About this course:
The purpose of this module is to provide a foundational understanding of the basics of pharmacology, outlining the principles of pharmacokinetics, pharmacodynamics, and the fundamental aspects of safe medication prescribing to enhance advanced practice registered nurse (APRN) practice and safeguard patient care.
Course preview
Introduction to Pharmacology for APRNs
The purpose of this module is to provide a foundational understanding of the basics of pharmacology, outlining the principles of pharmacokinetics, pharmacodynamics, and the fundamental aspects of safe medication prescribing to enhance advanced practice registered nurse (APRN) practice and safeguard patient care.
By the completion of this learning activity, learners should be able to:
- Discuss the general principles of pharmacology and define the concepts of pharmacotherapeutics, pharmacokinetics, and pharmacodynamics.
- Discuss the relevance of the four aspects of pharmacokinetics (absorption, distribution, metabolism, and excretion), routes of medication administration, and factors that affect a drug's pharmacokinetics.
- Describe the various biochemical changes that occur in the body due to the pharmacodynamics of a drug.
- Differentiate between drug tolerance, cumulative drug effects, and drug toxicity.
- Identify factors that cause variability in drug disposition and drug response.
- Explore the aspects of drug side effects and adverse effects, including drug-drug and drug-food interactions, and their relationship to the development of adverse effects and toxicity.
- Describe the etiology of hypersensitivity and anaphylactic reactions and their clinical manifestations and management.
- Discuss the elements of medication reconciliation, the core components of safe medication prescribing, and contraindications that can make prescribing a drug dangerous or detrimental to a patient.
- Outline the ethical responsibilities when prescribing medications and the core aspects of patient education.
Medications are chemicals used to treat illness, slow disease progression, improve quality of life, alleviate symptoms, and improve patient outcomes. There is an increased reliance on medication therapy in modern-day health care to support vital physiological processes and remedy illnesses. More than 20,000 prescription medications are available in the US (Food and Drug Administration [FDA], 2020b). According to the Centers for Disease Control and Prevention (CDC, 2023), 48.6% of individuals take at least one prescription medication, 24% of individuals take three or more, and 12.8% take five or more medications simultaneously. While medication therapy can be lifesaving or life-prolonging, every medication has the potential to cause harm inadvertently as a consequence of unintended side effects or medication errors (Agency for Healthcare Research and Quality [AHRQ], 2019).
The primary responsibility in patient care and medication therapy is patient safety. APRNs must learn the various classes of medications, understand how medications work, recognize both the generic and brand names of medications, follow the restrictions regarding controlled substances, be familiar with credible resources to obtain specific information about medications (drug handbooks, online resources), and ensure patients are adequately educated on all medications they are taking. They must understand the components of safe medication administration and their ethical responsibilities in prescribing and administering medications. APRNs must ascertain a comprehensive understanding of medications and their effects on the body to determine whether the intended effects of the medication have been achieved and recognize unintended effects (Craven et al., 2021; Smith & Pacitti, 2020).
Key Definitions
The following terms are briefly defined to outline the concepts that will be reviewed within this learning activity (Frandsen & Pennington, 2020; Lilley et al., 2022; Vanderah, 2024):
- ABSORPTION is the movement of a drug from the site of administration to various tissues in the body.
- ADVERSE DRUG EFFECT is an unintended and unexpected effect of a drug that can be severe and life-threatening at a therapeutic dose.
- AGONIST is a drug that stimulates (or activates) receptor(s) in the body.
- ALLERGIC REACTION is an immunologic-based hypersensitivity response that results from administering a drug to an individual sensitive to that drug.
- ANTAGONIST is a drug that inhibits the activity of receptor(s) in the body.
- BIOTRANSFORMATION is a biochemical reaction that occurs primarily in the liver and produces an active or inactive metabolite of the original drug.
- BLOOD-BRAIN BARRIER is a protective barricade that selectively restricts the passage of chemicals from the bloodstream to the brain.
- CONTRAINDICATION is a disease state or patient characteristic that renders a drug inappropriate to be used due to the potential for adverse effects.
- CUMULATIVE EFFECTS occur when the body cannot metabolize and excrete a drug before the next dose is given.
- DISTRIBUTION is the movement of a drug by the circulatory system to its intended site of action.
- DRUG-DRUG INTERACTION occurs when two or more drugs are given that can radically change the action of either or both drugs in the body by reducing absorption or increasing toxicity.
- DRUG-FOOD INTERACTION occurs when a drug is given alongside a specific type of food that can radically change its action by reducing absorption or increasing toxicity.
- DRUG METABOLISM is the change (chemical alteration) that occurs within a drug, making it more or less potent.
- DRUG'S BRAND NAME refers to the advertised name under which a drug is sold.
- DRUG'S GENERIC NAME refers to the chemical makeup, structure, or formula of a drug rather than the advertised brand name under which it is often sold.
- DURATION OF ACTION is the time a drug is in the blood in sufficient amounts to elicit a response.
- EXCRETION is the elimination of a drug or its metabolites through various parts of the body.
- FIRST-PASS EFFECT is the effect the liver has on a drug as it passes through for the first time, deactivating a portion of the drug.
- HALF-LIFE is the time it takes for the amount of a drug that enters the body to decrease by half.
- HYPERSENSITIVITY REACTION occurs secondary to administering a drug that a patient's body perceives as a foreign substance, precipitating a mild to moderate histamine release.
- METABOLITE is a chemical form of a drug remaining after biotransformation that may or may not have a pharmacologic effect.
- ONSET OF ACTION is the time it takes for a drug to exert its therapeutic effect.
- PEAK LEVEL of a drug is the point in time when a drug is at its highest concentration in the body.
- PHARMACEUTICS are the various pharmaceutical properties a drug possesses based on its form and chemical composition. The discipline of pharmaceutics involves developing a chemical entity into a medication that can be safely and effectively administered in the body.
- PHARMACODYNAMICS reflects the biochemical changes that occur in the body as a result of a drug.
- PHARMACOKINETICS refers to how a drug travels through the body and undergoes the biochemical processes of absorption, distribution, metabolism, and excretion.
- PHARMACOLOGY is the study of drugs and their effects on the body.
- PRECAUTIONS refer to actions that should be employed when medications are prescribed that can cause adverse effects in specific populations or with other drugs or foods.
- SIDE...
...purchase below to continue the course
Pharmacology
Pharmacology is the study of chemicals (drugs) used to prevent, diagnose, or treat disease and their effects on the body. Pharmacology analyzes the actions of drugs, incorporating knowledge from two primary and interrelated domains: pharmacokinetics and pharmacodynamics. The history of pharmacology dates back thousands of years, beginning with the use of medicinal plants and herbs to relieve manifestations associated with different diseases (OpenStax, 2024; Vanderah, 2024). A drug can have three effects on the body:
- Therapeutic effect: the intended, positive influence on the body
- diphenhydramine (Benadryl) is administered to alleviate pruritus, swelling, and erythema caused by a bee sting
- Side effect: a non-therapeutic effect on the body
- diphenhydramine (Benadryl) is administered to alleviate pruritus, swelling, and erythema caused by a bee sting, also leading to side effects of drowsiness and sedation
- Adverse effect: a negative, undesirable, unintended, or harmful consequence to the body
- diphenhydramine (Benadryl) is administered to alleviate pruritus, swelling, and erythema caused by a bee sting inadvertently inducing hypotension and syncope in an older adult (Vanderah, 2024)
The goal of medication therapy is to achieve a desired beneficial effect with minimal adverse effects or toxicity. When administered for therapeutic purposes, drugs are often referred to as medications since the intent is to elicit a specific response. In most clinical settings, these terms are used interchangeably and carry the same meaning. All prescribed medications have three names: the chemical name, the generic name, and the brand (or trade) name. A chemical name is built around the drug's specific chemical structure or composition and often has little meaning for nurses. For example, the chemical name for ibuprofen is 2-(4-Isobutylphenyl) propanoic acid.
In clinical practice, nurses must be familiar with the generic and brand names of various medications since they can be ordered and labeled with either name. An example of a generic name is acetaminophen, and its corresponding brand name is Tylenol. A generic name typically begins with a lowercase letter, whereas the first letter of a brand name is capitalized: acetaminophen (Tylenol). A generic drug may have multiple brand names, such as the nonsteroidal anti-inflammatory drug (NSAID) ibuprofen, sold under the brand names Motrin and Advil. Due to the wide variability in brand names, best-practice guidelines recommend the use of generic names to prevent confusion. For this education module, both generic and brand names will be utilized when referencing medications (Craven et al., 2021; OpenStax, 2024; Smith & Pacetti, 2020).
Drug Development
The FDA regulates the safety and efficacy of all drugs (prescription and over-the-counter [OTC]) sold in the US. All drugs are toxic to some individuals at some dose. For a manufacturer to obtain FDA approval for a new drug, they must demonstrate the drug's safety and efficacy according to specified criteria outlined in federal law and FDA regulations. In addition, the drug must pass FDA inspection and obtain approval for its labeling (all written material about the drug, including its packaging, prescribing information for providers, and patient brochures). The drug's safety, dosing, and usefulness in treating disease are established through rigorous laboratory research, in vitro studies, and clinical trials involving human participants.
Clinical trials for drug development have four primary phases (see Figure 1 below). Prior to the four phases, a pre-clinical discovery and screening phase occurs. In the pre-clinical phase, the drug sponsor develops a new drug compound and tests it on animals for toxicity. Once the drug sponsor has determined the safety and efficacy in animals, they must submit an Investigational New Drug application to the FDA.
Phase I evaluates the drug's safety in humans and typically involves a few (usually 20 to 80) healthy adult volunteers, although there are exceptions, such as anti-cancer therapies. Phase II assesses the drug's effectiveness in treating disease, starts to evaluate the drug in the target population, and establishes the final dosage. Phase III tests the drug on a larger scale of human participants (up to 3,000) and brings the drug to market. Phase III studies are typically operationalized with randomized control trials. The drug's clinical utility is tested against another drug or placebo to ensure the drug is safe and effective in the target population and disease entity. Phase IV consists of post-marketing trials, such as when the manufacturer is trying to get the drug approved for another indication. Once a drug is on the market, the FDA maintains oversight of the drug's long-term safety and effectiveness and can remove the drug from the market if necessary (Dabrowska & Thaul, 2018; FDA, n.d., 2018b; Vanderah, 2024).
Figure 1
Clinical Trial Phases
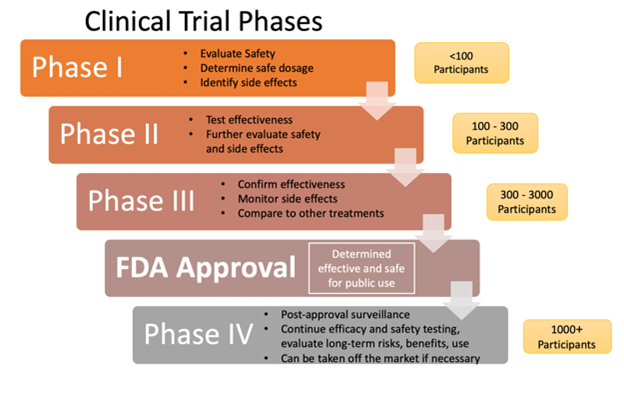
(National Institutes of Health, 2022; Selchick, 2021)
Prescription Medications, Herbals, and Dietary Supplements
Drugs are either prescribed (obtainable only by prescription from a licensed provider) or available OTC without a prescription. OTC agents, including herbal preparations and dietary supplements, are readily available and can be purchased at a store or online. Patients use these agents for diverse indications, such as managing symptoms or enhancing wellness. Some prescription medications are available for purchase as OTC preparations in smaller doses. For example, ibuprofen (Motrin) is available OTC at 200-mg doses, whereas the prescription strength is 800 mg. Diphenhydramine (Benadryl) is available OTC in 25-mg doses, and the prescription strength is 50 mg. While the FDA regulates prescription and OTC medications to ensure their safety and efficacy, herbal preparations and dietary supplements are not subjected to the same scrutiny and oversight.
Herbals and supplements represent various substances ranging from vitamins and minerals to enzymes, probiotics, and botanicals. Dietary supplements are available in many forms, such as pills, gummies, powders, energy bars, and drinks. Furthermore, most supplements and herbal preparations have not undergone rigorous scientific testing for safety or effectiveness. The lack of FDA oversight and regulation of these agents poses concerns regarding their consistency, ingredient purity, and safety profiles. Although patients do not require a prescription to obtain these substances, they still pose a risk for adverse effects and dangerous interactions. The federal government established the National Center for Complementary and Integrative Health (NCCIH) in 1998 to encourage the investigation of herbal medication and other botanicals (National Institute on Aging, 2021; NCCIH, 2021; OpenStax, 2024; Vanderah, 2024). For more information on this topic, refer to the Dietary Supplements NursingCE course.
Drug Classification
Drugs are grouped according to their similarities. A drug can be classified by its chemical composition (pharmacologic classification), effect on a specific body system, or ability to treat a particular disease (therapeutic classification). For example, morphine sulfate (MS Contin) can be classified as a narcotic or opioid analgesic or as a central nervous system (CNS) depressant. The overall purpose of drug classification is to ensure patient safety and guide provider selection. Table 1 provides a few examples of prescription drug classifications based on chemical makeup and the intended mechanism of action. While there are numerous drug categories (which extend beyond the scope of this module), many drugs fit into multiple categories because they exert various effects on numerous body systems (Ernstmeyer & Christman, 2023; Frandsen & Pennington, 2020; Lilley et al., 2022; Vanderah, 2024).
Table 1
Examples of Drug Classification Groups
Drug classification | Mechanism of action | Uses and examples |
Benzodiazepines | Increase the levels of the neurotransmitter gamma-aminobutyric acid-A in the brain. | Treat anxiety and insomnia by inducing a calming and relaxing effect. Examples:
|
Antidepressants | Act on various neurotransmitters in the brain (most commonly target the three neurotransmitters traditionally associated with depression: serotonin, norepinephrine, and dopamine). | Treat depression, anxiety, bipolar disorder, and many other mental health conditions. Examples:
|
Stimulants | Increase the activity of neurotransmitters in the brain, particularly dopamine and norepinephrine. | Treat ADHD and narcolepsy by increasing alertness, energy, and attention. Examples:
|
Antihypertensives | Variable mechanisms based on the subcategory (diuretics, beta-blockers) | Treat hypertension. Examples:
|
Laxatives | Substances that soften loosen stools and stimulate bowel movements through variable mechanisms based on their subcategory (osmotic, bulk-forming, stimulants, suppositories) | Treat and prevent constipation. Examples:
|
(Frandsen & Pennington, 2020; Lilley et al., 2022; Vanderah, 2024)
Pharmaceutics
Pharmaceutics refers to the process of drug formulation and manufacturing. To understand the actions of drugs on the body and what happens to the drug in the body, it is first essential to understand drug composition and formulation. A drug's ability to be absorbed in the body primarily depends on its physicochemical properties, formulation, and route of administration. The dosage forms (capsules, solutions, tablets) are made up of the drug and other ingredients and are formulated to be given by various routes of administration. The choice of routes that a drug will be delivered is based on convenience, adherence, and the drug's pharmacokinetic and pharmacodynamic profile.
Drugs can be administered in various ways: enterally, parenterally, and other routes (nasally, inhalation, vaginally, topically). Parenteral forms bypass the gastrointestinal (GI) tract and are administered intradermally, intramuscularly (IM), subcutaneously (SC), or intravenously (IV; see Figure 2). Parenteral forms allow for immediate absorption since they avert the body's need to dissolve the drug. Topical preparations are administered via aerosols, creams, foams, gels, and patches. These drugs may act directly on or be absorbed through the skin for a systemic response. Enteral forms are given via the intestinal tract through the mouth (oral, sublingual, buccal), the rectum, or direct instillation into the intestine by a catheter device (such as a nasogastric tube; Kim & De Jesus, 2023; Le, 2022a; Lilley et al., 2022; Vanderah, 2024).
Figure 2
Parenteral Drug Administration
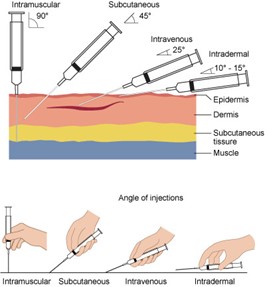
Drugs may be chemically formulated to have an effect over a specific period. Modified-release preparations include altered timing or rate of release of the medication to the body. In contrast, immediate-release (IR) dosage forms release the drug upon contact with the GI tract. Modified-release drugs may have a delayed (delayed-release [DR]) or a prolonged (extended-release [ER]) effect. With DR formulations, an initial amount may be released immediately after administration; the most common example is enteric-coated acetylsalicylic acid (Aspirin), which delays absorption until after the medication passes through gastric acids. ER preparations release the drug over a prolonged period and consist of sustained-release (SR) and controlled-release (CR) subtypes, but may also be abbreviated as XR, XL, or XT. SR delivers the drug over a sustained period to maintain steady drug concentration levels in the body, but not at a constant rate. CR maintains drug release over a sustained period at a nearly fixed rate.
APRNs must be cognizant of the formulation they are prescribing because ER medications are dosed less frequently due to their slow release over time. Orally disintegrating tablets break apart rapidly upon contact with the saliva and may be used without water. The drug is dispersed in saliva and swallowed with little or no water. There is no industry standard for these modified-release dosing abbreviations and acronyms, and there may be multiple abbreviations for each indication (see Table 2). Therefore, confusion and misreading have caused prescribing and administration errors. This has been mitigated, to some extent, with the advent of electronic health records (EHRs) and electronic medication prescribing (e-scribe) programs (Frandsen & Pennington, 2020; Le, 2022a; Lilley et al., 2022; Vanderah, 2024).
Table 2
Common Medication Acronyms
Acronym | Meaning |
CD | controlled-delivery |
DR | delayed-release |
EC | enteric-coated |
ER | extended-release |
LA | long-acting |
ODT | orally disintegrating tablet |
SA | sustained-action |
SR | sustained-release |
TR | time-release |
XR | extended-release |
XT | extended-time |
(Frandsen & Pennington, 2020; Le, 2022a; Lilley et al., 2022; Vanderah, 2024)
Pharmacokinetics and Pharmacodynamics
Pharmacokinetics (what the body does to a drug) refers to the movement of a drug through the body and the series of biochemical processes it undergoes. Pharmacodynamics (what a drug does to the body) studies how drugs act at target sites of action in the body, as summarized in Figure 3 (Le, 2022e; Lilley et al., 2022; Vanderah, 2024).
Figure 3
Pharmacokinetics and Pharmacodynamics
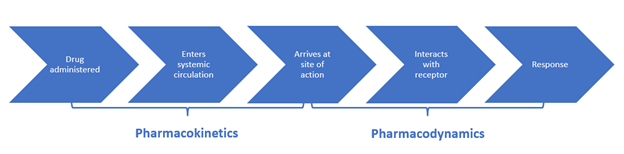
Pharmacokinetics
Pharmacokinetics refers to the movement of a drug into, through, and out of the body and includes the four primary stages that drugs undergo: absorption, distribution, metabolism, and excretion (abbreviated as ADME; see Figure 4). The pharmacokinetics of a drug determine the onset, duration, and intensity of a drug's effect and are also dependent on patient-related factors (genetic makeup, age, sex, renal function). For example, the half-life of drugs that require metabolism and excretion may be considerably longer for older adults. APRNs must understand pharmacokinetics to dose medications accurately. These stages will be outlined in this section (Le, 2022e; Lilley et al., 2022; Vanderah, 2024).
Figure 4
Pharmacokinetics
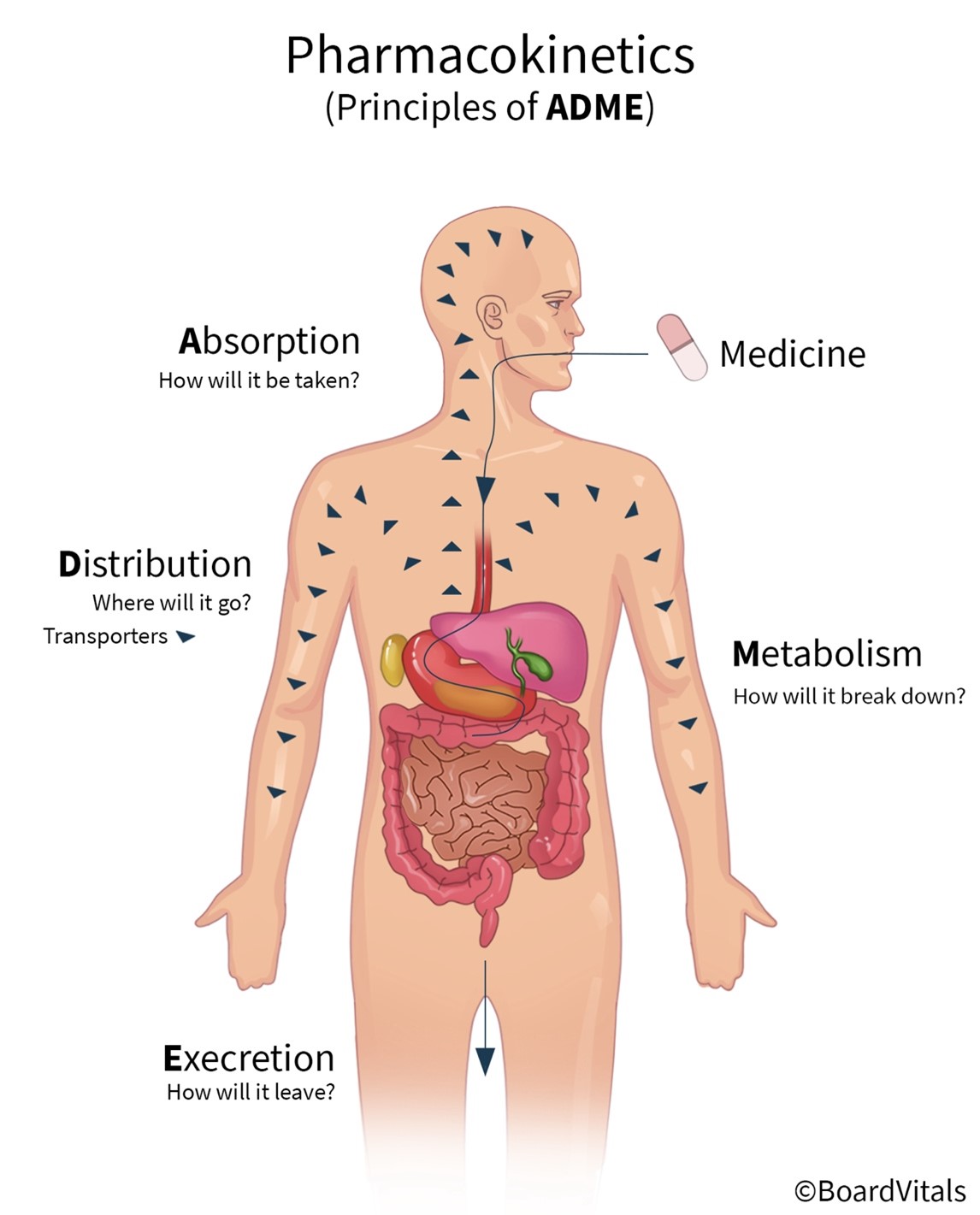
Absorption
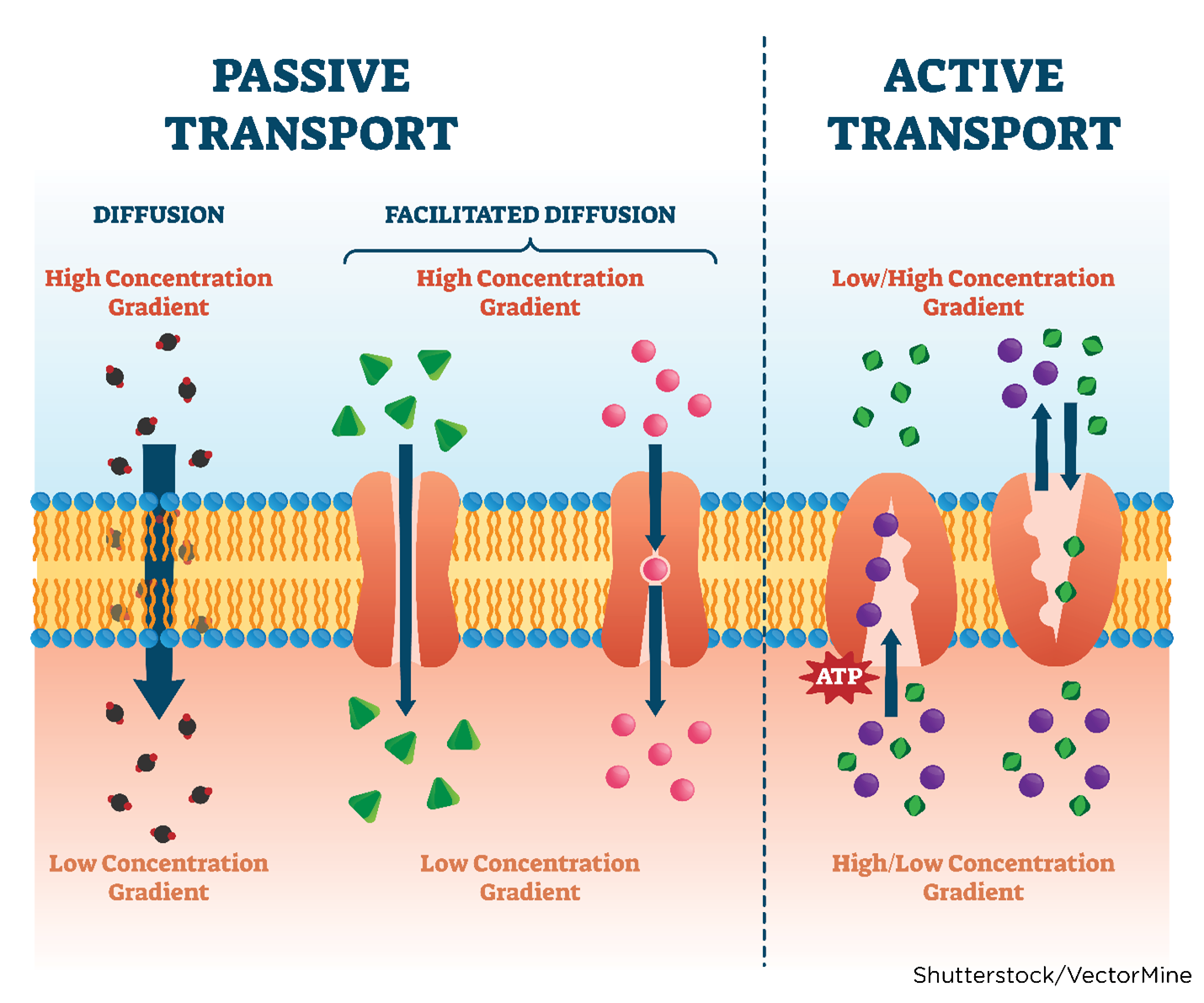
Absorption is the process that brings a drug from the administration (tablet, solution) into the systemic circulation of the body. For a drug to produce its intended effect(s), it needs to enter the body and reach its primary action site. Factors that determine drug absorption include its biochemical properties, formulation, and route of administration. While drugs are available in various forms, all drugs must be liquefied (dissolved into a solution) to be absorbed by the body. Thus, the body more rapidly absorbs liquid formulations than it does the same agent in pill (tablet, capsule) form, which needs to disintegrate and de-aggregate before absorption can occur. Unless administered intravenously, all drugs must cross several semipermeable cell membranes to reach systemic circulation and gain access to their target site. Cell membranes are biological barriers that selectively inhibit the passage of drug molecules. Drugs cross these barriers by passive diffusion, facilitated passive diffusion, active transport, or pinocytosis (see Table 3 and Figure 5; Le, 2022e; Vanderah, 2024).
Table 3
Mechanisms of Drug Transport
Mechanism | Description |
Passive diffusion | The drug moves across a concentration gradient (from an area of high concentration to low concentration, such as from the GI fluid to the blood). The diffusion rate depends on several factors, such as the molecule's size, lipid solubility, degree of ionization, and the area of the absorptive surface. Lipid-soluble drugs diffuse most rapidly due to the cell membrane's lipid composition. Smaller molecules diffuse faster than larger molecules. Most drugs are weak organic acids or bases and can be ionized or un-ionized. The un-ionized form is lipid-soluble and readily diffuses across cell membranes. Most absorption occurs in the small intestine because of the larger surface area and permeability (see Figure 5). |
Facilitated passive diffusion | Some molecules penetrate the membrane more rapidly than expected due to facilitated passive diffusion. Facilitated passive diffusion occurs when a carrier molecule in the cell membrane combines with the drug to form a carrier-substrate complex that can diffuse rapidly across the cell membrane. Since this process does not require energy expenditure, transport against a concentration gradient cannot occur. |
Active transport | The active transport process is selective and limited to drugs that are structurally similar to endogenous substances, such as ions, amino acids, and sugars. Energy expenditure is required and may involve transporting drugs against a concentration gradient. These drugs are usually absorbed from specific sites within the small intestine. |
Pinocytosis | Pinocytosis occurs when a fluid or particle is engulfed by a cell. As the cell membrane invaginates, it encloses the fluid or particle, forming a vesicle that later detaches and moves to the interior of the cell. Pinocytosis does require energy expenditure. |
(Le, 2022a)
Figure 5
Cellular Diffusion
Some agents have an enteric coating or a polymer applied to their exterior surface to delay dissolution until they reach the intestines. Without this coating, the acidic pH of the stomach would break down the chemical composition of a drug, rendering it ineffective. Enteric-coated pills should never be crushed because their action may be lost before leaving the stomach. There are various oral formulations of medications, each of which has variable absorption rates, as illustrated in Table 4 (Lilley et al., 2022; Vanderah, 2024).
Table 4
Rate of Absorption of Oral Medication
Drug form | Rate of absorption |
Liquids, syrups, and elixirs | Fastest
Slowest |
Suspension solutions | |
Powders | |
Capsules | |
Tablets | |
Coated tablets | |
Enteric-coated tablets |
The medication administration route also has a strong influence over the absorption process as follows (Kim & De Jesus, 2023; Lilley et al., 2022; Smith & Pacitti, 2020; Vanderah, 2024):
- Oral drugs (swallowed pills) must pass through a layer of epithelial cells that line the GI tract. The absorptive pattern of drugs administered orally varies due to the solubility and stability of the drug, the pH of the GI tract and its motility, the presence of food in the stomach or intestines, concomitant drugs or supplements, and the drug's formulation. The primary site of absorption is the small intestine, and the bioavailability is influenced by the amount of drug absorbed across the intestinal epithelium. Orally administered drugs are subject to the first-pass effect, where the concentration of the drug is significantly diminished before it reaches systemic circulation (see table above).
- Sublingual and buccal drugs are placed under the tongue (sublingual) or between the gums and the cheek (buccal) and are rapidly absorbed before swallowing. This route prevents the gastric pH from inactivating the drug and optimizes absorption through the highly vascular mucous membranes. Sublingual tissue is highly permeable and has a faster drug absorption than buccal tissue.
- Rectal and vaginal drugs (inserted rectal or vaginal suppositories) are easily absorbed and can precipitate both local and systemic effects. However, the presence of stool in the rectum or discharge in the vagina can limit tissue contact with the medication and hinder absorption. Many vaginal suppositories (such as an estradiol [Vagifem] tablet) are primarily used for their local effects on the vaginal mucosa and limited systemic absorption.
- Inhaled drugs (medications breathed from an inhaler or nebulizer) through the mouth or nose are rapidly absorbed through capillary networks in the nose or lung alveoli.
- Transdermal drugs (patches applied to intact skin) allow for slow, gradual absorption. The effects of transdermal drugs can be local (such as a lidocaine [Lidoderm] patch) or systemic (such as a fentanyl [Duragesic] patch).
- SC (injected into fat tissue) and IM (injected into a muscle) drugs may be absorbed quickly or slowly. For example, water-soluble drugs (such as insulin) are absorbed rapidly, whereas less water-soluble agents (such as leuprolide acetate [Lupron]) have a slower absorption rate. In general, drugs given IM are absorbed relatively quickly due to the high vascularity of muscles; however, patients with poor peripheral perfusion may experience delayed absorption. Subcutaneous tissue has few blood vessels and has a slow, sustained absorption rate.
- IV drugs (infused through a venous access device) are absorbed the most rapidly and entirely of all routes. This rapid absorption is because IV drugs are transmitted directly into the bloodstream, do not need to be broken down, and therefore reach tissues in their original chemical form.
Distribution
Distribution is the transportation of drugs to sites of action by bodily fluids. Once a drug enters systemic circulation, it needs to be dispersed into interstitial and intracellular fluids to reach its target. Drugs are primarily designed to bind to a receptor site to induce or thwart a specific action predictably. However, secondary side effects occur when the drug binds to other sites in addition to the target. Due to their high vascularity and extensive blood supply, the heart, liver, kidneys, and brain are the most common organs exposed to the drug first. Many drugs are considered potentially hepatotoxic (can cause liver injury) or nephrotoxic (can cause kidney injury) because they pass through these organs in higher concentrations, increasing their potential to cause damage. Drug distribution is generally uneven due to various factors, such as blood perfusion, permeability of cell membranes, regional pH, and tissue binding (lipid content; Le, 2022b; Lilley et al., 2022; Smith & Pacitti, 2020; Vanderah, 2024).
- Circulation can either enhance or inhibit the perfusion of drugs throughout the body. Health alterations such as cardiovascular disease or peripheral vascular disease can cause poor circulation and delay medication distribution. Hydration status (dehydration and overhydration) can impact circulation and affect the drug's ability to perfuse in the intended manner.
- The permeability of cell membranes is another factor that affects drug perfusion throughout the body. As described earlier, drugs must pass through several cell membranes before they reach their target tissue. Oral drugs must pass through cell membranes in the GI tract and capillaries to enter the circulation and then leave the circulation to bind with receptors on target cells. They subsequently return to circulation and pass through the liver. A certain amount of the drug is metabolized by liver enzymes (first-pass effect) and re-enters circulation before being excreted by the body.
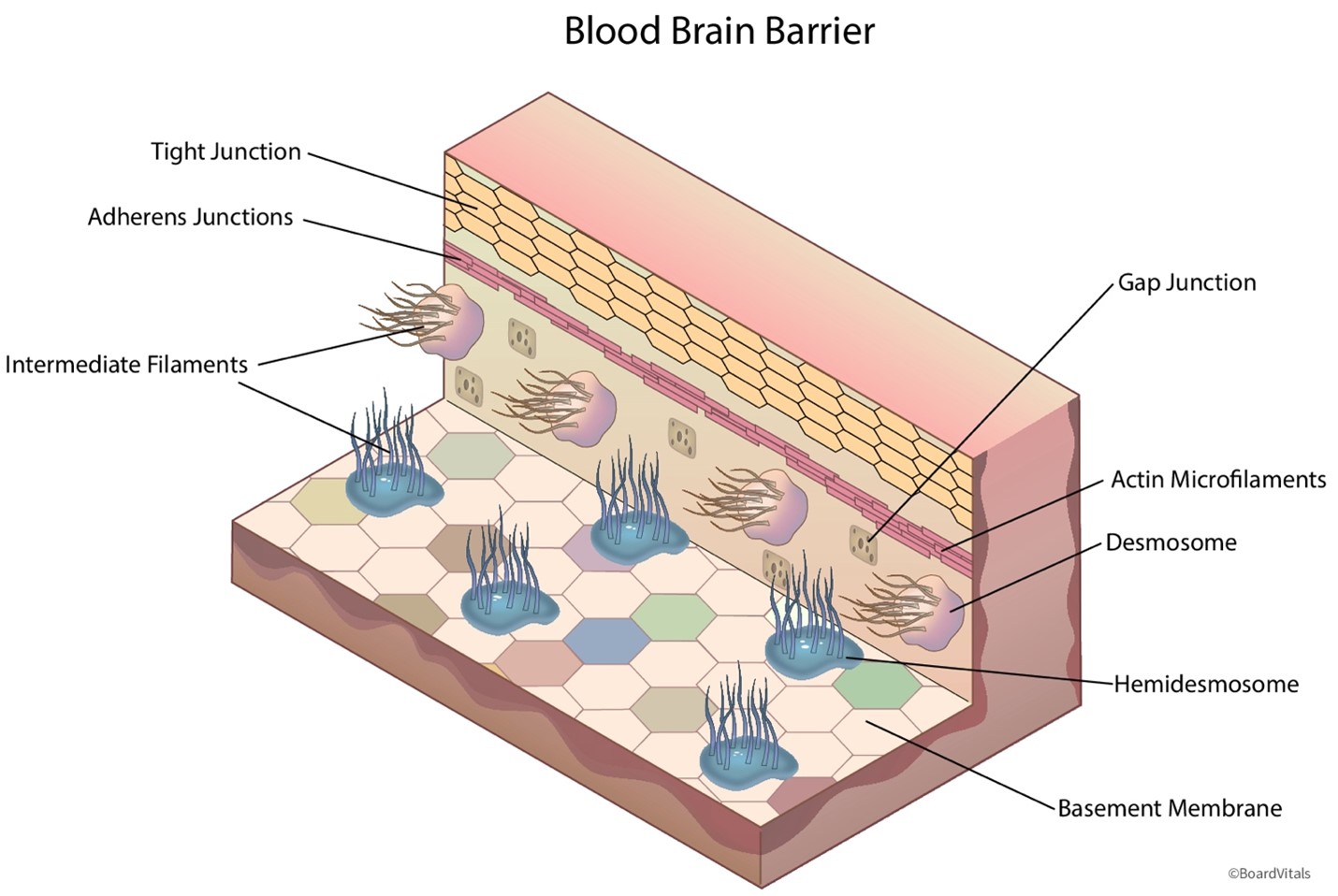
- The blood-brain barrier and the placental barrier protect the brain and the developing fetus, respectively, from potentially dangerous substances, such as poisons or viruses. As demonstrated in Figure 6, the blood-brain barrier is a blockade of tightly interwoven capillaries that prevent the passage of most drugs. Only certain medications comprised of lipids or attached to a carrier molecule can pass through this barrier. The placental barrier is more permeable than the blood-brain barrier. While some drugs can harm a fetus, many have not been studied in pregnant patients. The blood-brain barrier becomes less effective with aging, allowing increased passage of medications into the brain. Drugs can also enter the ventricular cerebrospinal fluid directly through the choroid plexus and then passively diffuse into the brain tissue. Drug penetration into cerebrospinal fluid is determined by the extent of protein binding, lipid-water partition coefficient of the drug, and degree of ionization.
- Plasma protein-binding sites affect the distribution of drugs within the bloodstream. A certain percentage of most drugs are bound to plasma proteins upon entering the bloodstream, forming a drug-protein complex. Their distribution is affected by how much of the drug is given, the plasma protein level in the blood, and the drug's transport to target tissues. The drug-protein complex is usually too large to pass through the capillaries into tissues; only unbound or "free" amounts of the drug are pharmacologically active and can exert a therapeutic effect. The portion of the drug that is "protein-bound" remains inactive while bound, whereas the part that escapes the initial protein binding is immediately "free" to bind to the target tissue. Patients with diminished protein stores, such as those who are malnourished or have underlying liver disease, are at higher risk for drug toxicity. Diminished protein stores increase the amount of free drug; thus, these patients typically require lower drug dosages to prevent toxicity.
- In addition to the examples cited above, several disease states can alter drug distribution or cause drug-disease interactions:
- Obesity allows for greater accumulation of lipid-soluble agents within the patient's adipose tissue, increasing their distribution and extending their half-life.
- Pregnancy increases the patient's intravascular volume.
- Edema or an edematous state (heart failure, cirrhosis, nephrotic syndrome) prolongs distribution, increases half-life, and delays drug clearance.
Figure 6
Blood-Brain Barrier
Metabolism
Metabolism is the chemical alteration of a drug by the body. Once a drug has been absorbed and distributed, the metabolism (breakdown) of the drug molecule ensues. Through enzyme activity and chemical reactions, drugs are dissolved into their less-active or inactive forms called metabolites. Metabolism occurs primarily in the liver through the action of enzymes, but limited metabolism can also occur in the kidneys, lungs, intestines, and blood. Cytochrome P450 (CYP450) enzymes are primarily capable of and responsible for metabolizing most drugs, toxins, and normal cellular components. They also play a chief role in synthesizing essential endogenous substances in the body, such as hormones, steroids, and fatty acids. Most drugs are lipid-soluble, and the kidneys can only excrete water-soluble substances. Therefore, the liver must convert lipid-soluble drugs to water-soluble substances as they pass through the liver to allow easier excretion via the urine. Several factors affecting drug metabolism are outlined below (Carpenter et al., 2019; Le, 2022d; Lilley et al., 2022; Smith & Pacitti, 2020; Vanderah, 2024):
- Age is an important consideration that can widely affect a drug's metabolism. Infants have a limited capacity to metabolize medications because their liver is still developing, and enzymes are not fully generated. Metabolism also tends to decline with age, and older adults frequently require a dosage reduction to avoid hepatic toxicity. The liver's capacity for metabolism through the CYP450 enzyme system is reduced by more than 30% in older adults due to decreased hepatic volume and blood flow.
- Increased medication-metabolizing enzymes can cause a drug to be metabolized faster than anticipated. This phenomenon can result from receiving the same drug over an extended period. It may warrant an increase in dosage to maintain a therapeutic level or a change in drug therapy. An increase in medication-metabolizing enzymes can also elevate the metabolism of other drugs being administered concurrently.
- The first-pass effect (or first-pass hepatic metabolism) is a common phenomenon with oral drugs. As the liver enzymes break down a drug, some of it escapes into the general circulation and becomes protein-bound or free. Several doses are typically required before enough free drug stays active in the circulation to exert its desired effect. Examples of drugs that undergo a significant first-pass effect include morphine sulfate (MS Contin), propranolol (Inderal), diazepam (Valium), and midazolam (Versed). Drugs inactivated by the liver need to be given by a route not involving the GI tract (such as a parenteral route). There is wide variability regarding the extent to which each patient experiences the first-pass effect, making it an unpredictable phenomenon. For drugs that undergo considerable first-pass metabolism, monitoring the blood levels of these drugs is the most viable way to maintain a therapeutic concentration.
- Similar metabolic pathways can alter the metabolism of two drugs administered concurrently. The rate of metabolism can decrease for either or both drugs, leading to toxicity. An example of this is the commonly used anticoagulant warfarin (Coumadin) and two widely utilized cholesterol-lowering medications known as statins (rosuvastatin [Crestor] and simvastatin [Zocor]). Rosuvastatin (Crestor) and simvastatin (Zocor) inhibit warfarin (Coumadin) metabolism by a specific metabolic pathway (such as CYP2C9), generating increased concentrations of warfarin (Coumadin) and heightening bleeding risk.
- Nutritional status also impacts drug metabolism. Since nutritional status alters drug distribution and plasma protein-binding sites, malnourished patients with limited protein and fat stores have an increased risk of drug toxicity due to the reduced availability of metabolizing enzymes.
Excretion
Excretion is the process by which the body eliminates waste (including drug metabolites). As the kidneys filter the blood, they screen the excess free metabolites; a portion is reabsorbed into the bloodstream, and the remainder is excreted through the urine. Renal excretion is supported by glomerular filtration, active tubular reabsorption, and active tubular secretion. The free or unbound water-soluble form of a drug or metabolite enters the kidney through passive glomerular filtration. The vasculature surrounding the nephron may also help transport metabolites into the nephron via tubular secretion. Active reabsorption can pull some of a drug back into circulation and redistribute it throughout the body.
Kidney dysfunction can impair the body's ability to eliminate a medication adequately, leading to increased circulating levels and consequential toxicity. Kidney function is most commonly measured through serum laboratory values (creatinine [Cr], estimated glomerular filtration rate [eGFR], blood urea nitrogen [BUN], and creatinine clearance [CrCl]). The eGFR is the best indicator of kidney function and is often used as a surrogate for CrCl when dosing medications in clinical practice. These levels need to be monitored closely for patients with underlying kidney dysfunction, acute kidney injury, or chronic renal disease when prescribing medications. While the primary site of excretion is through the kidneys, this process can also occur via the liver (excretion of byproducts and waste into bile), lungs (exhalation of gases and alcohol), intestines (feces), and exocrine glands (sweat). Biliary excretion permits the excretion of a drug through the GI tract via feces (Le, 2022c; Lilley et al., 2022; Smith & Pacitti, 2020; Vanderah, 2024).
Core Elements of Drug Dosing
Dosing considerations are essential to understanding the effect drugs can have on a patient. The APRN must pay close attention to the anticipated effect, patient response, and safe dose range for each agent when prescribing medications. The APRN must be aware of the overall dose-response based on the prescribed dosage. As the dose of the drug increases, the response should increase, as well as the potential for toxicity. The dose-response helps the provider determine the effective or therapeutic dose of the drug. Some medications can have a very small therapeutic window, which increases the risk of toxicity. Similarly, if a medication has a short half-life, it will be eliminated from the body quickly, resulting in a shorter therapeutic effect. These medications will likely require repeated doses in order to achieve a steady state (the point where the amount of drug entering the body is equal to the amount of drug being eliminated) and sustained therapeutic effect (Ernstmeyer & Christman, 2023; Longo, 2019; Vanderah, 2024).
Every drug has an onset, peak, and duration of efficacy. The onset of action refers to when the medication begins to take effect. It is influenced by the route of administration, functional capacity of the GI tract, and patency of the circulatory system. For example, a medication administered intravenously will take effect much more quickly than a medication administered orally, which will be delayed due to the first-pass effect. The peak refers to the maximum concentration (Cmax), typically where the patient demonstrates the most significant therapeutic effect. The duration of efficacy refers to the length of time the medication yields its desired therapeutic effect. Every drug has a minimally effective dose (minimal effective concentration [MEC]) and a minimally toxic dose (minimal toxic concentration [MTC]). The therapeutic window lies between the MEC and the MTC and represents the range of the safest and most effective treatment or the ideal plasma drug concentration (Cp, see Figure 7). Since it is not feasible to determine the amount of a drug that reaches its target site of action after administration, the Cp is measured. An example of this concept is warfarin (Coumadin), commonly prescribed to prevent blood clotting. Optimal warfarin (Coumadin) drug levels are monitored with a blood test called the international normalized ratio (INR). If the dose of warfarin (Coumadin) is too high, the INR increases above the therapeutic window, heightening the risk of bleeding (a toxic drug effect). In contrast, if the dose of warfarin (Coumadin) is too low, the INR level declines below the therapeutic window, reducing the drug's efficacy and increasing the risk of blood clotting. Therefore, APRNs must monitor the INR levels of patients receiving warfarin (Coumadin) to ensure the dosage appropriately reaches the therapeutic window without increasing the risk of harm. Patients on warfarin (Coumadin) often require periodic dosage adjustments based on their INR level (Ernstmeyer & Christman, 2023; Longo, 2019; Vanderah, 2024).
Figure 7
Principles of Pharmacokinetics
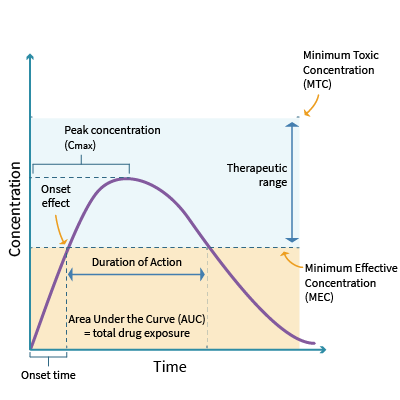
Unless a drug is administered by continuous infusion, variations in concentration will depend on its dosing frequency. The drug's half-life and the body's ability to metabolize or excrete the drug determines the trough concentration. Therapeutic drug monitoring with peak and trough levels is performed to adjust drug doses based on the Cp at defined intervals. The goal is to prevent and manage an undesirable overdose or under-dose. If the peak level is too high, the patient is at risk of drug toxicity. If the peak level is too low, the patient is at risk of receiving a non-therapeutic dose of the drug, mitigating its intended effect. The trough level should be collected just before the next dose is administered. For example, gentamycin (Garamycin), an aminoglycoside antibiotic, must maintain plasma concentration at a steady state (Cpss) to treat the underlying infection for which it has been prescribed. However, gentamycin (Garamycin) is also highly nephrotoxic and requires diligent monitoring of the peak level and renal function tests to ensure the dose is not reaching toxic levels. The prescriber is responsible for ensuring that peak and trough levels are monitored when indicated (Lilley et al., 2022; Vanderah, 2024).
The body's rate of excretion influences a medication's duration of action. The area under the curve (AUC) represents the total drug exposure over time and is an essential parameter for both pharmacokinetic and pharmacodynamic analyses. It is based on the body's elimination rate and the dose administered and reflects the optimal concentration of a specific drug in the body. The higher the AUC for a given dose, the lower the drug clearance. The AUC is clinically useful for monitoring drugs with a narrow therapeutic index, such as gentamycin (Garamycin) and phenytoin (Dilantin). Clinical trials can indicate whether two drug formulations of the same dosage (a 10-mg capsule and a 10-mg tablet, for example) result in equal tissue or plasma exposure (Lilley et al., 2022; Vanderah, 2024).
APRNs must be knowledgeable of the distinctions in medication dosing. A single dose is the recommended amount of a drug given at a single time (for example, clarithromycin [Biaxin] ER may be dosed at 1,000 mg daily for certain low-risk adults with pneumonia). A course dose, or divided dose, is the recommended amount of a drug given over a defined period (pediatric pneumonia patients may be treated with amoxicillin [Amoxil] 90 mg/kg/day dosed twice daily or 45 mg/kg/day dosed three times daily, for example). Specific high-risk drugs such as antineoplastic therapies (chemotherapy or radiation therapy) have cumulative dose restrictions. A cumulative dose refers to the total dose of the drug after repeated exposure to treatment, adding up each time the patient has received it. Some drug toxicities are more severe or more likely to occur as the cumulative dose of the drug increases. For example, the cumulative dose for doxorubicin (Adriamycin), a potent chemotherapy agent, is 550 mg/m² (or 450 mg/m² if the patient received prior radiation therapy to the chest) due to the heightened risk of cardiotoxicity. Tracking the cumulative dose of these agents is critical for patient health and safety. While some EHRs have features that automatically calculate and document the dosing of drugs, others lack such features and require APRNs to devise a metric for tracking and logging these doses (Olsen et al., 2019).
Drug Clearance and Half-Life
Drug clearance refers to the overall process of eliminating drugs from the body. Drug clearance is among the most important pharmacokinetic parameters because it determines the maintenance dose rate (dose per unit of time) required to maintain the Cp of the drug. The half-life is when the drug has lost half its Cmax; this decline reflects how quickly and efficiently the drug is metabolized and excreted from the body. A drug's half-life is analogous to the concept of CrCl, which measures Cr (a waste product produced by muscles from the breakdown of a compound called creatinine) levels in the blood. The amount of Cr produced in the body depends on muscle mass and is relatively constant for each patient. The amount of Cr removed from the blood relies on the filtering capacity and functioning of the kidneys and the rate at which blood is carried to the kidneys. The same principle applies to drug clearance; however, unlike CrCl, the clearance of a drug is rarely measured directly. Instead, drug clearance is calculated as the volume of plasma that gets filtered of the drug per unit of time (volume/time). Typically, four to five half-lives are needed for the Cp to reduce to below 10% of the starting value, so drugs are considered excreted from the body when approximately five half-lives have occurred. If an additional dose of the drug is administered every half-life, 50% of the peak plasma is eliminated each half-life. Therefore, Cpss is achieved after four to five half-lives, regardless of whether the drug was given by constant IV infusion or repeated intermittent doses.
Drugs with a short half-life typically need to be administered several times per day, whereas drugs with a long half-life may only require once-daily administration. Since most drugs are metabolized in the liver and excreted by the kidneys, a decrease in the functioning of either of these organ systems can increase the half-life of a drug. Thus, patients with liver or kidney dysfunction may experience the toxic effects of drugs more easily. Many drugs require periodic lab monitoring of a patient's liver (liver function tests such as alanine transaminase, aspartate transaminase, and alkaline phosphatase) and kidney function (BUN and Cr; Lilley et al., 2022; Vanderah, 2024).
Pharmacodynamics
Pharmacodynamics refers to the mechanisms and effects of medication on a person's body. Pharmacodynamics studies the relationship between the drug's concentration at the target site and its post-receptor effects, including therapeutic and adverse drug effects and interactions. For most drugs, the concentration at the receptor site determines the intensity of the drug's effect. A drug's pharmacodynamics can be affected by physiologic changes associated with other drugs, aging, and various diseases or disorders (malnutrition, myasthenia gravis, genetic mutations, Parkinson's disease, thyrotoxicosis, and some forms of insulin-resistant diabetes mellitus).
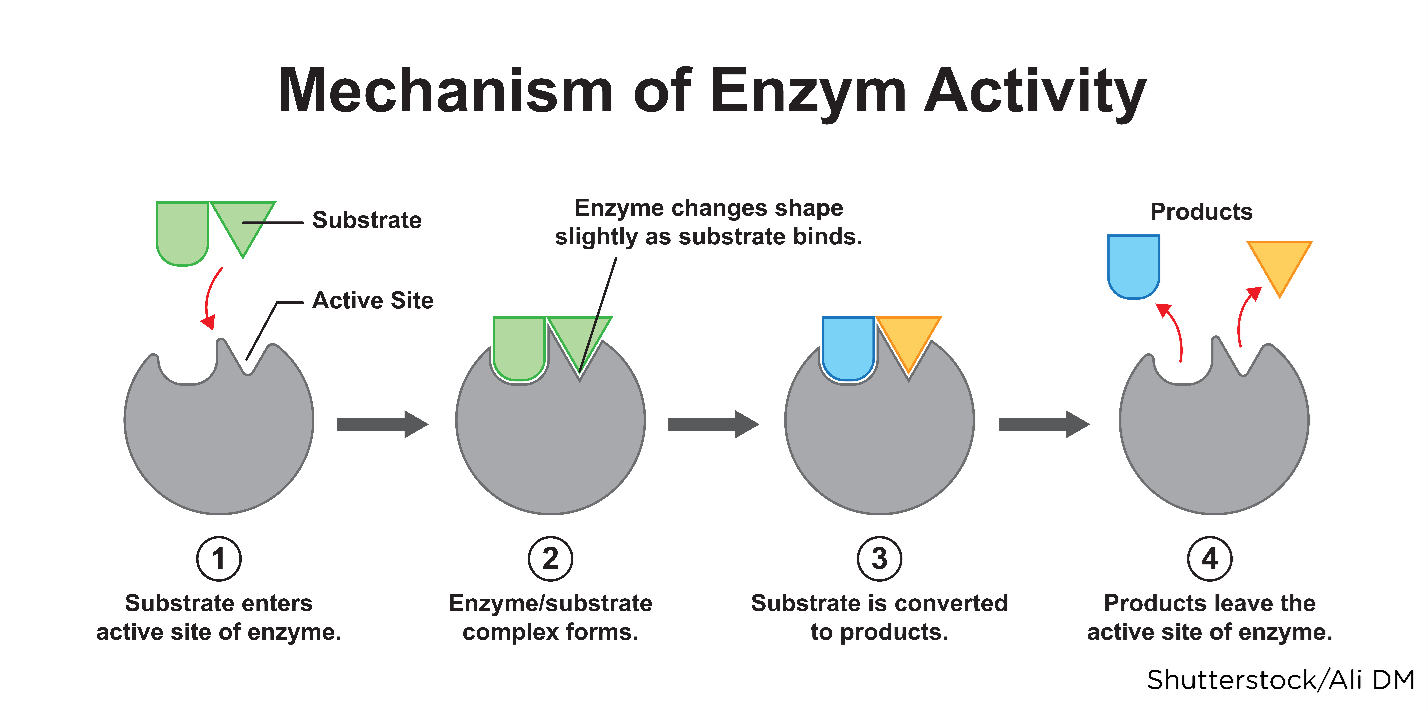
Understanding a drug's mechanism of action (how it functions within a person's body) is vital to comprehend the processes drugs endure to produce their intended effects. After a drug enters systemic circulation, it comes in contact with the cells of nearly all of the body's organs and tissues. The drug has to reach and bind to its target site (receptor) to produce an effect. Receptors are specialized proteins found inside or on a cell membrane located on various tissues (cardiac muscle, neurons in the CNS, GI tract). The joining or binding of a drug with a cell is called the drug-receptor interaction, creating a chemical bond between the receptor and the active site on the drug molecule (or substrate). When drugs bind to the receptor on a cell, they can alter its shape or activity, thereby changing its normal behavior. This relationship is often described as a "lock-and-key" model in which the substrate is the key that fits into the lock (the receptor), causing it to open (or activate) as demonstrated in Figure 8 (Farinde, 2023a, 2023b; Lilley et al., 2022; Vanderah, 2024).
Figure 8
Substrate Binding to a Receptor
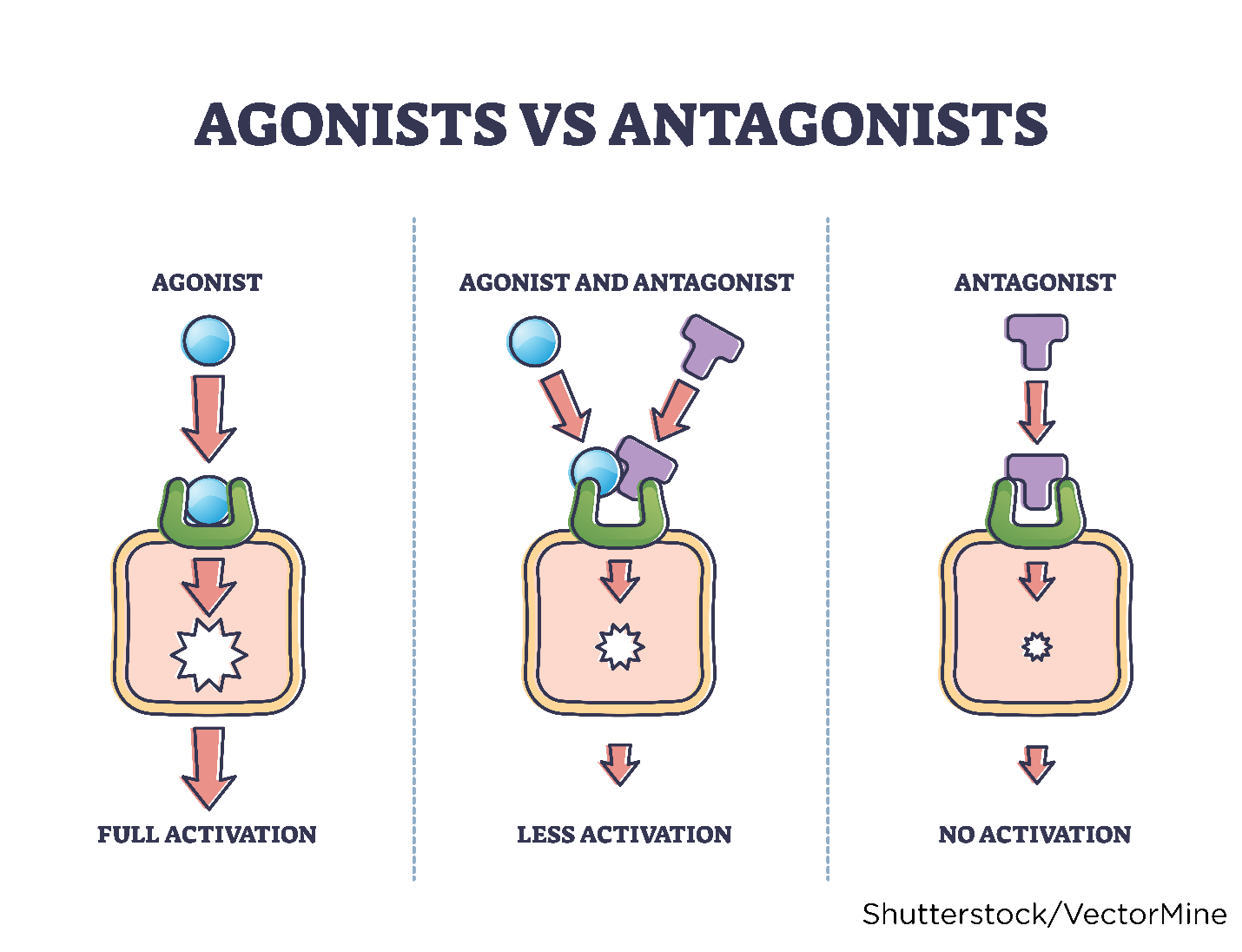
A receptor is impacted by altering the cellular function, cellular environment, or enzymatic action. Drugs are classified into two categories (agonists or antagonists) based on their functional impact on the receptor. Drugs given to enhance a physiologic response are called agonists. A drug agonist binds tightly to a receptor, activating it to produce the desired effect. Morphine sulfate (MS Contin) is an example of an agonist because it binds with receptors to generate the desired effect of analgesia (pain reduction). Drugs given to block or lessen a typical response are called antagonists. Antagonists compete with other molecules to block a specific action at a receptor site and decrease the receptor's ability to become activated by another agonist. Ranitidine (Zantac), a histamine-2 antagonist (H2 blocker), is an example of an antagonist. Ranitidine (Zantac) attaches to the H2 receptor on the parietal cells in the gastric mucosa to prevent the release of histamine-induced gastric acid (to treat or prevent heartburn or gastric ulcers). Figure 9 depicts drug agonists and antagonists (Farinde, 2023a; Lilley et al., 2022; Vanderah, 2024).
Figure 9
Drug Agonist vs. Antagonist
Alterations in the cellular environment can also occur when a drug changes the structure of a cell, such as modifying the cell wall or revising a critical process, such as replication. For example, penicillin-type antibiotics (cephalosporins, monobactams, β-lactams, carbapenems) inhibit the cell wall synthesis of certain bacteria to destroy them. Sulfa-type antibiotics (sulfonamides and trimethoprim [Primsol]) inhibit bacterial replication by preventing folic acid synthesis, thereby disrupting DNA and RNA replication. Through a process called selective interaction, a drug can change a target molecule's normal response by inhibiting or enhancing the action of an enzyme that affects the target molecule. For example, angiotensin-converting enzyme (ACE) inhibitors (lisinopril [Zestril], enalapril [Vasotec], benazepril [Lotensin]) block the activity of ACE, which is required to create the hormone angiotensin II. By blocking ACE, these medications decrease the production of angiotensin II, cause vasodilation, and reduce blood pressure, making it easier for the heart to pump blood to the rest of the body (Lilley et al., 2022; Vanderah, 2024).
Interactions
Drug-drug and drug-food interactions can dramatically change the action of a drug in a patient's body. Precautions should be taken to limit or restrict certain types of food or the concurrent administration of interacting drugs. Interactions may increase or decrease the therapeutic effect of drugs, produce a new effect, or increase incidences of adverse effects. The simultaneous administration of two or more drugs can result in impaired excretion of the more slowly metabolized agent, prolonging or potentiating its effects. For example, when trimethoprim-sulfamethoxazole (TMP-SMX; Bactrim) is administered with warfarin (Coumadin), the antibiotic interferes with the metabolism of warfarin (Coumadin), increasing levels in the blood and heightening bleeding risk. Likewise, simvastatin (Zocor) should not be administered with antifungal agents such as fluconazole (Diflucan), as the combination can lead to drug-induced hepatitis (inflammation of the liver) and rhabdomyolysis (the breakdown of skeletal muscles). Medications that are potent inhibitors of CYP450 enzymes (macrolide antibiotics and antifungals) are responsible for numerous drug-drug interactions. CYP450 inhibitors slow the metabolism and clearance of many drugs, thereby increasing drug concentration and heightening the risk of adverse effects, including drug overdose. See Table 5 for examples of drugs that interact with specific enzymes and drug-drug interactions (Carpenter et al., 2019; Lilley et al., 2022; Vanderah, 2024).
Table 5
Drugs That Interact with CYP450 Enzymes and Drug-Drug Interactions
Enzymes | Substrates | Inhibitors | Inducers |
CYP1A2 |
|
|
|
CYP2C9 |
|
|
|
CYP2C19 |
|
|
|
CYP2D6 |
|
|
|
CYP2E1 |
|
|
|
CYP3A4 |
|
|
|
(Le, 2022d)
There are four primary mechanisms of drug-drug interactions: additivity, synergism, potentiation, and antagonism. Additivity, or an additive effect, occurs when the combined influence of two drugs is the sum of the expected individual responses (for example, acetaminophen [Tylenol] combined with codeine sulfate [Codeine] can provide superior analgesia). An additive effect typically occurs when drugs work together positively. Still, this relationship can also induce unintentional harm, such as when ibuprofen (Motrin) is combined with acetylsalicylic acid (Aspirin). Since each agent can cause GI bleeding and gastric ulcers individually, this risk is significantly increased when they are taken together. In addition, a patient who ingests different CNS depressants, such as alcohol and opioids, can experience the combined effects of CNS depression (fatigue, sedation, impaired cognition, slowed reflexes, respiratory depression), which can be fatal (Carpenter et al., 2019; Lilley et al., 2022; Vanderah, 2024).
Another example of a harmful drug-drug interaction is the risk of serotonin syndrome with various antidepressant agents prescribed for major depressive disorder, obsessive-compulsive disorder, panic disorder, posttraumatic stress disorder, and social anxiety disorder. Serotonin syndrome results from the concurrent administration of SSRIs (citalopram [Celexa], escitalopram [Lexapro], fluoxetine [Prozac], paroxetine [Paxil], sertraline [Zoloft]), serotonin-norepinephrine reuptake inhibitors (SNRIs; duloxetine [Cymbalta], venlafaxine [Effexor], desvenlafaxine [Pristiq]), monoamine oxidase inhibitor (MAOIs; tranylcypromine [Parnate], phenelzine [Nardil], isocarboxazid [Marplan]), or any other drug that enhances serotonin neurotransmission. Serotonin syndrome is characterized by agitation, anxiety, confusion, high fever, sweating, tremors, lack of coordination, dangerous fluctuations in blood pressure, and rapid heart rate. It is a potentially life-threatening condition for which patients must seek immediate medical attention, as it can progress to delirium and coma.
MAOIs were the first type of antidepressant medications developed. They impair the metabolism of serotonin and block monoamine oxidase, an enzyme that breaks down excess tyramine in the body. Tyramine is an amino acid that helps regulate blood pressure, and it occurs naturally in the body and in certain foods. Due to the risk of serious adverse effects, the use of MAOIs for the treatment of depression is generally reserved for patients for whom all other options have failed. MAOIs have dangerous drug and food interactions. In particular, patients should be advised to avoid foods containing high levels of tyramine, such as aged cheese (aged cheddar, Swiss, parmesan, and blue cheeses); cured, smoked, or processed meats (pepperoni, salami, hot dogs, bologna, bacon, corned beef, smoked fish); pickled or fermented foods (sauerkraut, kimchi, tofu); broths and sauces (soy sauce, miso, and teriyaki); soybean products; and alcoholic beverages (beer, red wine, liquors; Carpenter et al., 2019; Lilley et al., 2022; Sub Laban & Saadabadi, 2023; Vanderah, 2024).
A synergistic effect occurs when a drug is enhanced when administered alongside another drug. The most-described example of a beneficial synergistic effect between drugs is the use of combined antibiotic therapy with an aminoglycoside (such as gentamicin [Gentacin]) and penicillin (penicillin G benzathine [Bicillin L-A]). Since penicillin is bactericidal, it destroys the bacterial cell wall, facilitating the intracellular uptake and transport of aminoglycosides into the cell, thereby enhancing the bactericidal effect. Without penicillin, there is little intracellular uptake of the aminoglycoside and, therefore, a reduced response. To differentiate between additive and synergistic drug effects, consider simple mathematic equations where additive effects are represented as 2+2=4, and synergistic effects are 2+2=10 (Carpenter et al., 2019; Lilley et al., 2022; Vanderah, 2024).
Antagonism, or an antagonistic effect, denotes the interaction of two or more drugs in which one agent lessens the action of the other, decreasing or blocking its effect. For example, administering an antacid such as famotidine (Pepcid) with ciprofloxacin (Cipro) reduces the absorption of the antibiotic, thereby diminishing its efficacy in treating an underlying infection. The use of opioids and naloxone (Narcan) is another example; naloxone (Narcan) is administered to reverse the effects of opioids during an acute overdose (Carpenter et al., 2019; Lilley et al., 2022; Vanderah, 2024).
Potentiation, or a potentiated effect, occurs when two unrelated drugs are combined, resulting in the increased effect of only a single drug. For example, the simultaneous use of hydroxyzine (Vistaril) and morphine sulfate (MS Contin) increases the analgesic effect of morphine sulfate (MS Contin) but does not impact the therapeutic effect of the hydroxyzine (Vistaril). Similarly, adding fluoxetine (Prozac) to lisinopril (Zestril) can decrease the patient's blood pressure after a steady state of fluoxetine (Prozac) is attained but does not further enhance the antidepressant effects (Carpenter et al., 2019; Lilley et al., 2022; Vanderah, 2024).
Harmful drug interactions can also ensue when patients combine dietary supplements or OTC agents with prescribed drugs. Omega-3 fatty acid (fish oil, algae oil) is a dietary supplement used to prevent cardiovascular disease and reduce inflammation. Omega-3 fatty acids (fish oil, algae oil) can interact with anticoagulants such as warfarin (Coumadin), increasing bleeding risk, and antihypertensive medications, causing severe hypotension.
Hypericum perforatum (St. John's wort) is a supplement with chemical properties similar to SSRIs and should not be combined with SSRIs, SNRIs, or any serotonin-modulating agent. Hypericum perforatum (St. John's wort) can expedite or diminish the prescribed agent's metabolism, leading to reduced efficacy or higher toxicity. It is notorious for interacting with several other prescription drugs and can decrease the efficacy of variable medications, including cyclosporine (Neoral), digoxin (Lanoxin), oral contraceptives, and warfarin (Coumadin; NCCIH, 2021). Antacids and calcium supplements interfere with the absorption of thyroid hormone-replacement medications such as levothyroxine (Synthroid), making them less effective (Carpenter et al., 2019; Frandsen & Pennington, 2020; Lilley et al., 2022; Vanderah, 2024).
Drug-food interactions occur when a drug is given with food that reduces its absorption or increases its toxicity. Among several well-cited drug-food interactions, the most common is the interaction between grapefruit (or grapefruit juice) and statins (atorvastatin [Lipitor], lovastatin [Mevacor], rosuvastatin [Crestor], simvastatin [Zocor]). Grapefruit contains a chemical that interferes with the body's ability to metabolize these medications and can significantly increase the blood levels of statins, leading to greater risks of hepatitis, liver failure, and rhabdomyolysis. Ingesting fruit or fruit juice within 2 hours of taking fexofenadine (Allegra), an OTC antihistamine, can inhibit its absorption and impair its ability to block histamine release. The dietary intake of dark green leafy vegetables, beef liver, and soybean-containing foods should remain consistent for patients taking warfarin (Coumadin). These foods contain high amounts of vitamin K, which diminishes the blood-thinning properties of warfarin (Coumadin), thereby lessening its therapeutic effects (Carpenter et al., 2019; Frandsen et al., 2020; Lilley et al., 2022; Vanderah, 2024).
Side Effects, Adverse Effects, and Toxicity
Side effects, adverse effects, cumulative effects, and drug toxicity are undesirable results of medication administration. Every drug carries a risk of side effects due to its activity in the body. For example, the most common side effects of diphenhydramine (Benadryl) include dry mouth and drowsiness, whereas the most common side effects of the antiemetic agent ondansetron (Zofran) include constipation and headaches. APRNs are responsible for ensuring patients are adequately educated on the potential side effects of prescribed drugs and their management. For example, a patient experiencing dry mouth from diphenhydramine (Benadryl) may benefit from mitigation strategies, such as sucking on sugar-free candy to stimulate saliva production or using a saliva substitute. A patient experiencing constipation from ondansetron (Zofran) should be counseled on preventing and treating constipation by increasing dietary fiber, ensuring adequate oral hydration, exercising, and using a stool softener and/or laxative as needed.
Adverse effects are unexpected, are more severe than side effects, and can occur at standard therapeutic dosages. All drugs produce some type of adverse effect, which can be mild to life-threatening in some populations. Adverse effects of diphenhydramine (Benadryl) in older adults can include confusion, lack of coordination, and dizziness. Adverse effects of ondansetron (Zofran) can consist of ECG changes such as QT-interval prolongation (Frandsen & Pennington, 2020; Lilley et al., 2022; Vanderah, 2024).
Cumulative effects occur from the repeated administration of a drug, becoming more pronounced than those produced by the first dose. Cumulative effects happen when the body cannot metabolize and excrete a drug before the next dose is given. If the next dose is administered while some of the previous dose is still in the patient's body, the drug accumulates. A cumulative drug effect may occur in liver or kidney disease since these organs are the primary sites of drug metabolism and excretion. This is a common phenomenon among older adults with decreased cardiac, liver, or kidney function. Chemotherapeutic agents carry a high risk for cumulative effects, such as bone marrow suppression, fatigue, and neuropathy; these effects worsen with each dose. The body's hematologic system has a more challenging time recovering from repeated insults of cytotoxic treatments, thereby prolonging the time to bone marrow recovery (prolonged pancytopenia). Renal toxicity associated with aminoglycoside antibiotics such as gentamycin (Gentacin) occurs from drug accumulation in the kidneys with repeated doses (Frandsen & Pennington, 2020; Lilley et al., 2022; Vanderah, 2024).
Drug toxicity most commonly occurs when drugs are prescribed in higher-than-recommended dosages; it can also arise from impaired drug excretion secondary to impaired metabolism or elimination mechanisms. If a toxic level is reached, the patient will experience severe and possibly fatal adverse effects. Drugs with a small margin of safety can rapidly accumulate to a toxic level; thus, patients receiving drugs with a small margin of safety need to have their serum drug level drawn regularly and be closely monitored for signs and symptoms of toxicity. The effects of drug toxicity may be irreversible and life-threatening. For example, if vancomycin (Vancocin) is administered in toxic quantities, the patient can experience permanent damage to cranial nerve VIII, resulting in hearing impairment or deafness. Acetaminophen (Tylenol) administered in doses greater than 4,000 mg per day can cause temporary or permanent liver damage. Acetaminophen (Tylenol) is a component of many medications, and APRNs must remain vigilant about all the medications a patient is receiving before prescribing drugs containing acetaminophen (Tylenol). A common example is oxycodone-acetaminophen (Percocet) 5/325 mg, which is an opioid medication that contains 5 mg of oxycodone (Roxicodone) and 325 mg of acetaminophen (Tylenol) per tablet (Frandsen & Pennington, 2020; Lilley et al., 2022; Vanderah, 2024).
Hypersensitivity Reactions and Anaphylaxis
A hypersensitivity reaction (HSR) is an allergic reaction mediated by immunoglobulin E mast cell activation. It occurs when a foreign substance overstimulates the immune system and forms antibodies that cause an immune response. HSRs occur secondary to the administration of a drug that the body recognizes as foreign. HSRs can occur within minutes of the initial administration (immediate) or several hours after the drug was administered (delayed). They can also arise after subsequent administrations of the same agent (repeated exposure). HSR manifestations result from histamine release and can be localized or systemic. Localized HSRs are typically limited to venous inflammation (redness at the injection site) and dermatologic manifestations (hives or wheals). Systemic HSRs can cause generalized inflammation and swelling of tissues, increased mucous production, and bronchiole constriction in severe cases. Initial signs and symptoms of HSRs most commonly include hives, urticaria, pruritis, swelling, back pain, facial flushing, rhinitis, abdominal cramping, chills, and anxiety. However, manifestations may suddenly progress to life-threatening anaphylaxis and anaphylactic shock, which is an exaggerated response of the body's immune system to a drug that precipitates a massive release of histamine and other chemical mediators into the body. Manifestations of anaphylactic shock can occur almost immediately after exposure and include hypotension, difficulty breathing (wheezing, bronchospasm, stridor), angioedema (swelling of the oral cavity, lips, and/or tongue), orbital edema, and cardiac arrest (Fernandez, 2022; Nettina, 2019; Olsen et al., 2019).
The likelihood of HSRs can be reduced for high-risk drugs by pre-medicating patients with a combination of agents such as corticosteroids, antihistamines such as diphenhydramine (Benadryl), acetaminophen (Tylenol), and/or H2 blockers such as famotidine (Pepcid). Management of HSRs focuses on decreasing vascular permeability, increasing vasoconstriction of the peripheral veins, and inducing smooth muscle relaxation of the bronchioles. Mild to moderate HSRs can be treated with an antihistamine such as diphenhydramine (Benadryl). A patient in anaphylactic shock requires immediate medical attention, including cardiopulmonary support and rescue drugs to prevent fatality. The treatment of anaphylactic shock focuses on reestablishing and securing an airway, oxygen therapy, and the administration of epinephrine 0.1 to 0.5 mg (1:10,000 solution for adult patients) to treat hypotension and induce bronchodilation (dilate the respiratory bronchi) and diphenhydramine (Benadryl) to block the additional release of histamine. Corticosteroids are commonly administered to decrease vascular permeability, enhance the effects of epinephrine, and block inflammatory mediators circulating in the blood. APRNs prescribing high-risk medications by IV (chemotherapy, certain antibiotics, immunotherapy, immune modulators) should be familiar with their institution's specific HSR protocols and policies and access their rapid response or code blue teams when needed (Fernandez, 2022; Nettina, 2019; Olsen et al., 2019).
APRNs must inquire about patients' allergies to drugs before prescribing any agents. Even minor drug reactions are noteworthy. If a patient reports an allergy to a drug, the APRN should inquire about the type of reaction experienced and document this information in the medical record. Most institutions require that patients wear an identification wristband, alerting providers that the patient has a documented allergy or allergies in their EHR. The provider should then check the EHR for a list of all known allergies at the time of admission. Some drugs demonstrate cross-sensitivity to another drug, especially among antibiotics. For example, patients who have an allergy to penicillin may have a cross-sensitivity to a cephalosporin antibiotic. Before prescribing medications, APRNs must verify the patient's allergies and confirm the absence of any medications that may cause a cross-sensitivity reaction (Burchum & Rosenthal, 2019; Carpenter et al., 2019; Fernandez, 2022; Vanderah, 2024).
Drug Tolerance, Dependence, Abuse, and Misuse
APRNs must understand the distinctions between physiological and behavioral adaptations to medication therapy, such as tolerance, dependence, and addiction, as there are many misconceptions related to these concepts. Drug tolerance is the body's decrease in response to a drug it receives over time. For the drug to continue to exert its desired therapeutic effects, the dosage must be increased. Tolerance is not synonymous with addiction. The National Institute on Drug Abuse (NIDA, 2020) defines drug tolerance to medication as the gradual need for an increased dose of a particular medication to obtain a similar effect. Tolerance development varies significantly from individual to individual and from medication to medication. Tolerance occurs because of the body's ability to adapt to its environment physically and is not limited to pain medication or illicit drugs. A patient can develop tolerance to a drug and not be addicted. However, if the patient continues to take the drug in increasing doses over a longer-than-recommended period, addiction can occur with specific drugs. Addiction is a combination of physical dependence and compulsive drug-seeking behaviors despite significant negative repercussions from use. Addiction is a chronic, neurobiological disease that has contributing genetic, psychosocial, physical, and environmental influences (NIDA, 2020; Vanderah, 2024).
Physical dependence is the body's physiological adaptation to a drug that develops with consistent and regular use. Physical dependence is also a component of addiction. The medication becomes necessary for normal body functioning and homeostasis and is typically accompanied by the negative manifestations of withdrawal when the medication is no longer present in the body. Misuse of prescription drugs is the ingestion or utilization of these medications in a manner, at a dose, or by an individual other than those prescribed. This misuse includes taking (stealing) another person's medication or using pain medication to induce feelings of euphoria instead of alleviating somatic pain. The medical terms substance abuse and substance dependence have been replaced in recent years by substance use disorder (SUD). SUD may refer to an individual who has become addicted to nicotine, alcohol, prescription medications, or illicit drugs (NIDA, 2020). In another phenomenon called pseudoaddiction, the individual becomes intensely fearful of being in pain. This is common in postoperative patients and usually manifests as clock-watching, asking to be awoken to receive pain medication, and hypervigilance in documenting and monitoring pain medications. Pseudoaddiction usually resolves with effective pain management treatments and the decline of painful stimuli (in a postoperative patient, this involves the healing of the surgical site). Psychological dependence occurs when medication ingestion becomes associated with alleviating discomfort, such as pain, anxiety, and depression. The presence of the drug then becomes a calming and reassuring presence in the patient's life, similar to a comfort or security object (Hudspeth, 2016a, 2016b).
The three categories of controlled substances that are most commonly misused or abused include opioids, sedatives/anxiolytics, and stimulants. These medications can only be administered with a valid prescription or licensed prescriber's order. Controlled substances are categorized by schedule (Schedules I through V) based on their therapeutic use, perceived risk of addiction, and potential for misuse, as outlined by the US Drug Enforcement Administration (DEA; see Table 6). Approximately 20% of people who require pain relief for acute pain (such as postoperative pain or chronic pain related to a health issue) are prescribed opioids. Opioids are narcotics and have addictive properties. They are safe if taken over a short period; however, if taken for extended periods or in higher-than-prescribed amounts, a patient has a significant risk of addiction (DEA, n.d.; NIDA, 2020; Preuss et al., 2023; Vanderah, 2024).
Table 6
Drug Schedules I-V
Schedule | Description | Drug examples |
I | Drugs, chemicals, or substances with a high potential for abuse that have no currently accepted medical use or treatment in the US |
|
II | Drugs, chemicals, or substances with a high potential for abuse leading to severe psychological or physical dependence Accepted medical use in the US or a currently accepted medical use with severe restrictions |
|
III | Drugs, chemicals, or substances with a moderate to low potential for physical and psychological dependence Lower abuse potential than Schedule I and Schedule II drugs but higher than Schedule IV Currently accepted medical use in the US |
|
IV | Drugs, chemicals, or substances with a low potential for abuse and a low risk of dependence Currently accepted medical use in the US |
|
V | Drugs, chemicals, or substances with lower abuse potential than Schedule IV agents Currently accepted medical use in the US for preparations containing limited quantities of certain narcotics Abuse may lead to limited physical dependence or psychological dependence relative to the drugs or other substances in Schedule IV |
|
(DEA, n.d.; Preuss et al., 2023)
APRNs must monitor for signs of tolerance if a medication is prescribed over an extended period or if the specified amount of medication is no longer managing the underlying issue. In light of the ongoing opioid epidemic across the US, providing adequate pain management without promoting an opioid use disorder remains a significant challenge. Safer alternatives should always be explored and attempted before initiating a controlled substance if those alternatives exist. APRNs have been professionally trained to prescribe medications effectively and safely to treat and manage medical conditions within an overall treatment plan. However, the unique risks associated with controlled substances, their use, and potential drug interactions are significant (Hudspeth, 2016a, 2016b; NIDA, 2020; Vanderah, 2024).
Providers must uphold several responsibilities when providing safe, effective care for patients receiving opioids. State laws, regulations, and policies delineate prescriber responsibilities regarding the prescribing and dispensing of controlled substances. The US Department of Health and Human Services (2017) increased grant funding for developing novel strategies to impede this growing and deadly problem. These efforts have brought a new level of urgency to the matter, with heightened surveillance, restriction, and patient monitoring on long-term opioid therapy. Prescribers must be registered with the DEA, be granted prescriptive authority, and know the legislation regarding the prescribing and monitoring of opioids in the governing state. It is equally imperative for healthcare professionals to remain vigilant in screening for the signs and symptoms of misuse and abuse (Schiller et al., 2023).
As part of the national movement to mitigate the opioid epidemic, most states (49 states, Washington, D.C., and Guam) have established statewide electronic databases or prescription drug-monitoring programs (PDMPs) to track and monitor opioid prescriptions. A PDMP is a statewide electronic database that collects designated data on controlled substances dispensed to or for each patient. PDMPs are among the most promising state-level interventions to improve opioid prescribing, inform clinical practice, and protect patients at high risk. PDMPs are housed and operated by state regulatory, administrative, or law enforcement agencies. The housing agency disseminates information from the database to individuals authorized under state law to receive the information for purposes identified by state law. States arrange individual systems to track and monitor prescriptions. The details about use, access, and drug inclusion, and the regulations and implications for prescribers, vary by state. Most states have a method by which prescribers can access their patients' records in other or neighboring states. The key to the efficacy of PDMP systems is the mandate that all providers check the system before initiating opioid therapy. Reviewing each patient's history of controlled substance prescriptions using the PDMP database helps determine whether a patient is already receiving opioids or potentially dangerous combinations that place them at risk for overdose. Differences exist between states regarding how frequently providers should monitor this system and which controlled substances are included. Reporting systems help reduce the diversion of illegitimate opiate prescriptions. The consistent and diligent review of best practices and the most recent evidence in pharmacology are necessary components of a safe and effective medical practice. APRNs are encouraged to remain aware of best-practice prescribing guidelines, seek continuing education hours to stay informed, and ensure familiarity with prescribing guidelines and federal and state laws (CDC, 2021; D'Souza et al., 2023; Hudspeth, 2016a, 2016b).
For more information on the safe and effective prescribing of controlled substances, refer to the following NursingCE courses:
- Substance Abuse and Addiction for APRNs
- Safe and Effective Prescribing of Controlled Substances for APRNs
- Pain Management for APRNs
Considerations, Precautions, and Special Populations
Certain drugs should only be given after careful consideration of the underlying precautions or be avoided entirely. Patients with underlying medical conditions, those of a specific age group, and pregnant or lactating women are considered special populations. APRNs must know contraindications if a disease state or patient characteristic renders a drug inappropriate for use due to the potential for adverse effects. For example, tobacco users should use oral contraceptives cautiously due to the heightened risk of vascular events (such as venous thromboembolism [VTE]). Tamoxifen (Soltamox) should be used with caution by women who have a history of VTEs, such as deep vein thrombosis or pulmonary embolism, due to the combination's increased risk for blood clotting. Bisphosphonates are the most widely used medications for treating osteoporosis, a bone-thinning disorder associated with aging, heightening the risk of bone fractures. Bisphosphonates, such as denosumab (Prolia), alendronate (Fosamax), and risedronate (Actonel), inhibit the action of osteoclast cells to decrease bone turnover and increase bone mineral density. While these drugs are widely utilized and highly effective in reducing the risk of bone fractures, they are associated with serious adverse effects such as medication-related osteonecrosis of the jaw (a chronic condition that affects the oral cavity, leading to mucosal ulceration and refractory exposure of underlying necrotic bone), and atypical femur fracture (a fracture of the femoral shaft). These risks are associated with significant morbidity and mortality in some patients. Before prescribing medications with serious warnings, APRNs must ensure patients are fully educated on the possible complications to facilitate informed decision-making (Al-Worafi, 2020; Porter & Varacallo, 2023).
The Risk Evaluation and Mitigation Strategy Program
Some drugs require strict monitoring as outlined by the FDA Risk Evaluation and Mitigation Strategy (REMS) program, which is reserved for drugs with serious safety concerns to help ensure the benefits of a medication choice outweigh the associated risks. REMS is designed to mitigate the occurrence and severity of certain risks by enhancing the safe use of high-risk medications, as described within the FDA-approved prescribing information. REMS programs heighten the monitoring and surveillance of patients receiving high-risk medications. REMS programs consist of information communicated to and/or required activities to be undertaken by one or more participants (health care providers, pharmacists, patients) who prescribe, dispense, or take the medication. Each REMS program addresses a specific safety concern related to the medication. While the requirements for each REMS program vary, most require prescribers to enroll in the program, complete the specified training, document patient counseling, enroll patients, and monitor and confirm compliance with the safe use of the medication (FDA, 2023). Isotretinoin (Claravis), for example, is used to treat severe recalcitrant nodular acne; it carries an extremely high risk of congenital deformities if pregnancy occurs while taking the medication in any amount, even for a short period. Any fetus exposed during pregnancy could be affected. Due to the severity of this toxicity, isotretinoin (Claravis) can only be marketed under a restricted distribution REMS program called iPLEDGE. This specialized REMS program for isotretinoin (Claravis) aims to (a) prevent fetal exposure and (b) inform prescribers, pharmacists, and patients about its severe risks and safe-use conditions. Prescribers must be registered with and activated by the iPLEDGE program and can only prescribe isotretinoin (Claravis) to registered patients who meet all the requirements of iPLEDGE. Furthermore, the medication can only be dispensed by a pharmacy registered with and activated by the iPLEDGE program (FDA, 2021a).
Olanzapine (Zyprexa Relprevv) is a long-acting injectable antipsychotic medication used to treat schizophrenia in adults that can cause a serious post-injection reaction called delirium sedation syndrome. Manifestations include sedation, coma, or confusion that occurs within a few hours of administration. Although the risk of post-injection delirium sedation syndrome is small (less than 1%), there is a risk after each injection. The FDA required the manufacturer of olanzapine (Zyprexa Relprevv) to develop a REMS to ensure that the drug is administered only in certified healthcare facilities that can observe patients for at least 3 hours after injection and provide medical care if an adverse event occurs (FDA, 2023).
Boxed Warnings
The FDA requires boxed warnings for certain medications that carry severe safety risks. These warnings communicate the rare but potentially dangerous adverse effects of a drug and essential instructions for safe use. Boxed warnings typically appear in bold font on the medication. They are surrounded by a black border on the medication package insert and drug manufacturer's website (hence the moniker black box warning, now box warning; Delong & Preuss, 2023; FDA, 2020c; Vanderah, 2024).
In 2004, the FDA required a boxed warning on all antidepressant medications (SSRIs, SNRIs) regarding the risk of increased suicidality among children and adolescents taking these medications. A few years later, the warning was expanded to include all young adults, especially those under the age of 25, stating that these individuals may experience an increase in suicidal thoughts or behaviors during the first few weeks of taking an antidepressant and warning clinicians to monitor patients for this effect. Patients with underlying depression treated with SSRIs and SNRIs may experience worsening depression and/or the emergence of suicidal ideation and suicidality. This risk may persist until significant remission occurs. Research has demonstrated that SSRIs and SNRIs increase the risk of suicidality when compared to placebo controls. The FDA also requires manufacturers to provide a Patient Medication Guide to recipients of these medications to advise them of risks and precautions to reduce the risk of suicide. APRNs should inquire directly about suicidal thoughts before prescribing antidepressants to young persons. All patients treated with antidepressants (for any indication) should be counseled on the risk of increased suicidality and encouraged to monitor and report worsening symptoms, suicidality, and unusual changes in behavior, especially during the first few months of treatment. Table 7 provides the points that must be included in an antidepressant box warning (FDA, 2018a).
Table 7
FDA Antidepressants Boxed Warning Points
|
|
|
|
|
(FDA, 2018a)
Contraindications
Some drugs are contraindicated in specific populations due to their potential to cause severe or life-threatening adverse effects. For example, acetylsalicylic acid (Aspirin) is contraindicated in children suffering from a viral infection due to the risk of Reye's syndrome. Reye's syndrome is a rare but serious condition that causes swelling in the liver and brain and most commonly affects children recovering from influenza or chickenpox. Another example is that children under 8 years old should not be given tetracycline antibiotics (minocycline [Minocin], doxycycline [Monodox]) because they can permanently stain developing teeth. APRNs should only consider using drugs in these situations under particular circumstances (Shutter & Akhondi, 2023; Vanderah, 2024).
Pregnant and Lactating Women
Pregnant women have a high risk of maternal and fetal adverse drug effects, and extreme caution must be exercised before prescribing medications to these patients. Some drugs are teratogenic (can disturb the development of the embryo or fetus) and cause significant congenital disabilities if administered during pregnancy, typically during the first trimester. For example, women with type 2 diabetes taking oral diabetic agents such as metformin (Glucophage) usually need to switch to insulin, as the safety of oral antihyperglycemics during pregnancy has not yet been established. Pregnancy-related insulin resistance also decreases the effectiveness of oral diabetic agents (Al-Worafi, 2020; Vanderah, 2024).
Before 2015, pregnancy- and lactation-related drug risks were assigned categories of A, B, C, D, and X. These five categories indicated a drug's risk of causing fetal injury when taken during pregnancy; however, they were often criticized for leading to prescribing errors based on false conclusions drawn from the categories. Effective June 30, 2015, the FDA replaced these categories with the Pregnancy and Lactation Labeling Rule (PLLR). The PLLR provides a concise, standardized summary of available evidence and detailed animal data on the medication, including a background risk statement and the estimated risk of significant congenital disabilities and miscarriage. APRNs should consistently reference the most recent, evidence-based drug resources before prescribing medications to pregnant patients (FDA, 2021b; Pernia & DeMaagd, 2016).
Older Adults
The known physiological changes accompanying advancing age should be considered when prescribing medications to patients older than 65. Although the absorption rate of most medications does not change with age, common drugs can alter the absorption of other medications by this population. Furthermore, certain conditions may reduce the acidity of the GI tract or the availability of intrinsic factors, thereby interfering with proper medication absorption. The percentage of body fat typically increases with age, while total body water decreases. This gradual change can lead to longer half-lives for fat-soluble medications and an increase in the concentration of water-soluble medications. In addition, hepatic blood flow tends to decrease with age (along with size/mass), altering the body's ability to process and clear drugs. Hepatic impairment may further increase a medication's half-life and alter how frequently it should be dosed. Evaluating these changes is especially crucial for patients taking medications that are processed via CYP450. Patients with decreased hepatic metabolism who take medications processed via CYP450 may experience higher circulating levels of medications, especially warfarin (Coumadin) and phenytoin (Dilantin; Al-Worafi, 2020; Rochon, 2023; Ruscin & Linnebur, 2021b; Saljoughian, 2019).
Similarly, drug elimination can be impacted by renal impairment associated with advanced age, such as diminished renal blood flow, fewer functioning nephrons, decreased glomerular filtration rate, and tubular secretion. Like hepatic impairment, renal deficiency can also increase a medication's half-life, shifting its dosing frequency. Reduced lean muscle mass can diminish CrCl, rendering this an inaccurate indicator of medication elimination. Several medications that rely heavily on renal excretion are commonly used for older adults, such as allopurinol (Aloprim), H2 blockers such as famotidine (Pepcid), digoxin (Lanoxin), lisinopril (Zestril, Prinivil), atenolol (Tenormin), ciprofloxacin (Cipro), lithium (Lithobid), vancomycin (Vancocin), amantadine (Symmetrel, Osmolex ER), and memantine (Namenda). See the table below for additional information regarding medications to avoid for patients with impaired renal function. Increasing levels of unbound or free medication may result from a reduction in serum albumin levels related to malnutrition in a geriatric patient experiencing feeding or eating problems. Decreased cardiac output may lead to inadequate drug distribution. Due to changes in pharmacodynamics in older adults, many patients will also develop a heightened sensitivity to certain drugs. Thus, a lower dosage of most medications may be required to achieve the same Cp and therapeutic effects (Al-Worafi, 2020; Rochon, 2023; Ruscin & Linnebur, 2021b; Saljoughian, 2019).
Various tools have been developed to help screen for potentially inappropriate medications (PIMs) in older adults. The American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medication Use in Older Adults was last updated in 2023 and includes the following categories: PIMs, medications that are potentially inappropriate with certain conditions, medications that should be used with caution, drug-drug interactions that should be avoided, and medications that should be avoided or reduced in patients with kidney dysfunction (see Table 8). For example, drugs with substantial anticholinergic effects, such as first-generation antihistamines (diphenhydramine [Benadryl], dimenhydrinate [Dramamine]), should be avoided due to the reduced renal clearance in older adults and risks of confusion, dry mouth, constipation, and other anticholinergic effects or toxicity. In addition, the use of meperidine (Demerol) is discouraged for this population since impaired renal function can lead to an accumulation of normeperidine, a neurotoxic metabolite (AGS Beers Criteria Update Expert Panel, 2023; Nguyen et al., 2020; Rochon, 2023; Ruscin & Linnebur, 2021a).
Table 8
Medications to Avoid for Older Adults with Impaired Renal Function
Medication | Recommendation | Rationale |
Patients with a CrCl greater than 95 mL/min | ||
edoxaban (Savaysa) | Avoid | Lack of evidence regarding efficacy/safety |
Patients with a CrCl of less than 81 mL/min | ||
levetiracetam (Keppra) | Reduce dose | CNS effects |
Patients with a CrCl less than 60 mL/min | ||
dofetilide (Tikosyn) | Reduce dose | QTc prolongation; risk of torsades de pointes |
gabapentin (Neurontin) | Reduce dose | CNS effects |
pregabalin (Lyrica) | Reduce dose | CNS effects |
Patients with a CrCl less than 50 mL/min | ||
cimetidine (Tagamet) | Reduce dose | Mental status changes |
edoxaban (Savaysa) | Reduce dose | Lack of evidence regarding efficacy/safety |
famotidine (Pepcid) | Reduce dose | Mental status changes |
nizatidine (Axid) | Reduce dose | Mental status changes |
rivaroxaban (Xarelto) | Reduce dose | Lack of evidence regarding efficacy/safety |
Patients with a CrCl of less than 30 mL/min | ||
amiloride (Midamor) | Avoid | Hyperkalemia and hyponatremia |
ciprofloxacin (Cipro) | Reduce dose | CNS effects; risk of tendon rupture |
colchicine (Colcrys) | Reduce dose/monitor | Increased GI and neuromuscular adverse effects; bone marrow toxicity |
dabigatran (Pradaxa) | Avoid | Lack of evidence regarding efficacy/safety; may also require dose reduction for patients with CrCl greater than 30 mL/min if combined with other high-risk medications |
duloxetine (Cymbalta) | Avoid | Increased GI adverse effects |
enoxaparin (Lovenox) | Reduce dose | Increased risk of bleeding |
fondaparinux (Arixtra) | Avoid | Increased risk of bleeding |
nitrofurantoin (Macrobid) | Avoid | Potential for pulmonary and hepatotoxicity and peripheral neuropathy |
NSAIDs | Avoid | Increased risk of acute kidney injury and worsening kidney function |
probenecid (Probalan) | Avoid | Lack of effectiveness |
spironolactone (Aldactone) | Avoid | Hyperkalemia |
TMP-SMX (Bactrim) | Reduce dose | Risk of worsening renal function/hyperkalemia |
tramadol (Ultram) IR | Reduce dose | CNS effects |
tramadol (Ultram) ER | Avoid | CNS effects |
triamterene (Dyrenium) | Avoid | Hyperkalemia and hyponatremia |
Patients with a CrCl of less than 20 mL/min | ||
dofetilide (Tikosyn) | Avoid | QTc prolongation; risk of torsades de pointes |
Patients with a CrCl of less than 15 mL/min | ||
edoxaban (Savaysa) | Avoid | Lack of evidence regarding efficacy/safety |
rivaroxaban (Xarelto) | Avoid | Lack of evidence regarding efficacy/safety |
TMP-SMX (Bactrim) | Avoid | Risk of worsening renal function/hyperkalemia |
Patients with eGFR less than 60 | ||
baclofen (Lioresal) | Avoid; when it cannot be avoided, use the lowest effective dose and monitor for signs of CNS toxicity | Increased encephalopathy |
(AGS Beers Criteria Update Expert Panel, 2023)
Older Adults and Polypharmacy. Roughly one-half (44% of men and 57% of women) of those over 65 take at least five medications (prescription or OTC) every week. In this age group, 12% of patients take at least 10 medications. Polypharmacy (the simultaneous use of multiple medications) becomes an issue if it contributes to negative outcomes, such as adverse events, nonadherence, and increased cost. The use of greater numbers of drug therapies has been independently associated with an increased risk for an adverse drug event (ADE), irrespective of age, and an increased risk of hospital admission. For example, polypharmacy is established as an independent risk factor for hip fractures in older adults. Being prescribed various medications by multiple providers increases the risk of adverse events and drug-drug interactions for older adults. Polypharmacy leads to therapeutic duplication, unnecessary medication use, increased side effects, and poor adherence. Risk factors for polypharmacy include advancing age, lack of education, ethnicity, health status, and access to a pharmacy. A patient taking 5 to 9 different medications has a 50% chance of experiencing a drug-drug interaction, and that probability increases to 100% in a patient taking 20 or more medications. While polypharmacy increases the risk of poor adherence, older adults may also have other issues specific to their population that affect adherence. These include forgetting to take their medications due to cognitive impairment, poor vision, limited financial resources, or limited access to or transportation to a pharmacy, all of which have been shown to reduce medication regimen adherence. A prescribing cascade is a common phenomenon among older adults with multiple prescribed medications that occurs when additional medications are prescribed due to the misdiagnosis of an adverse effect as a new medical condition. For example, many antipsychotic medications may lead to parkinsonism (manifestations that mimic Parkinson's disease in patients without the underlying diagnosis), prompting the prescription of an antiparkinsonian medication, which then causes orthostatic hypotension and delirium. APRNs must ensure that they consistently and accurately communicate all medication changes to the other members of the interdisciplinary healthcare team, including pharmacists, intensivists, specialists, and primary care providers (Nguyen et al., 2020; Rochon, 2023; Saljoughian, 2019; Ward & Reuben, 2022).
For more information on older adults and polypharmacy, refer to the NursingCE course Care Considerations for Older Adults: The Assessment and Management of Polypharmacy for APRNs.
Medication Errors
Attention toward medication errors has grown since 2000 when the Institute of Medicine (IOM), now called the National Academy of Medicine (NAM), released its landmark report, To Err Is Human. The report called to light the astronomical data surrounding medication errors as common, costly, and preventable medical mishaps, citing nearly 98,000 hospitalized patient deaths per year due to preventable medical errors. This momentous study ignited a focus on medical practices, spawning new policies and procedures and setting performance standards and expectations for patient safety and quality improvement (IOM US Committee on Quality of Health Care in America, 2000). In a 2007 report entitled Preventing Medication Errors, the IOM released alarming statistics that more than 1.5 million people suffered an injury each year in US hospitals; the average hospitalized patient experienced at least one medication error daily (IOM, 2007).
In a 2016 study by Johns Hopkins University, researchers compared medical errors to the annual list from the CDC of the most common causes of death in the US Their findings demonstrated that 10% of all deaths in the US are attributable to medical errors, branding medical errors as the third-highest cause of death (Makary & Daniel, 2016). To date, the FDA (2019) receives at least 100,000 reports annually associated with a suspected medication error. Each year, between 7,000 and 9,000 people die due to a medication error (Tariq et al., 2023). Safe medication administration involves the entire healthcare team and carries legal and ethical implications. The primary focus of effective medication administration is patient safety. Although many measures have been employed over the past few decades to promote and enhance patient safety, medication errors and ADEs continue to occur. ADEs are temporally associated with the use of a drug but are not necessarily causally related. ADE encompasses all drug-related incidents, including adverse reactions and medication errors. The World Health Organization (n.d.) estimates that the global cost associated with medication errors is $42 billion annually.
According to the AHRQ (2019), a medication error is a preventable event defined as "an error (of commission or omission) at any step along the pathway that begins when a clinician prescribes a medication and ends when the patient receives the medication." ADEs account for nearly 700,000 emergency department visits and 100,000 hospitalizations each year. Almost 5% of hospitalized patients experience an ADE, making them a prevalent inpatient medical error. Incorporating the NAM competencies for nursing, the Quality and Safety Education for Nurses (QSEN) framework identified the following six competencies necessary for safe practice: patient-centered care, teamwork and collaboration, evidence-based practice, quality improvement, safety, and informatics. Nursing programs are tasked with integrating these principles into their curricula to support safe medication administration (QSEN, n.d.). As part of their national patient safety goals (NPSG), The Joint Commission (TJC) has identified particular areas of interest regarding medication administration; one such area addresses errors that occur when reading and interpreting new medication orders. A "Do Not Use" list of abbreviations has been established to avert confusion that could lead to a medication error, as summarized in Table 9 (TJC, 2024).
Table 9
TJC "Do Not Use" List
Do not use | Potential problem | Use instead |
U, u (unit) | Mistaken for "0" (zero), the number "4", or "cc" | Unit |
IU (International Unit) | Mistaken for IV (intravenous) or the number "10" (ten) | International Unit |
Q.D., QD, q.d., qd (daily) | Mistaken for each other | Daily |
Q.O.D., QOD, q.o.d., qod (every other day) | The period after the Q can be mistaken for "I" or the "O" mistaken for "I" | Every other day |
Trailing zero (X.0 mg) Lack of leading zero (.X mg) | Decimal point missed | X mg 0.X mg |
MS | Can mean magnesium sulfate or morphine sulfate | Morphine sulfate |
MSO4 and MgSO4 | Can be confused with each other | Morphine sulfate Magnesium sulfate |
(TJC, n.d.)
Appropriate medication labeling and the appropriate use of anticoagulant medications have also been implemented to reduce the likelihood of patient harm. The 2024 version of the TJC NPSG discusses medication reconciliation in response to the large percentage of patients taking multiple medications and the complexity of managing them (see Table 10). Medication reconciliation is the process of directly comparing the medications a patient is prescribed with newly ordered medications. It is intended to identify and resolve discrepancies; it addresses duplications, omissions, and interactions. The medication list must include the drug name, dosage, frequency, and route. Potential errors during medication reconciliation include omitting a medication, recording the drug more than once, or recording an incorrect dose or frequency (Institute for Healthcare Improvement, 2018; TJC, 2024).
Table 10
TJC 2024 National Patient Safety Goals: Improve the Safety of Using Medications
Goal | Description |
NPSG.03.04.01 | Before a procedure, label medicines that are not labeled (medicines in syringes, cups, and basins). Perform this task in the area where medicines and supplies are set up. |
NPSG.03.05.01 | Take extra care with patients who take anticoagulants. |
NPSG.03.06.01 | Record and pass along correct information about a patient's medications. Determine which medicines the patient is taking and compare those to new medicines given to the patient. Provide the patient with written information about the medicines they need to take and encourage them to bring their updated list of medications every time they visit a doctor. |
(TJC, 2024)
The FDA and the Institute for Safe Medication Practices (ISMP) collaboratively generated a list of high-alert medications that pose an enhanced risk of causing significant patient harm if given inappropriately. The ISMP Look-Alike/Sound-Alike (LASA) medication list refers to visually similar drugs (physical appearance or packaging) and names of medications with similar spelling or phonetics. Tall man lettering (TML) is a technique that uses uppercase lettering to help differentiate lookalike drug names. TML distinguishes between similar drug names by capitalizing dissimilar letters (see Table 11). TML is often used alongside color changes or bold-style font to draw attention to the dissimilarities between lookalike drug names and alert healthcare professionals that the drug name is easily confused with another (FDA, 2020a; ISMP, 2016).
Table 11
Examples of TML for LASA Medications
Established name | Recommended name |
bupropion buspirone | buPROPion busPIRone |
hydralazine hydroxyzine | hydrALAZINE hydrOXYzine |
nicardipine nifedipine | niCARdipine NIFEdipine |
prednisone prednisolone | predniSONE prednisoLONE |
trazodone tramadol | traZODone traMADol |
(FDA, 2020a; ISMP, 2016)
American Nurses Association Code of Ethics
The American Nurses Association's Code of Ethics guides nursing responsibilities consistent with quality nursing care and ethical obligations. Several of the provisions cited within the document influence how nurses should ethically administer medications. The following points summarize how each provision affects medication administration (American Nurses Association, 2015; Ernstmeyer & Christman, 2023):
- Provision 1 focuses on respect for human dignity and the right to self-determination, instructing nurses to deliver care with compassion and respect for each patient's dignity, worth, and unique attributes.
- Provision 2 denotes that each nurse's primary commitment is to the patient, embracing the patient's right to accept, refuse, or terminate any treatment, including medications.
- Provision 3 refers to a nurse's responsibility to promote, advocate for, and protect each patient's rights, health, and safety, upholding a culture of safety. If medication errors occur, a nurse must report them and ensure the responsible disclosure of errors to patients, including an appropriate disclosure of any questionable practices, such as drug diversion or impaired practice by any healthcare professional or colleague.
- Provision 4 refers to a nurse's authority, accountability, and responsibility to follow and carry out all legal requirements, such as state practice acts and professional standards of care.
- Provision 5 involves a nurse's responsibility to promote health and safety.
- Provision 6 identifies a nurse's morals and virtues, such as their accountability to exercise clinical judgment to avoid causing harm to patients (maleficence) and do good (beneficence) when administering medications and caring for patients.
- Provision 7 refers to a nurse's responsibility to practice within the professional standards set forth by their state nurse practice act and standards established by professional nursing organizations.
- Provision 8 focuses on a nurse's role in addressing the social determinants of health, such as poverty, education, safe medication use, and health care disparities.
APRN Responsibilities in Preventing Medication Errors
APRNs must assess a patient's condition before prescribing medication and anticipate possible side effects, interactions, contraindications, and precautions. Various sources refer to the five rights of medication administration, and some list up to 10 rights of medication administration (see Table 12; AHRQ, 2019; Hanson & Haddad, 2020; Vanderah, 2024).
Table 12
Medication Administration Rights
Original five rights of medication administration | → | Additional five rights of medication administration |
Right patient | Right education | |
Right drug | Right documentation | |
Right dose | Right to refuse | |
Right route | Right assessment | |
Right time | Right evaluation |
(AHRQ, 2019; Hanson & Haddad, 2024; Vanderah, 2024)
Medication errors can occur from the time a health care provider writes the prescription to the pharmacy filling the drug to the actual delivery of medication to the patient, and the entire multiprofessional team is responsible for promoting safe medication administration. Mistakes can occur at various steps in the medication administration process, but consistently following the steps can reduce or avoid costly errors. The appropriate medication, route, dose, and duration should be selected. APRNs should note that while EHRs have improved prescribing safety by alerting the user when a medication incompatibility is detected, an overabundance of these reminders can lead to reminder fatigue (also called alert or alarm fatigue). The list below summarizes the APRN responsibilities regarding preventing medication errors (AHRQ, 2019; Burcham & Rosenthal, 2019; Saljoughian, 2019; Vanderah, 2024).
Strategies to Mitigate Medication Errors
- Knowing and understanding federal, state (nurse practice acts, including prescriptive authority, prescribing laws, and scope of practice), local, and facility policies that govern the prescribing, dispensing, and administration of medications
- Prescribing, preparing, monitoring, and evaluating patient response to medications
- Developing and maintaining current knowledge of all prescribed medications, including indications, mechanisms of action, routes of administration, safe dosage range, side effects, adverse drug effects, precautions, contraindications, and interactions
- Maintaining knowledge of acceptable practice and skills competency, including medication calculations
- Determining the accuracy of medication prescriptions
- Reporting all medication errors
- Safeguarding and storing medications
- Educating patients on medication actions, dosing schedule, anticipated side effects, and the importance of reporting any signs of adverse drug effects (Burcham & Rosenthal, 2019; Lilley et al., 2017; Smith & Pacitti, 2020; Vanderah, 2024)
Summary
APRNs must be knowledgeable of all drugs they prescribe to patients. Understanding the pharmacokinetics and pharmacodynamics of a drug is vital for safely administering the drug while monitoring for adverse drug effects, signs of an HSR, and toxicity. Patient characteristics such as age, health alterations, and liver or kidney impairment must also be considered. APRNs must recognize which medications require peak and trough level monitoring and ensure they are ordered and monitored as directed. APRNs should routinely and meticulously review all drugs prescribed to a patient to ensure accuracy and safety and avert drug interactions. APRNs are responsible for safeguarding patient care by serving as each patient's advocate. Education and proper communication can help avoid many medication errors and adverse effects. Patients should be encouraged to maintain an accurate medication list; they should update it regularly, bring it with them to all medical appointments (or keep a digital copy on their phone), and share it with all their medical providers to ensure accuracy and awareness across disciplines and specialists. APRNs should educate patients and caregivers on any new medications, the rationale for use, possible side effects, and any dietary restrictions, as well as alert them to LASA medications to avoid dosing errors at home. Patients should be encouraged not to stop or change how they take a medication without first discussing this action with the prescriber. Patients should be directly asked about medication adherence at each visit. Education about medication safety can also prevent mishaps, including not saving or sharing medications and storing medication in a secure location. For older adults or patients with memory deficits, APRNs should encourage them to explore various solutions available, such as pill dispensers, color-coded pillboxes, or technology aids offering reminder features, such as smartphone applications. A simple alternative is to link the medication dose with routine daily activity, such as brushing their teeth, shaving, or drinking coffee (Saljoughian, 2019; Vanderah, 2024).
For more information on medication errors and medical errors, refer to the Medical Errors NursingCE Course.
References
Agency for Healthcare Research and Quality. (2019). Medication errors and adverse drug events. https://psnet.ahrq.gov/primer/medication-errors-and-adverse-drug-events
Al-Worafi, Y. M. (2020). Chapter 13 – Safety of medications in special population. Drug Safety in Developing Countries, 143–162. https://doi.org/10.1016/B978-0-12-819837-7.00013-3
American Geriatrics Society Beers Criteria Update Expert Panel. (2023). American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society, 71(7), 2052–2081. https://doi.org/10.1111/jgs.18372
American Nurses Association. (2015). Code of ethics for nurses with interpretive statements. https://www.nursingworld.org/coe-view-only
Burchum, J. R., & Rosenthal, L. D. (2019). Lehne’s pharmacology for nursing care (10th ed.). Elsevier.
Carpenter, M., Berry, H., & Pelletier, A. L. (2019). Clinically relevant drug-drug interactions in primary care. American Family Physician, 99(9), 558–564. https://www.aafp.org/pubs/afp/issues/2019/0501/p558.html
Centers for Disease Control and Prevention. (2021). Prescription drug monitoring programs (PDMPs). https://www.cdc.gov/drugoverdose/pdmp
Centers for Disease Control and Prevention. (2023). Therapeutic drug use. https://www.cdc.gov/nchs/fastats/drug-use-therapeutic.htm
Craven, R. F., Hirnle, C. J., & Henshaw, C. M. (2021). Fundamentals of nursing: Concepts and competencies for practice (9th ed.). Wolters Kluwer.
Dabrowska, A., & Thaul, S. (2018). How FDA approves drugs and regulates their safety and effectiveness. Congressional Research Service, 1–13. https://fas.org/sgp/crs/misc/R41983.pdf
Delong, C., & Preuss, C. V. (2023). Box warning. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK538521
D'Souza, R. S., Lang, M., & Eldridge, J. S. (2023). Prescription drug monitoring program. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK532299
Ernstmeyer, K., & Christman, E. (Eds.). (2023). Nursing pharmacology (2nd ed.). Open Resources for Nursing. https://www.ncbi.nlm.nih.gov/books/NBK595000
Farinde, A. (2023a). Drug-receptor interactions. https://www.merckmanuals.com/professional/clinical-pharmacology/pharmacodynamics/drug%E2%80%93receptor-interactions
Farinde, A. (2023b). Overview of pharmacodynamics. https://www.merckmanuals.com/professional/clinical-pharmacology/pharmacodynamics/overview-of-pharmacodynamics
Fernandez, J. (2022). Drug hypersensitivity. https://www.merckmanuals.com/professional/immunology-allergic-disorders/allergic,-autoimmune,-and-other-hypersensitivity-disorders/drug-hypersensitivity
Frandsen, G., & Pennington, S. S. (2020). Abrams’ clinical drug therapy: Rationales for nursing practice (12th ed.). Wolters Kluwer.
Hanson, A., & Haddad, L. M. (2023). Nursing rights of medication administration. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK560654
Hudspeth, R. S. (2016a). Safe opioid prescribing for adults by nurse practitioners: Part 1. Patient history and assessment standards and techniques. The Journal for Nurse Practitioners, 12(3), 141–148. https://doi.org/10.1016/j.nurpra.2015.10.012
Hudspeth, R. S. (2016b). Safe opioid prescribing for adults by nurse practitioners: Part 2. Implementing and managing treatment. The Journal for Nurse Practitioners, 12(4), 213–220. https://doi.org/10.1016/j.nurpra.2015.11.032
Institute for Healthcare Improvement. (2018). New thinking on medication reconciliation. https://www.ihi.org/insights/new-thinking-medication-reconciliation
Institute of Medicine. (2007). Preventing medication errors. The National Academies Press. https://doi.org/10.17226/11623
Institute of Medicine Committee on Quality of Health Care in America. (2000). To err is human: Building a safer health system. The National Academies Press. https://doi.org/10.17226/9728
Institute for Safe Medication Practices. (2016). FDA and ISMP lists of look-alike drug names with recommended tall man letters. https://www.ismp.org/sites/default/files/attachments/2017-11/tallmanletters.pdf
The Joint Commission. (n.d.). Do not use list fact sheet. Retrieved March 25, 2024, from https://www.jointcommission.org/resources/news-and-multimedia/fact-sheets/facts-about-do-not-use-list
The Joint Commission. (2024). Hospital: 2024 National Patient Safety Goals. https://www.jointcommission.org/standards/national-patient-safety-goals/hospital-national-patient-safety-goals
Kim, J., & De Jesus, O. (2023). Medication routes of administration. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK568677
Le, J. (2022a). Drug absorption. Merck Manual. https://www.merckmanuals.com/professional/clinical-pharmacology/pharmacokinetics/drug-absorption
Le, J. (2022b). Drug distribution to tissues. Merck Manual. https://www.merckmanuals.com/professional/clinical-pharmacology/pharmacokinetics/drug-distribution-to-tissues
Le, J. (2022c). Drug excretion. Merck Manual. https://www.merckmanuals.com/professional/clinical-pharmacology/pharmacokinetics/drug-excretion
Le, J. (2022d). Drug metabolism. Merck Manual. https://www.merckmanuals.com/professional/clinical-pharmacology/pharmacokinetics/drug-metabolism
Le, J. (2022e). Overview of pharmacokinetics. Merck Manual. https://www.merckmanuals.com/professional/clinical-pharmacology/pharmacokinetics/overview-of-pharmacokinetics
Lilley, L. L., Collins, S. R., & Snyder, J. S. (2022). Pharmacology and the nursing process (10th ed.). Elsevier.
Longo, D. L. (2019). Harrison's hematology and oncology (3rd ed.). McGraw-Hill Education.
Makary, M. A., & Daniel, M. (2016). Medical error—the third leading cause of death in the US. BMJ, 353, i2139. https://doi.org/10.1136/bmj.i2139
National Center for Complementary and Integrative Health. (2021). Depression and complementary health approaches. https://www.nccih.nih.gov/health/providers/digest/depression-and-complementary-health-approaches
National Institute on Aging. (2021). Dietary supplements for older adults. https://www.nia.nih.gov/health/vitamins-and-supplements/dietary-supplements-older-adults
National Institute on Drug Abuse. (2020). Misuse of prescription drugs research report. https://www.drugabuse.gov/publications/misuse-prescription-drugs/overview
National Institutes of Health. (2022). Clinical research trials. https://www.nih.gov/health-information/nih-clinical-research-trials-you/basics
Nettina, S. M. (Ed.). (2019). Lippincott manual of nursing practice (11th ed.). Wolters Kluwer.
Nguyen, T., Wong, E., & Ciummo, F. (2020). Polypharmacy in older adults: Practical applications alongside a patient case. The Journal for Nurse Practitioners, 16(3), 205–209. https://doi.org/10.1016/j.nurpra.2019.11.017
Olsen, M., LeFebvre, K., & Brassil, K. (2019). Chemotherapy and immunotherapy guidelines and recommendations for practice (1st ed.). Oncology Nursing Society.
OpenStax. (2024). Pharmacology for nurses. https://openstax.org/details/books/pharmacology
Pernia, S., & DeMaagd, G. (2016). The new pregnancy and lactation labeling rule. Pharmacy & Therapeutics, 41(11), 713–715. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5083079/pdf/ptj4111713.pdf
Porter, J. L., & Varacallo, M. (2023). Osteoporosis. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK441901
Preuss, C. V., Kalava, A., & King, K. C. (2023). Prescription of controlled substances: Benefits and risks. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK537318
Quality and Safety Education for Nurses. (n.d.). Graduate QSEN competencies. Retrieved March 25, 2024, from https://qsen.org/competencies/graduate-ksas
Rochon, P. A. (2023). Drug prescribing for older adults. UpToDate. Retrieved March 19, 2024, from https://www.uptodate.com/contents/drug-prescribing-for-older-adults
Ruscin, J. M., & Linnebur, S. A. (2021a). Drug categories of concern in older adults. https://www.merckmanuals.com/professional/geriatrics/drug-therapy-in-older-adults/drug-categories-of-concern-in-older-adults
Ruscin, J. M., & Linnebur, S. A. (2021b). Drug-related problems in older adults. Merck Manual. https://www.merckmanuals.com/professional/geriatrics/drug-therapy-in-older-adults/drug-related-problems-in-older-adults
Saljoughian, M. (2019). Polypharmacy and drug adherence in elderly patients. US Pharmacist, 44(7), 33–36. https://www.uspharmacist.com/article/polypharmacy-and-drug-adherence-in-elderly-patients
Schiller, E. Y., Goyal, A., & Mechanic, O. J. (2023). Opioid overdose. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/pubmed/29262202
Selchick, F. (2021). Clinical trial phases [Image].
Shutter, M. C., & Akhondi, H. (2023). Tetracycline. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK549905
Smith, B. T., & Pacitti, D. F. (2020). Pharmacology for nurses (2nd ed.). Jones & Bartlett Learning.
Sub Laban, T., & Saadabadi, A. (2023). Monoamine oxidase inhibitors (MAOI). StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK539848
Tariq, R. A., Vashisht, R., Sinha, A., & Scherbak, Y. (2023). Medication dispensing errors and prevention. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK519065
US Department of Health and Human Services. (2017). HHS acting secretary declares public health emergency to address national opioid crisis. https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html
US Drug Enforcement Administration. (n.d.). Drug scheduling. Retrieved March 14, 2024, from https://www.dea.gov/drug-information/drug-scheduling
US Food and Drug Administration. (n.d.). Drug approval process. Retrieved February 20, 2024, from https://www.fda.gov/media/82381/download
US Food and Drug Administration. (2018a). Suicidality in children and adolescents being treated with antidepressant medications. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/suicidality-children-and-adolescents-being-treated-antidepressant-medications
US Food and Drug Administration. (2018b). The drug development process. https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process
US Food and Drug Administration. (2019). Working to reduce medication errors. https://www.fda.gov/drugs/information-consumers-and-patients-drugs/working-reduce-medication-errors
US Food and Drug Administration. (2020a). FDA name differentiation project: FDA list of established drug names recommended to use tall man lettering (TML). https://www.fda.gov/drugs/medication-errors-related-cder-regulated-drug-products/fda-name-differentiation-project
US Food and Drug Administration. (2020b). Regulated products and facilities. https://www.fda.gov/media/143704/download
US Food and Drug Administration. (2020c). Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products – Content and format. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/warnings-and-precautions-contraindications-and-boxed-warning-sections-labeling-human-prescription
US Food and Drug Administration. (2021a). Isotretinoin (marketed as Accutane) capsule information. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/isotretinoin-marketed-accutane-capsule-information
US Food and Drug Administration. (2021b). Pregnancy and lactation labeling (drugs) final rule. https://www.fda.gov/drugs/labeling-information-drug-products/pregnancy-and-lactation-labeling-drugs-final-rule
US Food and Drug Administration. (2023). Risk evaluation and mitigation strategies / REMS. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems
Vanderah, T. W. (2024). Katzung's basic & clinical pharmacology (16th ed.). McGraw-Hill Education.
Ward, K. T., & Reuben, D. B. (2022). Comprehensive geriatric assessment. UpToDate. Retrieved March 23, 2024, from www.uptodate.com/contents/comprehensive-geriatric-assessment
World Health Organization. (n.d.). Medication without harm. Retrieved March 25, 2024, from https://www.who.int/initiatives/medication-without-harm