antiviral treatment should be started as soon as possible in patients who meet the above priority criteria. Treatment should not be delayed while awaiting laboratory confirmation. Early empiric antiviral therapy can be considered based on clinical judgment in moderate- to low-risk patients with suspected influenza who are seen for an outpatient visit. The IDSA makes the following treatment recommendations for patients who meet the above criteria for antiviral treatment for suspected or confirmed influenza (Uyeki et al., 2019; Zachary, 2024b):
- Start antiviral treatment as soon as possible with an NAI at the FDA-approved dosage guidelines.
- Do not combine NAI medications.
- Treat uncomplicated influenza in otherwise healthy ambulatory patients with one of the following regimens:
- oral oseltamivir phosphate (Tamiflu) or
- inhaled zanamivir (Relenza) or
- a single dose of IV peramivir (Rapivab)
- Consider a longer duration of treatment for patients with a documented or suspected immunocompromising condition or patients requiring hospitalization for severe lower respiratory tract disease (especially pneumonia or ARDS), as influenza viral replication is often protracted.
Amantadine (Symmetrel) and rimantadine (Flumadine) are older FDA-approved agents for influenza A that are no longer recommended due to developed resistance in many flu strains. Drug resistance occurs when a condition stops responding to the prescribed treatment. Flu viruses are constantly mutating and changing from one season to the next; they can sometimes vary within the course of a single flu season. As a virus replicates, the genetic material can alter in a way that renders it less susceptible to the antiviral drugs used to treat or prevent the illness. The flu viruses can become less susceptible to these drugs spontaneously, or resistance can emerge during treatment. The CDC routinely monitors and tests flu viruses collected throughout surveillance to identify reduced susceptibility to antiviral drugs. This data informs public health policy recommendations regarding the benefits and use of medications. In some cases, the influenza virus can develop resistance to multiple drugs, called multiple drug resistance, resulting in minimal cell death and the growth of drug-resistant microbes (Boktor & Hafner, 2023; CDC, 2024k; Katz, 2024; Rogers, 2022).
Symptom Management
Aside from antiviral therapy, influenza treatment primarily focuses on managing and alleviating the symptoms of the illness to promote comfort. There is a wide range of over-the-counter (OTC) medications to relieve common flu ailments. Antipyretics and analgesics such as acetaminophen (Tylenol) or NSAIDs such as ibuprofen (Motrin, Advil) can be used to treat fever, myalgias, and headache. Salicylate agents such as acetylsalicylic acid (Aspirin) are contraindicated in children and adolescents under 18 years due to the risk for Reye's syndrome, a rare but serious condition that causes swelling in the liver and brain and most commonly affects children recovering from influenza or chickenpox. Patients should be advised to maintain oral hydration to prevent dehydration (Boktor & Hafner, 2023; Katz, 2024; Zachary, 2024b).
In pediatric populations who more commonly endure GI side effects of the flu, preventing dehydration is the priority, as it is a significant cause of morbidity and mortality in infants and young children. Infants and young children are also more susceptible to volume depletion and dehydration due to their higher metabolic rate. They require proportionally larger volumes of water than adults to maintain their fluid balance, and their inability (or reduced ability) to communicate their needs and rehydrate themselves further compounds the issue. Significant fluid losses may occur rapidly, leading to intravascular volume depletion, and the younger the child, the greater the risk. Therefore, the priorities in managing pediatric dehydration include early recognition, stabilization, and replacement of fluids. According to the AAP (2023), oral rehydration is appropriate for infants and children with mild dehydration (vomiting, diarrhea, decreased oral intake, decreased urine output, fatigue). However, oral fluids with high sugar content can worsen diarrhea and should be avoided. Electrolyte solutions (Pedialyte or other homemade oral rehydration preparations) should be administered until GI losses decline, followed by a gradual advancement of dietary intake with bland and stool-bulking foods (applesauce, pears, gelatin, bananas, rice). Breastfed infants should continue to nurse, as they are less likely to become severely dehydrated. For infants and children with moderate to severe dehydration (altered mental status, lethargy, tachycardia, hypotension, weak thread pulses, delayed capillary refill), hospitalization and IV fluid rehydration may be necessary to thwart severe complications (hypovolemic shock, cardiac arrhythmias) and ensure safety (AAP, 2023; Daley & Avva, 2024; Munoz & Edwards, 2024).
Physical activity may need to be modified based on individual symptoms but can be resumed as tolerated. Cough suppressants can be used in those bothered by cough, although the cough associated with flu is self-limited in most cases. Numerous agents are available OTC to manage cough and typically fall into two categories: suppressants (antitussives) and expectorants (Vanderah, 2023). Dextromethorphan (DXM) is one of the most common antitussive ingredients in many cough and cold products (Delsym, Robitussin). It is a centrally acting agent that crosses the blood-brain barrier, activates receptors in the central nervous system (in the cough center), and suppresses the cough reflex. When taken as directed, it is highly effective, and side effects are rare but may include drowsiness, nervousness, and restlessness. Patients should be counseled to avoid alcohol, caffeine pills, or other stimulants, which can exacerbate these effects. Of note, DXM is known for its abuse potential, particularly in teenagers and young adults. When taken in high doses, it can induce psychotropic effects such as euphoria, auditory and visual hallucinations, confusion, agitation, paranoia, and inappropriate laughter (US Drug Enforcement Administration, 2020).
Guaifenesin (Mucinex) is one of the most commonly used expectorants, which helps loosen or thin secretions and alleviate congestion in the chest or throat. Common side effects include dizziness, headache, drowsiness, rash, stomach upset, and nausea/vomiting. Since acetaminophen (Tylenol) is a common ingredient in many OTC flu products and doses greater than 4,000 mg per day can cause temporary or permanent liver damage, HCPs must remain hypervigilant about all the medications a patient is receiving before prescribing drugs containing acetaminophen (Tylenol). Patients and caregivers should be educated on reading the ingredients of all OTC drug labels and tracking the quantity of acetaminophen (Tylenol) intake (Vanderah, 2023).
There are many remedies to alleviate discomfort associated with a sore throat, such as oral rinses with salt water (1 cup water with one-quarter to one-half teaspoon of table salt), oral anesthetic sprays (phenol [Chloraseptic]), and lozenges containing topical anesthetics (benzocaine/menthol [Cepacol, Chloraseptic]). In addition, nurses should counsel patients on the therapeutic effects of sipping warm beverages (honey/lemon tea, chicken soup), cold beverages, or frozen desserts such as ice cream or popsicles. Caregivers should be reminded that honey should not be given to infants younger than 12 months due to the risk of botulism (Stead, 2023).
Secondary Bacterial Pneumonia Management
HCPs should evaluate for and empirically treat potential bacterial co-infection in patients with the flu who present with severe disease (extensive pneumonia, respiratory failure, high fever, and hypotension) and patients who demonstrate signs of clinical deterioration after initial improvement. HCPs should evaluate for bacterial co-infection in patients who do not improve after 3 to 5 days of antiviral therapy. Likewise, during flu season, patients who present with community-acquired pneumonia or suspected community-acquired pneumonia should be tested for flu with a rapid molecular assay. Pneumonia should be treated with antibiotics in addition to the appropriate influenza antiviral agent (Metlay et al., 2019; Zachary, 2024b). For children with positive flu tests, antibacterial therapy is not necessary unless a bacterial co-infection is suspected. Antiviral agents should be used according to the current guidelines for pediatric flu patients, as outlined above and demonstrated in Tables 3 and 4 (AAP, 2020).
Infection Control and Prevention
According to the CDC (2022b), the single most effective way to prevent the flu is to get immunized every year. To optimally control flu outbreaks, it is essential to identify cases early and implement appropriate infection control measures as soon as possible. As a society, preventing and controlling the spread of the flu centers on everyday preventive actions to limit the spread of any communicable disease, such as avoiding contact with sick individuals, staying away from others when ill, and adhering to the CDC recommendations for infection control such as effective hand hygiene practices (CDC, 2024i).
Outpatient Counseling
Patients with suspected or confirmed influenza managed during an outpatient visit should be counseled on infection control measures to prevent transmission to others, including respiratory hygiene/cough etiquette. In 2013, the CDC recommended that respiratory hygiene/cough etiquette practices be incorporated into infection control as a component of standard precautions across all health care facilities. These practices include:
- covering the mouth and nose during coughing and sneezing
- using disposable facial tissues to contain respiratory secretions, with prompt disposal into a hands-free receptacle
- wearing a surgical mask when coughing to minimize contamination of the surrounding environment
- turning the head when coughing and staying at least 3 feet away from others, especially in common waiting areas
- washing hands with soap and water or alcohol-based hand rub after contact with respiratory secretions (CDC, 2009, 2023a)
These practices should be instituted at the first point of contact with a potentially infected person to prevent the transmission of all respiratory infections. They are backed by a robust evidence base underpinning their efficacy in reducing transmission rates in health care settings, at home, and in the community. In addition, patients with suspected or confirmed influenza should remain home from work, school, and other community-related activities and events until they are afebrile for at least 24 hours without taking antipyretics (CDC, 2009, 2023a, 2024i).
Hospitalized Patients
HCPs caring for hospitalized patients with suspected or confirmed influenza should adhere to their institution's policy on droplet precautions to control and prevent the spread of the virus. According to the CDC's isolation precautions guideline, the following fundamental principles of droplet precautions apply (CDC, 2009, 2023a):
- Put a mask on the patient for source control.
- Ensure appropriate patient placement:
- Patients on droplet precautions should be placed in a private room with a private bathroom to prevent cross-contamination. In addition, the CDC offers more specific recommendations based on the health care facility as follows:
- Acute care facilities/hospitals: If single rooms are unavailable, utilize the recommendations for alternative patient placement considerations in the Guideline for Isolation Precautions.
- Long-term care and other residential settings: Decisions regarding patient placement should be made on an individual basis regarding infection risks to other residents in the room and available alternatives.
- Ambulatory settings: Place patients in an exam room or cubicle as soon as possible and instruct patients to follow all the respiratory hygiene/cough etiquette standards described in the previous section.
- Patients on droplet precautions should be placed in a private room with a private bathroom to prevent cross-contamination. In addition, the CDC offers more specific recommendations based on the health care facility as follows:
- Wear appropriate personal protective equipment (PPE):
- Droplet precautions require healthcare workers to wear a surgical mask when within 3 feet of the patient. Don a mask before entering the patient room.
- Limit the transport and movement of patients outside of their designated room for medically necessary purposes only. When transporting a patient on droplet precautions outside their room, the following principles apply:
- Put a surgical mask on the patient.
- Instruct the patient to adhere to respiratory hygiene/cough etiquette standards as described above.
Immunization
During the 2019–2020 flu season, the CDC reported that flu vaccines prevented an estimated 7 million infections, 100,000 influenza-associated hospitalizations, and 7,000 deaths. Since flu vaccines vary in efficacy, immunized persons may still get sick with the flu. However, studies consistently demonstrate that immunized individuals endure a less severe clinical course and have fewer complications than unimmunized persons (CDC, 2024g). In a 2018 study led by the CDC, findings revealed that between 2012 and 2015, flu vaccines significantly reduced the severity of illness among adults, lowering the risk of admission to an ICU with flu by 82%. Among adults admitted to the hospital with the flu, immunized persons were 59% less likely to require ICU admission than those who had not been immunized. Further, among those requiring ICU care, immunized persons spent 4 fewer days in the hospital than those not immunized (Thompson et al., 2018). Receiving the flu vaccine is associated with reduced rates of cardiac events among those with underlying cardiovascular disease and reduced hospitalizations among those with diabetes and chronic lung diseases. In pregnant women, immunization is linked to a 50% reduction in the risk for flu-associated acute respiratory infections. Several studies have demonstrated that a flu vaccine during pregnancy helps protect the baby from the flu for several months after birth. In children, immunization can be life-saving (CDC, 2024g).
The CDC (2020c) Advisory Committee on Immunization Practices recommends that all individuals aged 6 months and older receive an annual influenza immunization unless contraindicated. While vaccines provide vital protection against the flu and its complications, their efficacy varies each year. The CDC conducts studies annually to determine how well the vaccine protects against the virus, and its effectiveness relies on several factors, such as:
- the characteristics of the recipient (age and underlying health conditions)
- the type of vaccine administered
- the type(s) of circulating flu viruses each season
- the degree of similarity between circulating viruses and those included in the vaccine (Grohskopf et al., 2023)
Epidemiologists and scientists study the ongoing trends and circulating strains of influenza viruses to determine the flu vaccine composition that provides the highest protection. The CDC estimates that flu vaccines, on average, reduce the risk of illness by 40% to 60%. Currently, available flu vaccines offer higher protection against influenza B and influenza A(H1N1) viruses and less protection against influenza A(H3N2) viruses (CDC, 2024g).
Types of Flu Vaccines
Flu vaccines work by impersonating the influenza virus, prompting the body to create antibodies against it to prevent the acquisition of the illness in the future. If the flu virus tries to invade the body after a person has been immunized, their adaptive immune system responds more quickly, producing additional antibodies to address the infection and thwart illness. Immunization stimulates the body's immune system to generate defenses against the flu without causing illness. The flu vaccine cannot cause the flu. The most common flu vaccines contain weakened or inactivated parts of the virus to trigger an immune response. Typically, the flu vaccine contains a minuscule, weakened, and non-dangerous fragment of the virus that teaches the body how to build the specific antibody in the event of an encounter with the actual antigen later. Select populations (infants, young children) should be given a two-dose series 4 weeks apart, but the majority of the population requires one dose annually (CDC, 2023f; WHO, 2020).
While various flu vaccines are licensed and recommended for use in the US, all must meet FDA safety and efficacy requirements. There are three FDA-approved technologies for producing flu vaccines: egg-based flu vaccine, cell-based flu vaccine, and recombinant flu vaccine. Egg-based manufacturing processes are the oldest and most common way flu vaccines are produced. The CDC (and other authorized laboratory partners) provide manufacturers with candidate vaccine viruses grown in eggs per FDA regulatory requirements. The candidate vaccine viruses are injected into fertilized hen's eggs and incubated to allow the viruses to replicate. The fluid containing the virus is harvested from the eggs and manipulated to generate vaccines (CDC, 2023f, 2024g). While the composition of flu vaccines depends on whether they are egg-based, cell-based, or recombinant, the WHO (2024) states that their formula differences do not substantially affect their efficacy.
The three major types of flu vaccines endorsed by the CDC (2023f) include inactivated influenza vaccine (IIV), recombinant influenza vaccine (RIV), and LAIV. IIVs comprise two primary subtypes (trivalent [IIV3] and quadrivalent [IIV4]) and are the most common type of flu vaccine administered in the US. Trivalent denotes a three-ingredient component, whereas quadrivalent denotes four ingredients. High-dose (HD) flu vaccines contain an adjuvant ingredient that helps generate a more robust immune response and are recommended for persons aged 65 and older. IIVs are produced by destroying (inactivating) the virus, usually with chemicals, heat, or radiation. IIVs are more stable and safer than live vaccines as the disabled viral components cannot mutate back to their disease-causing state. However, most IIVs stimulate a weaker immune system response when compared to LAIVs. LAIV subtypes contain a fragment of the live virus in a weakened form, so it cannot inflict serious disease in recipients with healthy immune systems. LAIV is the closest to natural immunity and, therefore, associated with the highest immunity compared to other vaccines. However, they are contraindicated in specific populations, particularly those with weakened immune systems. RIVs do not use an egg-grown vaccine virus or CVV component in the production process, thus making them an attractive alternative for patients with severe egg allergies. To create a recombinant vaccine, scientists isolate the DNA of the surface protein (H) and combine it with a baculovirus (a virus that infects invertebrates [animals lacking a backbone]). The baculovirus helps transport the DNA instructions for generating antigens for the flu virus into a host cell, thereby stimulating the growth and replication of protective antibodies (CDC, 2023f; Hibberd et al., 2024; Richards et al., 2020; Savoy, 2024).
Table 5 provides an overview of some of the flu vaccines available during the 2023–2024 season. Each flu vaccine has specific indications as denoted by the manufacturer guidelines (see Table 5).
Table 5
Flu Vaccines Examples
Class | Example | Indication: Dose/Administration |
IIV | FLUAD Quadrivalent | Approved for adults >65 years: 0.5 mL IM injection |
Fluzone HD Quadrivalent | >65 years: 0.5 mL IM injection | |
FluLaval Quadrivalent | >6 months: 0.5 mL IM injection | |
Fluzone Quadrivalent | >6 months: 0.5 mL IM injection | |
Fluarix Quadrivalent | >6 months: 0.5 mL IM injection | |
IIV | Afluria Quadrivalent | Age-based dosing:
|
RIV | Flublok Quadrivalent | >18 years: 0.5 mL IM injection |
LAIV | FluMist Quadrivalent | 2 to 49 years: 0.2 mL intranasal sprayer |
(CDC, 2023f; Dzintars & Chalk, 2024; Grohskopf et al., 2023)
Vaccine Indications, Timing, and Dosing
HCPs are encouraged to consult manufacturer guidelines before administration of any vaccine. Ideally, flu vaccines should be administered before the end of October but are offered as long as the influenza viruses are circulating and the vaccine supply is available. If the vaccine is administered too early in the season (July through September), it may lead to suboptimal protection later in the season, particularly among older adults and high-risk populations. For children who need two doses of the vaccine, the first should be given as early in the season as possible and the second a minimum of 4 weeks later (CDC, 2024g; Grohskopf et al., 2023).
Contraindications and Precautions
According to the CDC (2023f), absolute contraindications to flu immunization include infants under 6 months old and those who have experienced a severe (life-threatening) allergy to a previous dose of a flu vaccine or any ingredient in the vaccine. Individuals with mild egg allergies are safe to receive any flu vaccine. Persons with severe egg allergies can receive vaccines in a medical office or hospital, where HCPs can monitor them for 15 minutes following administration. LAIV immunization (FluMist Quadrivalent) has a more extensive list of contraindications than other flu vaccines (CDC, 2023f; Savoy, 2024). Most flu vaccines are administered by IM injection in the upper arm (deltoid). LAIV is administered via the intranasal route. Both administration routes require an appropriate technique to ensure the proper delivery of the agent (Vanderah, 2023).
Side Effects and Interactions
Side effects are typically mild and resolve within a few days. As denoted earlier, these are side effects and are not symptoms of infection, as the vaccine cannot cause the flu. Although serious side effects from the flu vaccine are rare, HSRs and other serious complications such as Guillain-Barré syndrome and Bell's palsy (unilateral facial paralysis) have been reported. The most common side effects include the following:
- pain, swelling, or redness at the injection site
- myalgias
- low-grade fever
- fatigue/malaise
- headache
- nausea (CDC, 2023f; Hibberd et al., 2024; Savoy, 2024)
The Vaccine Adverse Event Reporting System (VAERS) is a nationwide online system managed by the CDC and endorsed by the US Department of Health and Human Services (HHS), which monitors the safety of all vaccines licensed in the US VAERS collects and reviews reports of adverse events occurring with—and following—immunization (including immunization errors). The system is primarily concerned with monitoring adverse events. It encourages HCPs to report clinically significant adverse events so that officials can determine if further investigation is needed (HHS, n.d.; Hibberd et al., 2024; Savoy, 2024).
While there are minimal vaccine interactions, HCPs should perform a comprehensive medication reconciliation before administering a flu vaccine. The most notable interactions include immunosuppressive therapies (chemotherapy, corticosteroids, radiation); LAIVs should be avoided in these patients. While IIV subtypes can be administered to these patients, they should be counseled on the potential for diminished or suboptimal immunity due to a potentially weakened immune response. The flu vaccine can be safely co-administered with the pneumococcal vaccine. While there have been reports of the flu vaccine impeding the clearance of certain drugs (warfarin [Coumadin], phenytoin [Dilantin]), well-controlled studies have demonstrated inconsistent findings. Further, the CDC (2024g) cautions that antiviral drugs are the second line of defense against the flu and are not a substitute for getting immunized. LAIV should not be administered until 48 hours after completing antiviral therapies, and antiviral drugs should not be given until 2 weeks after receiving LAIV (AAP, 2020; Grohskopf et al., 2023).
Patient Education
Patients should receive education on their risk factors for flu-related complications, especially those over 65 and with underlying health conditions. In addition to the annual flu vaccine, other immunizations, including the COVID-19 vaccine and pneumococcal vaccines, should be discussed with patients. Patients covered by Medicare should be educated that their Medicare covers the influenza vaccine, and HCPs should be made aware that Medicare Part B reimburses for influenza vaccines. Each patient should be encouraged to avoid individuals with upper respiratory symptoms and crowded places during the flu and holiday season. They should be taught how to cough, turn, and breathe deeply, especially in the context of mobility impairments. Patients should receive education and support for smoking cessation and be counseled to avoid indoor pollutants like dust, smoke (firsthand or secondhand), and chemicals such as aerosol sprays. In addition, other measures to remain healthy and boost natural immunity against the flu are crucial aspects of patient teaching, such as getting adequate and consistent sleep, staying well hydrated and active, consuming a healthy and well-balanced diet, and managing stress (CDC, 2024i; Rogers, 2022).
2024–2025 Vaccine Projections
For the past several years, the influenza vaccine in many countries has been quadrivalent, containing two type A subtypes (A[H1N1] and A[H3N2]) and two type B lineages (B/Victoria and B/Yamagata). Given that viruses of the B/Yamagata lineage have not been identified globally since 2020, they will be removed for the 2024–2025 season. Therefore, all influenza vaccines available in the US for the 2024–2025 season will be trivalent, containing a representative strain of A(H1N1), A(H3N2), and B/Victoria. The WHO (2024) released recommendations for the 2024–2025 flu vaccine composition in February 2024:
- Egg-based vaccines:
- A/Victoria/4897/2022 (H1N1) pdm09-like virus
- A/Thailand/8/2022 (H3N2)-like virus
- B/Austria/1359417/2021 (B/Victoria lineage)-like virus
- Cell- or recombinant-based vaccines:
- A/Wisconsin/67/2022 (H1N1) pdm09-like virus
- A/Massachusetts/18/2022 (H3N2)-like virus
- B/Austria/1359417/2021 (B/Victoria lineage)-like virus (Hibberd et al., 2024; WHO, 2024)
All flu vaccines will contain the same lineages of influenza B/Victoria lineage (unchanged from the 2023–2024 season), as they were closely related to the strains found in circulation. Influenza A lineage will be the same for the A(H1N1) subtype and updated strain of H3N2 (an A/Thailand/8/2022 (H3N2)-like virus; Hibberd et al., 2024; WHO, 2024).
References
American Academy of Pediatrics. (2020). Recommendations for prevention and control of influenza in children, 2021–2021. Pediatrics, 146(4), 1–31. https://doi.org/10.1542/peds.2020-024588
American Academy of Pediatrics. (2023). Treating dehydration with electrolyte solution. https://www.healthychildren.org/English/health-issues/conditions/abdominal/Pages/Treating-Dehydration-with-Electrolyte-Solution.aspx
Benam, K. H., Denney, L., & Ho, L. (2019). How the respiratory epithelium senses and reacts to influenza virus. American Journal of Respiratory Cell and Molecular Biology, 60(3), 259–268. https://doi.org/10.1165/rcmb.2018-0247TR
Boktor, S. W., & Hafner, J. W. (2023). Influenza. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK459363
Centers for Disease Control and Prevention. (2009). Respiratory hygiene/cough etiquette in healthcare settings. https://www.cdc.gov/flu/professionals/infectioncontrol/resphygiene.htm
Centers for Disease Control and Prevention. (2016). Influenza A (H3N2) variant virus. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/flu/swineflu/variant/h3n2v-cases.htm
Centers for Disease Control and Prevention. (2020a). Algorithm to assist in the interpretation of influenza testing results and clinical decision-making during periods when influenza viruses are circulating in the community. https://www.cdc.gov/flu/professionals/diagnosis/algorithm-results-circulating.htm
Centers for Disease Control and Prevention. (2020b). Overview of influenza testing methods. https://www.cdc.gov/flu/professionals/diagnosis/overview-testing-methods.htm
Centers for Disease Control and Prevention. (2021). Pediatric flu deaths during 2019–2020 reach new high. https://www.cdc.gov/flu/spotlights/2020-2021/pediatric-flu-deaths-reach-new-high.htm
Centers for Disease Control and Prevention. (2022a). Flu symptoms & complications. https://www.cdc.gov/flu/symptoms/symptoms.htm
Centers for Disease Control and Prevention. (2022b). What are the benefits of flu vaccination? https://www.cdc.gov/flu/prevent/vaccine-benefits.htm
Centers for Disease Control and Prevention. (2023a). 2007 guideline for isolation precautions: Preventing transmission of infectious agents in healthcare settings. https://www.cdc.gov/infection-control/media/pdfs/guideline-isolation-h.pdf
Centers for Disease Control and Prevention. (2023b). Estimated influenza illnesses, medical visits, hospitalizations, and deaths in the United States—2019–2020 influenza season. https://www.cdc.gov/flu/about/burden/2019-2020.html
Centers for Disease Control and Prevention. (2023c). Guide for considering influenza testing when influenza viruses are circulating in the community. https://www.cdc.gov/flu/professionals/diagnosis/consider-influenza-testing.htm
Centers for Disease Control and Prevention. (2023d). Key facts about human infections with variant viruses. https://www.cdc.gov/flu/swineflu/keyfacts-variant.htm
Centers for Disease Control and Prevention. (2023e). People at high risk for flu complications. https://www.cdc.gov/flu/highrisk/index.htm
Centers for Disease Control and Prevention. (2023f). Seasonal influenza vaccine safety: A summary for clinicians. https://www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm
Centers for Disease Control and Prevention. (2023g). Types of influenza viruses. https://www.cdc.gov/flu/about/viruses/types.htm
Centers for Disease Control and Prevention. (2024a). 2023–2024 US flu season: Preliminary in-season burden estimates. https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm
Centers for Disease Control and Prevention. (2024b). Disease burden of influenza. https://www.cdc.gov/flu/about/burden/index.html
Centers for Disease Control and Prevention. (2024c). H5N1 bird flu: Current situation summary. https://www.cdc.gov/flu/avianflu/avian-flu-summary.htm
Centers for Disease Control and Prevention. (2024d). How flu spreads. https://www.cdc.gov/flu/about/disease/spread.htm
Centers for Disease Control and Prevention. (2024e). Influenza type A viruses. https://www.cdc.gov/bird-flu/about
Centers for Disease Control and Prevention. (2024f). Key facts about influenza (flu). https://www.cdc.gov/flu/about/keyfacts.htm
Centers for Disease Control and Prevention. (2024g). Key facts about seasonal flu vaccine. https://www.cdc.gov/flu/prevent/keyfacts.htm
Centers for Disease Control and Prevention. (2024h). Past seasons' flu season severity assessments. https://www.cdc.gov/flu/about/classifies-flu-severity.htm
Centers for Disease Control and Prevention. (2024i). Prevent flu: Preventative actions. https://www.cdc.gov/flu/prevent/prevention.htm
Centers for Disease Control and Prevention. (2024j). Similarities and differences between flu and COVID-19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm
Centers for Disease Control and Prevention. (2024k). What are flu antiviral drugs? https://www.cdc.gov/flu/treatment/whatyoushould.htm
Daley, S. F., & Avva, U. (2024). Pediatric dehydration. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK436022
Dolin, R. (2024a). Influenza: Epidemiology and pathogenesis. UpToDate. Retrieved June 1, 2024, from https://www.uptodate.com/contents/influenza-epidemiology-and-pathogenesis
Dolin, R. (2024b). Seasonal influenza: Clinical manifestations and diagnosis. UpToDate. Retrieved June 12, 2024, from https://www.uptodate.com/contents/seasonal-influenza-in-adults-clinical-manifestations-and-diagnosis
Dzintars, K., & Chalk, B. S. (2024). Johns Hopkins ABX guide: Influenza vaccine. https://www.hopkinsguides.com/hopkins/view/Johns_Hopkins_ABX_Guide/540287/all/Influenza_vaccine
Flerlage, T., Boyd, D. F., Meliopoulos, V., Thomas, P. G., & Schultz-Cherry, S. (2021). Influenza virus and SARS-CoV-2: Pathogenesis and host responses In the respiratory tract. Nature Reviews, 19, 425–441. https://doi.org/10.1038/s41579-021-00542-7
Grohskopf, L. A., Blanton, L. H., Ferdinands, J. M., Chung, J. R., Broder, K. R., & Talbot, H. K. (2023). Prevention and control of seasonal influenza with vaccines: Recommendations of the advisory committee on immunization practices—United States, 2023–24 influenza season. Morbidity and Mortality Weekly Report, 72(2), 1–25. http://dx.doi.org/10.15585/mmwr.rr7202a1
Hibberd, P. L., Monto, A., & Ortiz, J. R. (2024). Seasonal influenza vaccination in adults. UpToDate. Retrieved June 13, 2024, from https://www.uptodate.com/contents/seasonal-influenza-vaccination-in-adults
Kalil, A. C., & Thomas, P. G. (2019). Influenza virus-related critical illness: Pathophysiology and epidemiology. Critical Care, 23(258), 1–7. https://doi.org/10.1186/s13054-019-2539-x
Katz, S. (2024). Influenza. Merck Manual Professional Version. https://www.merckmanuals.com/professional/infectious-diseases/respiratory-viruses/influenza
Merced-Morales, A., Daly, P., Elal, A. I. A., Ajayi, N., Annan, E., Budd, A., Barnes, J., Colon, A., Cummings, C. N., Iuliano, A. D., DaSilva, J., Dempster, N., Garg, S., Gubareva, L., Hawkins, D., Howa, A., Huang, S., Kirby, M., Kniss, K., . . . Brammer, L. (2022). Influenza activity and composition of the 2022–23 influenza vaccine—United States, 2021–2022 season. Morbidity and Mortality Weekly Report, 71(29), 913–919. https://doi.org/10.15585%2Fmmwr.mm7129a1
Metlay, J., Waterer, G., Long, A., Anzueto, A., Brozek, J., Crothers, K., Cooley, L., Dean, N., Fine, M., Flanders, S., Griffin, M., Metersky, M., Musher, D., Restrepo, M., & Whitney, C. (2019). Diagnosis and treatment of adults with community-acquired pneumonia. American Journal of Respiratory and Critical Care Medicine, 200(7), 45–67. https://doi.org/10.1164/rccm.201908-1581ST
Munoz, F. M., & Edwards, M. S. (2024). Seasonal influenza in children: Management. UpToDate. Retrieved June 12, 2024, from https://www.uptodate.com/contents/seasonal-influenza-in-children-management
National Institute of Health. (2022). Is it flu, COVID-19, allergies, or a cold? https://newsinhealth.nih.gov/2022/01/it-flu-covid-19-allergies-or-cold
National Institute for Occupational Safety and Health. (2022). Chain of infection components. Centers for Disease Control and Prevention. https://www.cdc.gov/niosh/learning/safetyculturehc/module-2/3.html
Ng, K. E. (2019). Xofluza (baloxavir marboxil) for the treatment of acute uncomplicated influenza. Pharmacy & Therapeutics, 44(1), 9–11. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6336199
Palmore, T. N. (2024). Infection control measures for prevention of seasonal influenza. UpToDate. Retrieved June 1, 2024, from https://www.uptodate.com/contents/infection-control-measures-for-prevention-of-seasonal-influenza
Parra-Rojas, C., Nguyen, V. K., Hernandez-Mejia, G., & Hernandez-Vargas, E. A. (2018). Neuraminidase inhibitors in influenza treatment and prevention—Is it time to call it a day? Viruses, 10(9), 454. https://doi.org/10.3390/v10090454
Richards, K. A., Moritzky, S., Shannon, I., Fitzgerald, T., Yang, H., Branche, A., Topham, D. J., Treanor, J. J., Nayak, J., & Sant, A. J. (2020). Recombinant HA-based vaccine outperforms split and subunit vaccines in elicitation of influenza-specific CD4 T cells and CD4 T cell-dependent antibody response in humans. npj Vaccines, 5(77), 1–10. https://doi.org/10.1038/s41541-020-00227-x
Rogers, J. L. (2022). McCance & Heuther's Pathophysiology: The biologic basis for disease in adults and children. (9th ed.). Mosby.
Savoy, M. L. (2024). Influenza vaccine. Merck Manual Professional Version. https://www.merckmanuals.com/professional/infectious-diseases/immunization/influenza-vaccine
Stead, W. (2023). Symptomatic treatment of acute pharyngitis in adults. UpToDate. Retrieved June 13, 2024, from https://www.uptodate.com/contents/symptomatic-treatment-of-acute-pharyngitis-in-adults
Thompson, M. G., Pierse, N., Huang, Q. S., Prasad, N., Duque, J., Newbern, E. C., Baker, M. G., Turner, N., McArthur, C., & Centers for Disease Control and Prevention. (2018). Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012–2015. Vaccine, 36(39), 5916–5925. https://doi.org/10.1016/j.vaccine.2018.07.028
Tokars, J. I., Olsen, S. J., & Reed, C. (2018). Seasonal incidence of symptomatic influenza in the United States. Clinical Infectious Diseases, 66(10), 1511–1518. https://doi.org/10.1093/cid/cix1060
Tong, J.Y., Wong, A., Zhu, D., Fastenberg, J.H., & Tham, T. (2020). The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: A systematic review and meta-analysis. Otolaryngology Head Neck Surgery, 163(1), 3–11. https://doi.org/10.1177/0194599820926473
U.S. Department of Health and Human Services. (n.d.). VAERS: Vaccine adverse events reporting system. Retrieved June 13, 2024, from https://www.cdc.gov/vaccinesafety/pdf/vaers_factsheet1.pdf
U.S. Drug Enforcement Administration. (2020). Drug fact sheet: DXM. https://www.dea.gov/sites/default/files/2020-06/DXM-2020.pdf
U.S. Food & Drug Administration. (2018). Highlights of prescribing information: RELENZA (zanamivir inhalation). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021036s030lbl.pdf
U.S. Food & Drug Administration. (2019). Highlights of prescribing information: TAMIFLU (oseltamivir phosphate). https://www.gene.com/download/pdf/tamiflu_prescribing.pdf
U.S. Food & Drug Administration. (2022). Highlights of prescribing information: RAPIVAB (peramivir) injection. https://www.rapivab.com/themes/rapivab/pdf/Rapivab-Prescribing-Information.pdf
U.S. Food & Drug Administration. (2024). Highlights of prescribing information: XOFLUZA (baloxavir marboxil). https://www.gene.com/download/pdf/xofluza_prescribing.pdf
Uyeki, T. M., Bernstein, H. H., Bradley, J. S., Englund, E. A., File, T. M., Fry, A. M., Gravenstein, S., Hayden, F. G., Harper, S. A., Hirshon, J. M., Ison, M. G., Johnston, B. L., Knight, S. L., McGeer, A., Riley, L. E., Wolfe, C. R., Alexander, P. E., & Pavia, A. T. (2019). Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clinical Infectious Diseases, 68(6), e1–e47. https://doi.org/10.1093/cid/ciy866
Vanderah, T. W. (2023). Katzung's basic and clinical pharmacology (16th ed.). McGraw-Hill Education.
Wiersinga, W. J., Rhodes, A., Cheng, A. C., Peacock, S. J., & Prescott, H. C. (2020). Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA, 324(8), 782–793. https://doi.org/10.1001/jama.2020.12839
World Health Organization. (2020). How do vaccines work? https://www.who.int/news-room/feature-stories/detail/how-do-vaccines-work
World Health Organization. (2023a). Clinical management of COVID-19. https://www.who.int/teams/health-care-readiness/covid-19
World Health Organization. (2023b). Influenza (seasonal). https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal)
World Health Organization. (2024). Recommended composition of influenza virus vaccines for use in 2024–2025 northern hemisphere influenza season. https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2024-2025-northern-hemisphere-influenza-season
Zachary, K. C. (2024a). Seasonal influenza in adults: Role of antiviral prophylaxis for prevention. UpToDate. Retrieved June 12, 2024, from https://www.uptodate.com/contents/seasonal-influenza-in-adults-role-of-antiviral-prophylaxis-for-prevention
Zachary, K. C. (2024b). Seasonal influenza in nonpregnant adults: Treatment. UpToDate. Retrieved June 12, 2024, from https://www.uptodate.com/contents/seasonal-influenza-in-nonpregnant-adults-treatment