About this course:
This course reviews the anatomy and physiology of the heart, including blood flow and electrical activity. It also examines the principles for assessing a telemetry strip and the variations in telemetry monitoring. Finally, this course reviews the pathophysiology, risk factors, signs and symptoms, telemetry features, and essential treatment and management for various arrhythmias.
Course preview
Introduction to Telemetry Monitoring for Arrhythmias
This course reviews the anatomy and physiology of the heart, including blood flow and electrical activity. It also examines the principles for assessing a telemetry strip and the variations in telemetry monitoring. Finally, this course reviews the pathophysiology, risk factors, signs and symptoms, telemetry features, and essential treatment and management for various arrhythmias.
At the completion of this activity, learners will be prepared to:
- define the terms, and recall the anatomy and physiology of the heart
- briefly discuss the variations of telemetry monitoring and how to assess a telemetry strip
- identify the characteristics of normal sinus rhythm and its variations, including sinus arrhythmia, sinus bradycardia, sinus tachycardia, sinus arrest, and sick sinus syndrome
- identify supraventricular arrhythmias on telemetry, including premature atrial contractions, atrial tachycardia, atrial flutter, atrial fibrillation, and wandering pacemaker, and illustrate their pathophysiology, risk factors, protective factors, signs and symptoms, telemetry features, other associated diagnostic tests, nursing care, and basic treatment/management
- identify junctional arrhythmias on telemetry, including Wolff-Parkinson-White, premature junctional contractions, junctional escape rhythm, accelerated junctional rhythm, and junctional tachycardia, and illustrate their pathophysiology, risk factors, protective factors, signs and symptoms, telemetry features, other associated diagnostic tests, nursing care, and basic treatment/management
- identify ventricular arrhythmias on telemetry, including premature ventricular contractions, idioventricular rhythms, ventricular tachycardia, Torsades de Pointes, ventricular fibrillation, asystole, and pulseless electrical activity, and illustrate their pathophysiology, risk factors, protective factors, signs and symptoms, telemetry features, other associated diagnostic tests, nursing care, and basic treatment/management
- identify heart blocks on telemetry, including first-degree, second-degree type I, second-degree type II, or third-degree SA and AV blocks, as well as bundle branch blocks, and illustrate their pathophysiology, risk factors, protective factors, signs and symptoms, telemetry features, other associated diagnostic tests, nursing care, and basic treatment/management
Healthcare providers (HCPs) across various clinical environments will care for patients with known cardiac arrhythmias or risk factors for future arrhythmias. Arrhythmias encompass a broad spectrum of cardiac abnormalities related to heart rate (HR) and rhythm. The most common way to categorize arrhythmias is based on the rate of conduction, including bradyarrhythmia and tachyarrhythmia. Arrhythmias are further categorized based on their origin, means of transmission, and associated clinical syndromes. The prevalence of arrhythmias within the general population is 1.5% to 5%, with atrial fibrillation (AFib) the most commonly occurring arrhythmia. However, these statistics underrepresent the true prevalence of arrhythmias since many are paroxysmal (i.e., sudden and short in duration) and asymptomatic. Patients experiencing arrhythmias may exhibit significant variations in clinical presentation, ranging from asymptomatic to cardiac arrest. Given that arrhythmias are associated with higher morbidity and mortality, HCPs must be adequately prepared to recognize signs and symptoms of various arrhythmias, including the principles of telemetry monitoring and arrhythmia identification. In addition, HCPs must understand evaluation and treatment/management strategies for all types of arrhythmias. Arrhythmias that are left untreated can lead to damage to the heart, brain, and other organs (Desai & Hakouli, 2023; Levy & Olshansky, 2024; National Institute of Health, 2022).
Anatomy and Physiology
The heart is a strong, muscular pump that circulates blood throughout the body. Each day, the heart beats approximately 100,000 times and pumps 2,000 gallons of blood. The heart consists of four chambers: the right atrium, which accepts deoxygenated blood from the body via the superior and inferior vena cava; the right ventricle, which pumps that deoxygenated blood to the lungs via the pulmonary artery; the left atrium, which accepts newly oxygenated blood from the pulmonary veins; and the left ventricle, which pumps oxygenated blood to the rest of the body via the aorta. Four one-way, pressure-activated valves separate these four chambers. The tricuspid valve is a three-flap valve that separates the right atrium from the right ventricle. The pulmonary valve is a three-flap valve that separates the right ventricle from the pulmonary artery. The mitral valve is a double-leaflet valve that separates the left atrium from the left ventricle; it may also be called the bicuspid valve because of its construction. Finally, the aortic valve is a three-flap valve that separates the left ventricle from the aorta (refer to Figure 1; American Heart Association [AHA], 2024e; Hinkle et al., 2021; Rehman & Rehman, 2023).
Figure 1
Anatomy of the Heart
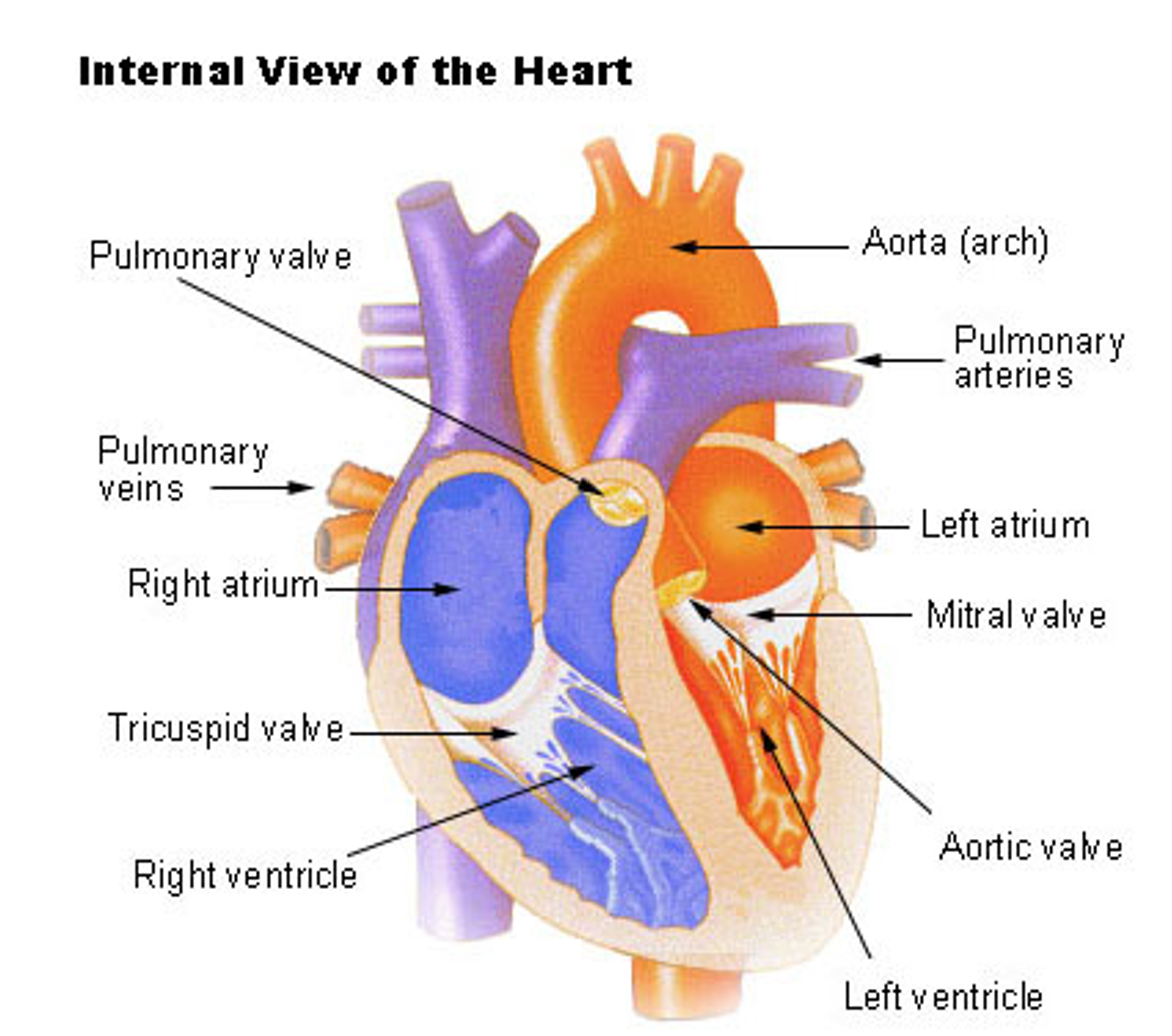
(National Cancer Institute, n.d.)
The contraction of the heart muscle is a highly coordinated and precisely timed series of events. During diastole, the atria fill from the venous system (i.e., the superior vena cava and left pulmonary veins), and passive filling of the ventricles occurs. The atria contract simultaneously, filling the ventricles with the remainder of the blood via the open mitral and tricuspid valves, also known as the atrioventricular (AV) valves. During systole, the full ventricles contract, closing the mitral and tricuspid valves (creating the S1 sound) and opening the aortic and pulmonary valves (or semilunar valves) to allow the blood into the pulmonary arteries and aorta. Once empty, decreased pressure inside the ventricles causes the semilunar valves to close again while the atria fill. This valve closure causes the second heart sound, or S2, which marks the start of diastole. Ventricular filling may be heard as S3 or, if the ventricles are resistant and filling is slow, S4 (refer to Figure 2; AHA, 2024e; Hinkle et al., 2021; Mitchell, 2025; Oberman et al., 2023; Rehman & Rehman, 2023).
...purchase below to continue the course
An electrical impulse, also known as nature’s pacemaker, initiates the sinoatrial (SA) node contraction. The impulse is transmitted from the SA node through the atria via Bachman’s bundle and the internodal pathways to the AV node. The impulse passes through the AV node down through the ventricles via the bundle of His, the right and left bundle branches, and the Purkinje fibers. A healthy, properly functioning SA node will keep the heart pumping between 60 and 100 beats per minute (bpm). If the SA node becomes damaged and is unable to send an impulse, the AV node, the heart’s first backup system, will create its own electrical impulse of 40–60 bpm. Finally, the Purkinje fibers can also act as a tertiary pacemaker, but only at 20–40 bpm. The ability of cardiac cells to contract after receiving a signal is called contractility. This electrical signal has a depolarizing effect on the cardiac cells. The ability of a cardiac cell to initiate an impulse is called automaticity, while the ability to react to the impulse is called excitability. The ability of a cell to transmit an impulse is called conductivity. The signal from the SA node triggers rapid depolarization, which consists of sodium (Na+) quickly entering the cells and calcium (Ca+) slowly moving into the cells. Repolarization can be broken down further into three stages: early repolarization, which consists of Na+ channels closing; the plateau phase, which consists of Ca+ moving into the cell and potassium (K+) moving out of the cell; and rapid repolarization, which consists of the Ca+ channels closing and K+ moving out of the cell rapidly. The resting phase between impulses actively pumps K+ in and Na+ out of the cell. Though the cell is impermeable to Na+ diffusion during the resting phase, K+ can slowly diffuse out of the cell (refer to Figure 3; AHA, 2024e; Hinkle et al., 2021; McGee, 2024; Mitchell, 2025; Oberman et al., 2023; Rehman & Rehman, 2023).
Figure 2
Circulation of Blood Through the Heart
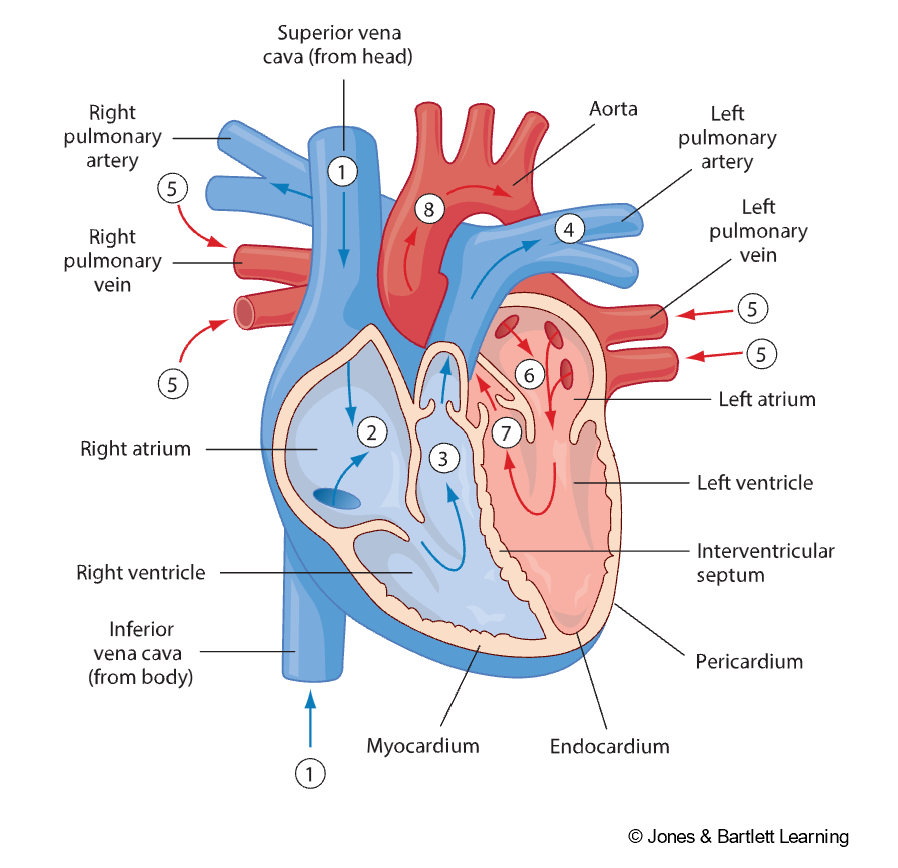
Figure 3
Basic Cardiac Electrophysiology
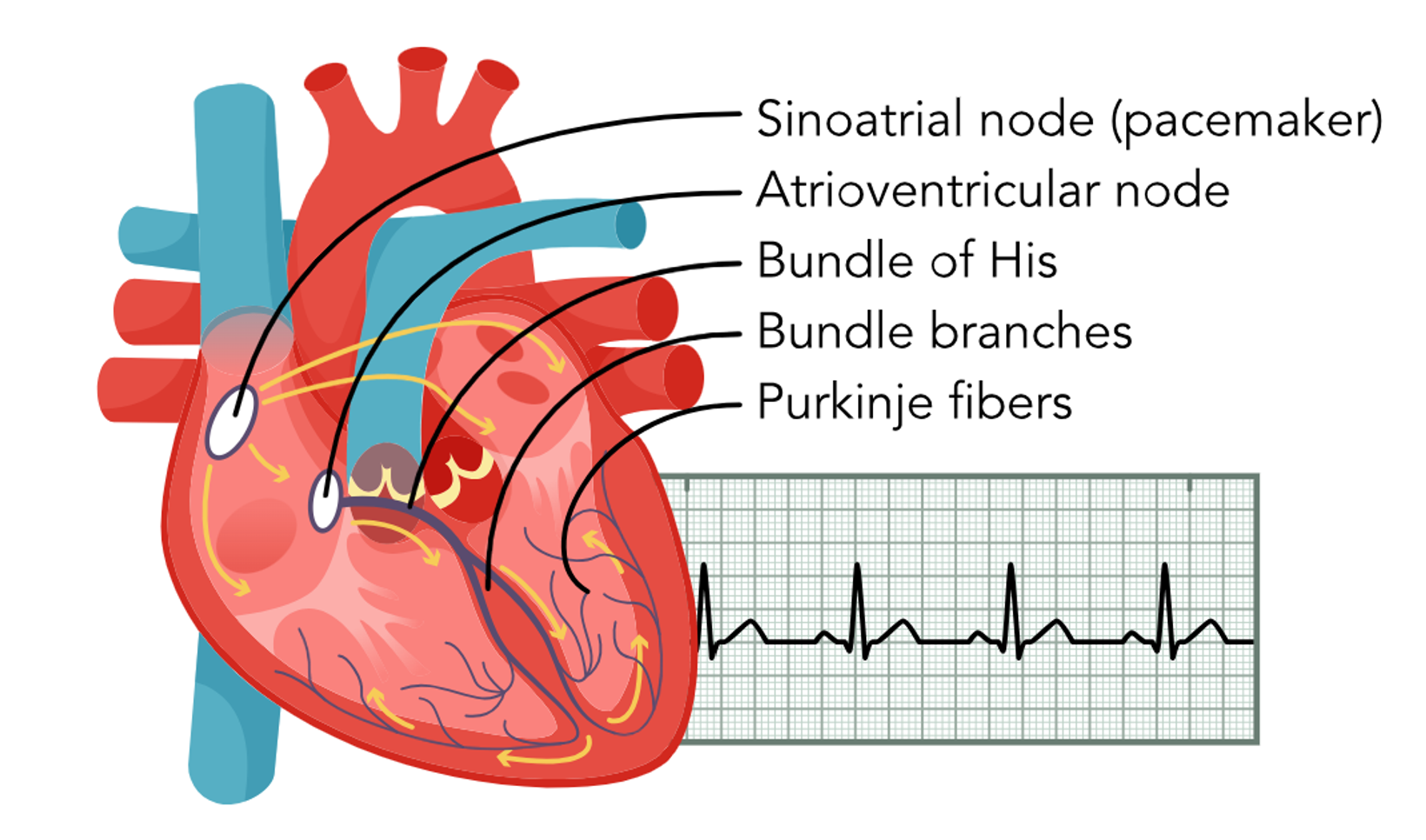
Risk Factors for Cardiac Arrhythmias
The term arrhythmia refers to abnormalities in HR or rhythm caused by changes in heart tissue and activity or in the electrical signals that control the heartbeat. Typically, arrhythmias are set off by a trigger, such as exertion or stress, and can persist if there is a problem with the heart (National Heart, Lung, and Blood Institute [NHLBI], 2022a). There can be various causes of arrhythmias, including:
- Changes to the heart
- anatomical changes
- reduction in blood flow to the heart
- damage to the heart’s electrical system
- restoring blood flow during treatment for a heart attack
- stiffening (i.e., fibrosis or scarring) of the heart tissue
- Stress or exertion
- Pain, anger, emotional stress, or startling can increase the workload of the heart, raise blood pressure, and cause the body to release stress hormones.
- Vomiting or coughing can trigger arrhythmias.
- Physical activity in individuals with heart disease can cause arrhythmias due to excess adrenaline.
- Imbalances in the blood
- Excessive thyroid hormone can cause tachycardia, while thyroid hormone deficiency can cause bradycardia.
- Dehydration can cause tachycardia.
- Hypoglycemia can cause bradycardia or extra heartbeats.
- Hypokalemia, hypomagnesemia, and hypocalcemia can cause arrhythmias.
- Medication
- Blood pressure medication, antidepressants, antipsychotics, antibiotics, and allergy or cold medicines can cause arrhythmias.
- Conduction disorders
- Electrical signals can be delayed or blocked.
- Another part of the heart produces electrical signals, disrupting the normal heartbeat (NHLBI, 2022a; National Library of Medicine, 2024).
Although signs and symptoms vary depending on the type of arrhythmia, general signs and symptoms include anxiety, blurry vision, chest pain, difficulty breathing, syncope, palpitations, confusion or difficulty concentrating, fatigue, excessive sweating, weakness, dizziness, and lightheadedness (AHA, 2024b, 2024d; Levy & Olshansky, 2024). The NHLBI and AHA list several risk factors for developing/experiencing an arrhythmia, including:
- increased age
- air pollutants (e.g., particulates and gases)
- family history or genetics
- There is an increased risk for arrhythmia if an immediate relative has been diagnosed with an arrhythmia and an increased risk with certain inherited cardiac conditions and genetic mutations (e.g., malfunctioning ion channels).
- alcohol use disorder
- tobacco or substance use (e.g., cocaine and amphetamines)
- certain medical conditions such as aneurysm, rheumatoid arthritis (ra), lupus, diabetes mellitus (DM), cardiomyopathy, eating disorders, myocardial infarctions (MIs), congestive heart failure (CHF), hypertension (HTN), hypoglycemia, chronic obstructive pulmonary disease (COPD), obesity, or obstructive sleep apnea
- race/ethnicity
- AFib is more common in White individuals; the risk for other arrhythmias is increased in Black individuals.
- sex
- AFib is more common in males, while supraventricular tachycardias (SVT) are more common in females.
- recent surgery
- Following surgery, there is an increased risk of atrial flutter in the weeks following significant heart, lung, or esophageal surgery (AHA, 2024d; Hinkle et al., 2021; NHLBI, 2022a; National Library of Medicine, 2024).
This learning activity will cover additional risk factors for specific arrhythmias later. The NHLBI also lists protective factors, including a heart-healthy lifestyle of regular exercise; a diet high in fruits, vegetables, and fiber and low in saturated fats and salt; avoiding illicit drugs, tobacco, and excessive alcohol; and managing stress on a regular daily basis (NHLBI, 2022a). HCPs should counsel patients regarding diet and lifestyle choices to limit their risk for arrhythmias, and caffeine consumption is often a recommended area in which patients can make beneficial changes. Many providers will recommend reducing caffeine intake. Although excessive caffeine may impact cardiac function, moderate intake is likely acceptable. Research on the relationship between caffeine consumption and arrhythmias has found some studies that suggest a link, while others have not supported this association. Kim and colleagues (2021) actually found that greater amounts of caffeine consumption were associated with a lower risk of arrhythmias.
The HCP should pay close attention to the auscultation of heart sounds, palpating peripheral pulses, and auscultating the apical HR if necessary for patients with a known or suspected arrhythmia. While auscultating, count the HR and note any irregularity in the heart rhythm and any audible murmurs. The HCP should check for lower extremity swelling, indicating CHF or fluid overload. The lungs should be auscultated, noting any crackles or other adventitious sounds. The nurse should ask the patient for a full history of present illness and all medications they are taking, including prescribed, over-the-counter (OTC), and herbal medications. In addition, the HCP should also gather information on signs, symptoms, and risk factors for arrhythmias (i.e., coughing may indicate exposure to pollutants or tobacco smoke). When completing the health history, physical activity, eating habits, and family history are also important to gather (AHA, 2024a; Hinkle et al., 2021; NHLBI, 2022b; Zimetbaum, 2023).
Blood work may help determine the cause or risk factors for arrhythmias, including thyroid-stimulating hormone (TSH), a metabolic profile with electrolytes, a toxicity screen, and any therapeutic medication levels, as these are common reversible causes of many arrhythmias. A chest x-ray may be ordered to assess for various lung abnormalities and determine the presence of cardiomegaly. An electrocardiogram (ECG) is the primary screening test for detecting arrhythmias. More invasive testing may be done if abnormalities are seen in labs, imaging, or ECG monitoring. This may include an echocardiogram (echo) to assess the heart’s size, shape, and function; a cardiac catheterization to assess for complications of heart disease; or an electrophysiology study (EPS) to assess the heart’s electrical activity. Cardiac catheterization carries an increased risk of bleeding, infection, vessel or heart damage, blood clot formation/embolization, or the development of a new arrhythmia. In an EPS, a wire is inserted to stimulate the heart and trigger an arrhythmia to assess risk and test potential treatments. Finally, a stress test may be ordered to assess for arrhythmias when the heart is working hard. A Holter or event monitor can be used to record the heart’s electrical activity over a longer period for patients who report transient palpitations. Another option is an implantable loop recorder placed under the skin to continuously record the heart’s electrical activity and transmit that information to the HCP (AHA, 2024a; Hinkle et al., 2021; Levy & Olshansky, 2024; NHLBI, 2022b).
Telemetry Monitoring Basics
Telemetry, or ECG monitoring, has become more widely used in hospital settings. The need for more ECG monitoring has moved beyond simple rhythm determination to include the diagnosis of complex arrhythmias, detection of MIs, and identification of drug-induced prolonged QT intervals. The AHA published guidelines in 2004 regarding patients that are appropriate candidates for ECG monitoring. Since then, the AHA has updated these guidelines (last in 2017) to address the overuse of ECG monitoring more comprehensively in some populations, the underuse of ST-segment and QT-interval monitoring in select populations, alarm fatigue, and documentation in the electronic health record (EHR). These guidelines exclude patients admitted to the intensive care unit (ICU), as these patients are more complicated and often require continuous monitoring. On a medical-surgical floor, the decision for telemetry monitoring is more nuanced (Sandau et al., 2017). The AHA outlines four primary rationales for monitoring a patient with telemetry on a medical or surgical floor in a hospital:
- to detect cardiac arrest sooner and therefore reduce the time to defibrillation
- to recognize deteriorating conditions (i.e., early afterdepolarizations or non-sustained arrhythmias)
- to facilitate the management of arrhythmias (even if not life-threatening)
- to facilitate the diagnosis of an arrhythmia or a cause for specific signs/symptoms (Sandau et al., 2017)
In addition to the general goals of telemetry monitoring, the AHA guidelines recommend monitoring in specific discrete patient populations. Cardiac arrest is the leading cause of death and the most common cause of death after an MI in adults in the United States. There is a substantial risk of death in the early hours to days after an MI, highlighting the importance of early recognition, evaluation, and ECG monitoring for patients with acute ischemic events. The most recent AHA (Sandau et al., 2017) recommendations for ECG monitoring for arrhythmias include:
- immediate monitoring for all patients at immediate or high risk of acute coronary syndrome (ACS) and those with documented ST-segment elevated MI (STEMI); monitoring should continue uninterrupted for 24 to 48 hours or until negative biomarkers (i.e., troponin) or successful revascularization
- monitoring not indicated for patients with ACS symptoms determined to be low-risk or noncardiac in origin
- immediately on presentation of an mi and continued for 12 to 24 hours after reperfusion
- immediately after an MI with no reperfusion or revascularization, continued for 24 to 48 hours until there is no evidence of ongoing modifiable ischemia or hemodynamic or electric instability
- patients treated with therapeutic hypothermia after cardiac arrest
- patients with vasospastic angina, until symptoms resolve
- patients with left ventricular apical ballooning, until symptoms resolve
- patients with a newly recognized critical left coronary artery stenosis while awaiting revascularization
- after nonurgent percutaneous coronary intervention (PCI) with suboptimal results or complications, beginning immediately and continuing for over 24 hours until the complication is resolved (monitoring after routine angiography is not recommended in low-risk patients beyond the immediate post-procedure area)
- patients with uncomplicated open-heart surgery for a minimum of 48 to 72 hours postoperatively; for patients at high risk for AFib, monitoring should be done for the duration of the hospital stay
- patients who are hemodynamically unstable with an immediate need for mechanical circulatory support
- patients in the postoperative period after ventricular assist device (VAD) implantation; monitoring is not indicated for patients with VADs in rehabilitation facilities or when admitted with noncardiac problems
- patients with a transcatheter aortic valve replacement (TAVR) should be monitored at least 3 days post-procedure and longer in patients with periprocedural conduction abnormalities
- patients undergoing transcatheter intervention should be monitored after the procedure (duration depends on the procedure and patient factors)
- patients with life-threatening ventricular arrhythmias, resuscitated from cardiac arrest, or unstable ventricular tachycardia (V-tach) should be monitored until implantable cardioverter-defibrillator (ICD) implantation or reversible cause is corrected
- patients with premature ventricular contractions (PVCs) and nonsustained V-tach, monitoring is not required unless there are other indicators present
- patients with new-onset or recurrent atrial tachyarrhythmias should be monitored throughout the evaluation; patients with symptomatic or hemodynamically unstable atrial arrhythmias should be monitored until stable; monitoring should also be done if rate control is deemed necessary
- monitoring is not indicated for patients with known AFib who have adequate rate control
- patients with symptomatic bradycardia (e.g., syncope) or if worsening baseline sinus bradycardia may be a concern when initiating chronotropic medications; monitoring is not recommended for asymptomatic or hemodynamically stable bradycardia
- inpatient initiation of the following antiarrhythmic medications: dofetilide (Tikosyn), sotalol (Betapace), flecainide (Tambocor), propafenone (Rythmol); monitoring may be considered for amiodarone (Cordarone) and dronedarone (Multaq) initiation
- patients with symptomatic second-degree AV block, asymptomatic second-degree AV block caused by distal conduction system disease, and third-degree AV block; patients with Wenckebach or transient AV block of any degree determined to be of vagal origin do not need monitoring
- patients with congenital or genetic arrhythmia syndromes who are hemodynamically unstable until appropriate therapy is delivered
- patients with Wolff-Parkinson-White (WPW) syndrome with rapid conduction via an accessory pathway until antiarrhythmic or ablation therapy is delivered
- patients with a congenital long QT interval who are unstable should be monitored until stabilization of ventricular arrhythmias, reversal of exacerbating metabolic or medical conditions, and return of QTc to baseline
- patients who are admitted for syncope with suspected cardiac origin should be monitored for at least 24 hours
- patients with uncomplicated SVT ablation may be discharged home after a short observation period
- patients with complex ablations (e.g., pulmonary vein isolation for AFib) or serious comorbidities (e.g., heart failure) should be monitored for 12 to 24 hours after the procedure; this includes patients receiving AV nodal ablation who experience prolonged tachycardia and those with chronic AFib with concomitant pacemaker implantation
- patients who receive transcutaneous pacing or standard temporary transvenous wires should be monitored until it is no longer required or the device is replaced with a permanent device
- patients receiving semipermanent transvenous pacing should be monitored for 24 hours
- patients receiving a permanent pacemaker or ICD should be monitored for 12 to 24 hours after implantation
- patients admitted after an ICD shock should be monitored for the duration of the hospital stay; monitoring is not recommended for patients with an ICD admitted for noncardiac conditions who do not meet another indication
- monitoring is not recommended for patients with a wearable cardiac defibrillator who are admitted for noncardiac reasons
- patients with acute decompensated heart failure should be monitored until the precipitating event is resolved
- patients with infective endocarditis should be monitored until clinically stable
- patients undergoing conscious sedation should be monitored until awake, alert, and hemodynamically stable
- monitoring is not recommended after noncardiac surgery for asymptomatic patients; exceptions include major thoracic surgery (e.g., pulmonary resection) who should be monitored for two to three days after surgery and patients with risk factors for afib
- patients admitted with stroke should be monitored for 24 to 48 hours
- patients with moderate to severe potassium or magnesium imbalance
- patients should be monitored following a drug overdose until they are clinically stable and free from the influence of the drug (i.e., psychotropic drugs, opiates, inhalants, cocaine, stimulants, and other drugs)
- efficacy of monitoring patients admitted to the hospital who receive chronic hemodialysis is not well established (Sandau et al., 2017)
Despite the comprehensive list of indications for ECG monitoring, initial ECG monitoring is often prompted by the patient’s report of signs or symptoms. In most cases, ECG monitoring is not prompted by a change in vital signs. Therefore, HCPs must pay close attention when taking a patient history to recognize and respond to signs and symptoms of arrhythmias. Research has also indicated that patient outcomes improved significantly across the entire hospital system when nurses and other staff were adequately and regularly educated about the various signs and symptoms of an MI or arrhythmia. HCPs should also be educated about ECG overuse, which can lead to increased financial costs, alarm fatigue, and increased length of stay. Studies have shown that up to 20% of ECG monitoring orders are for non-cardiac indications, which can be inappropriate (Chakravarthy et al., 2020; Mechanic et al., 2023).
The AHA revised the 2004 guideline recommendations for ST-segment ECG monitoring. The 2004 guidelines recommended continuous ST-segment monitoring for four patient populations:
- during the early phase of ACS
- patients presenting to the emergency department (ED) with chest pain
- after nonurgent PCI with suboptimal results
- possible variant angina caused by coronary vasospasm (Sandau et al., 2017)
The benefit of continuous ST-segment monitoring includes the early identification of ischemic events, which leads to prompt revascularization and prevention of myocardial tissue damage. However, continuous ST-segment monitoring can lead to false (i.e., no valid triggering event) and nonactionable (i.e., no clinical relevance) alarm signals. These false and nonactionable signals have led to alarm fatigue and sentinel events (Sandau et al., 2017).
The AHA guidelines call for technology and protocol improvement for telemetry monitoring while also addressing and exploring remedies for alarm fatigue (Sandau et al., 2017). Studies have shown that implementing the AHA guidelines can lead to a decrease in not only costs but also alarms and alarm fatigue. Telemetry use in inpatient medical units has been identified as an area of resource overutilization, which can be costly and have a negative impact on patient outcomes. Telemetry overutilization can cost $54 per patient per day, or approximately $1,400 per day for a hospital. Additional costs can be accrued related to increased length of stay and decreased hospital throughput. Pendharkar and colleagues (2020) utilized a Plan-Do-Study-Act approach to evaluate the impact of AHA guideline criteria for telemetry use. With this new protocol in place, they found that the average days on telemetry decreased from 7.20 to 3.51, while the number of patients on telemetry for appropriate diagnoses increased.
12-Lead ECG
To better identify an abnormal rate or rhythm, it is essential to understand regular cardiac rate and rhythm. As previously mentioned, a standard rate generated from the SA node is 60–100 bpm. Rhythm is more complicated to define, but not impossible. Because the electrical impulses in the heart trigger contraction, the heart's rhythm can easily and non-invasively be determined and monitored electronically via electrodes placed on the skin's surface in specific locations. These electrodes sense and record the electrical currents from the heart and use that information to graph a heart rhythm. A classic example of this is the 12-lead ECG, which uses 12 different views to electronically create a complete picture of the heart. An ECG is done using six limb leads and six precordial (V) leads. The limb leads provide information about the heart’s frontal (vertical) plane using three bipolar leads (I–III) and three unipolar (aVR, aVL, and aVF). All six limb leads produce a positive deflection on ECG apart from aVR, which creates a negative deflection (upside-down waves). The precordial leads (V1-6) are unipolar and provide information about the heart’s horizontal plane. A modified chest lead (MCL1-6) may be used instead of a precordial lead. They give the same information about the horizontal plane of the heart but are bipolar. A rhythm strip is a detailed picture of the electrical information gathered simultaneously from one or more lead(s). The transmission of the electrical activity to a computer that generates the pictograph can be hardwired, as is commonly seen in EDs and ICUs, or transmitted from a small portable box to a main computer for monitoring, as is seen in telemetry (Hinkle et al., 2021; McGee, 2024; Sattar & Chhabra, 2023; Shank Coviello, 2020). The 12 leads of an ECG are characterized as follows (Hinkle et al., 2021; McGee, 2024; Sattar & Chhabra, 2023; Shank Coviello, 2020):
- Lead I: a bipolar limb lead with a current that runs from right to left, with a negative electrode on the right arm (RA) and a positive electrode on the left arm (LA) or chest. It helps detect atrial arrhythmias and hemiblocks.
- Lead II: a bipolar limb lead with a current that runs from the negative electrode on the RA or below the right clavicle to a positive electrode down on the left lower extremity (LLE) or below the lowest palpable rib on the left midclavicular line. It produces a high-voltage deflection on ECG with tall P, R, and T waves. It is helpful for routine monitoring or to detect sinus or atrial arrhythmias.
- Lead III: the final bipolar limb lead with a current that runs down from the negative electrode on the LA to the positive electrode on the LLE. It is beneficial in detecting inferior wall MIs.
- aVR: an augmented unipolar limb lead with a negative deflection (the only limb lead with negative deflection). The positive electrode should be placed on the RA.
- aVL: an augmented unipolar limb lead with a positive deflection, the positive electrode on the LA, and useful in detecting lateral wall infarctions.
- aVF: an augmented unipolar limb lead with a positive deflection, the positive electrode on the LLE (or “foot”), and useful in detecting inferior wall infarctions.
- V1: the first precordial (or chest) lead, unipolar, biphasic, with the electrode placed to the right of the sternum at the 4th intercostal space. It provides views of the P wave, QRS complex, and ST-segment, and detects ventricular arrhythmias, ST-segment changes, a bundle branch block (BBB), and ectopic beats.
- V2: the second precordial (or chest) lead, unipolar, biphasic, with the electrode placed to the left of the sternum at the 4th intercostal space. It identifies ST-segment elevation.
- V3: the third precordial (or chest) lead, unipolar, biphasic, with the electrode placed on the left half of the chest, between the sternum and the midclavicular line at the 4th/5th intercostal space. It detects ST-segment elevation.
- V4: the fourth precordial (or chest) lead, unipolar, biphasic, with the electrode placed on the left half of the chest, at the midclavicular line, and the 5th intercostal space. It is useful in detecting ST-segment and/or T wave changes.
- V5: the fifth precordial (or chest) lead, unipolar, positive deflection, with the electrode placed on the left half of the chest, at the anterior axillary line, and the 5th intercostal space. It is useful in detecting ST-segment and/or T wave changes.
- V6: the sixth precordial (or chest) lead, unipolar, positive deflection, with the electrode placed on the left half of the chest, at the midaxillary line, and the 5th intercostal space.
As previously mentioned, the MCLs may be used in conjunction with the limb leads instead of the precordial leads. The MCL1 is like V, but the negative electrode is placed on the left upper chest, the positive electrode is placed just to the right of the sternum at the 4th intercostal space, and a ground electrode is placed on the right upper chest just below the clavicle. This placement produces a negative deflection waveform and helps detect PVCs, V-tach, SVT, bundle branch defects, and P wave changes, or can confirm pacemaker wire placement. MCL6 is another alternative to MCL1 to monitor ventricular conduction. The negative lead is placed below the left shoulder; the positive electrode is placed on the left half of the chest at the midaxillary line and the 5th intercostal space (same location as V6). A ground electrode is placed just below the right shoulder. As previously mentioned, in most cases, only one lead will be monitored at a time, and proper selection of the most appropriate lead for a particular patient is key to successful cardiac monitoring. The AHA recommends the V1 lead when attempting to distinguish between V-tach and aberrancy in adult patients. The AHA recommends that lead II be used in pediatric patients, as supraventricular arrhythmias are more common in children than ventricular arrhythmias and P waves are best visible in inferior leads (Hinkle et al., 2021; McGee, 2024; Sandau et al., 2017; Sattar & Chhabra, 2023; Shank Coviello, 2020).
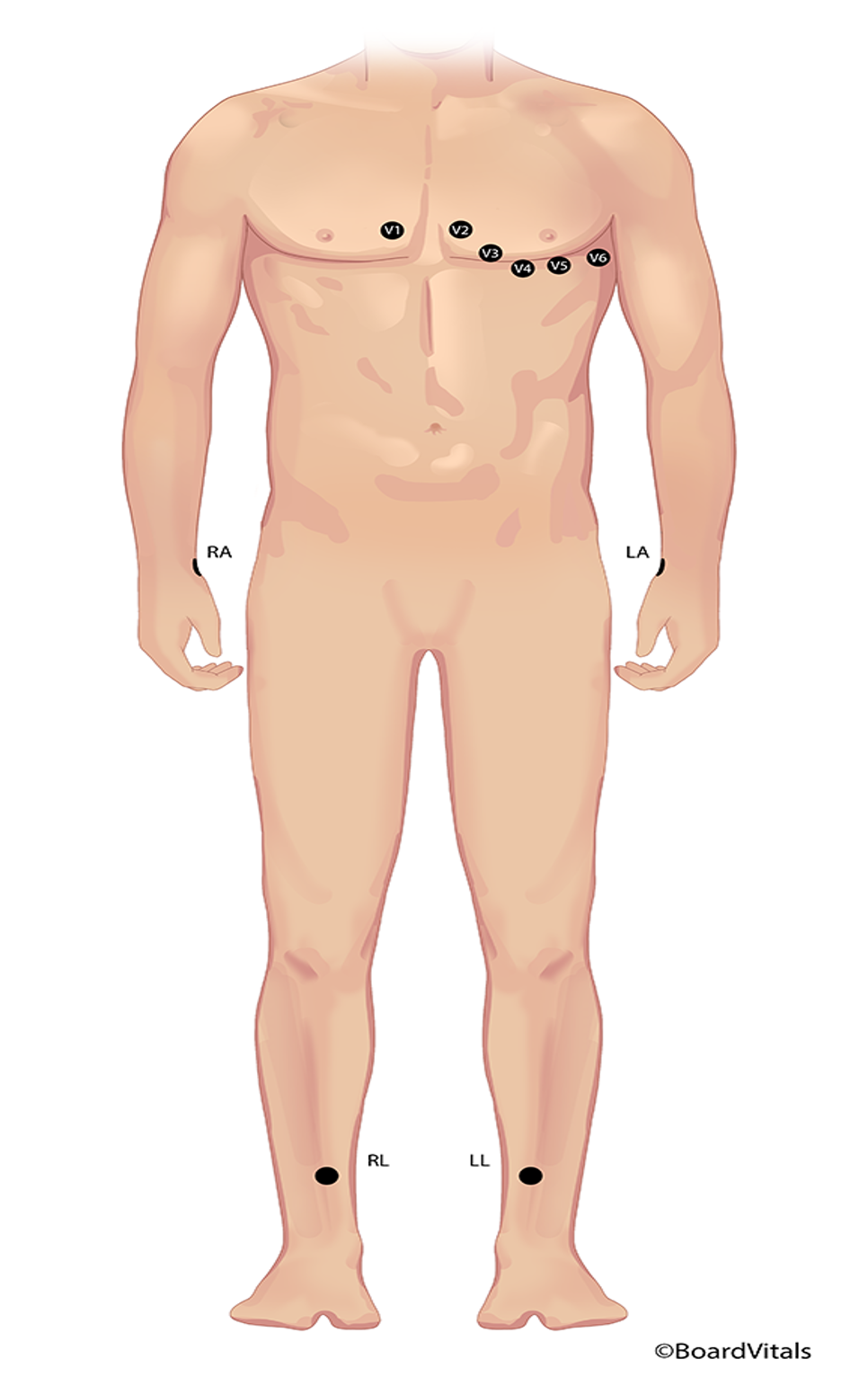
Before applying electrodes, the skin should be prepped. It should be washed, dried, and hair trimmed, and if needed, the skin may be rubbed briskly with a dry, clean washcloth to remove dead skin cells and improve electrode contact. If the electrode has a snap design, the lead wire should be attached to the electrode before applying it to the patient. If the electrode has a clip design, it can be applied to the patient first, and the lead wire can be attached second. Ten electrodes are placed in a traditional 12-lead ECG: on the RA, right lower extremity (RLE), LA, LLE, and V1-6 positions. While this is a complete picture of the heart, this system is not conducive to ambulatory patients who may need monitoring for hours or even days (refer to Figure 4; Hinkle et al., 2021; McGee, 2024; Sattar & Chhabra, 2023; Shank Coviello, 2020).
Figure 4
Traditional Ten-Electrode Placement for ECG Monitoring
The Mason-Likar system is a variation in which the four limb leads are moved to the torso to reduce motion artifact (refer to Figure 5). The RA/LA electrodes are placed medial to the deltoid muscle in the infraclavicular fossa about 2 cm below the clavicle, while the RLE/LLE electrodes are placed along the anterior axillary line halfway between the ribs and the iliac crest. The AHA guidelines recommend this variation to allow increased mobility and reduced artifact (Hinkle et al., 2021; Sandau et al., 2017; Shank Coviello, 2020).
Figure 5
The Mason-Likar System
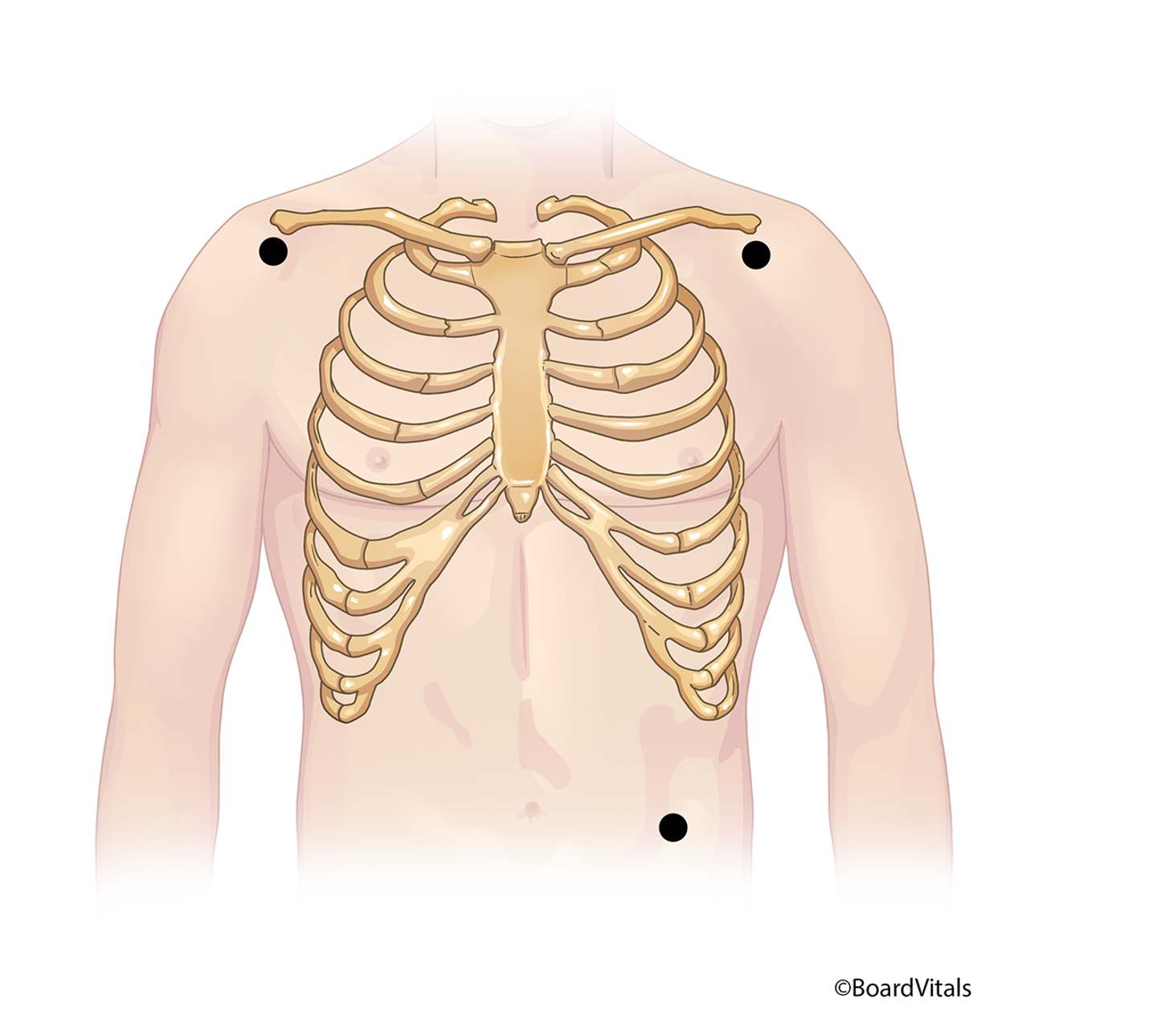
There are three streamlined options for placing lead wires on ambulatory patients, depending on the ECG monitoring system used in an individual facility. A three-lead wire system (refer to Figure 6), which is the simplest system, includes a positive, a negative, and either a left lower (LL) or a ground electrode, all of which can be placed and adjusted depending on the desired lead to be monitored (Shank Coviello, 2020).
Figure 6
Three-Lead Electrode Placement
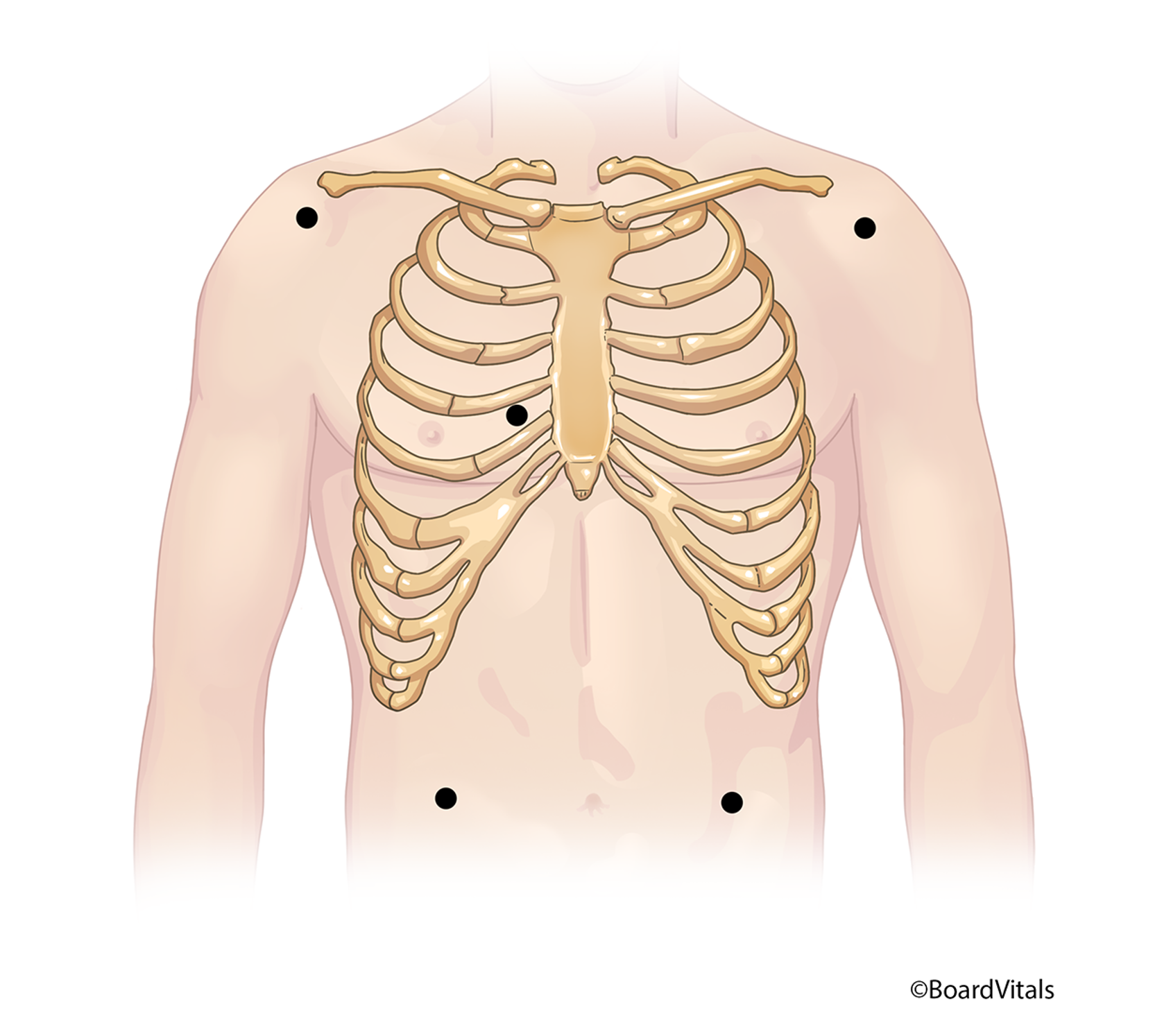
A five-lead Mason-Likar system (refer to Figure 7) utilizes an RA, right lower (RL; ground), LA, LL, and a single chest (C) electrode. Electrode positions may be identical for multiple leads, so a lead selector switch on the monitor needs to be adjusted instead of the electrodes themselves (Shank Coviello, 2020).
Figure 7
Five-Lead Electrode Placement
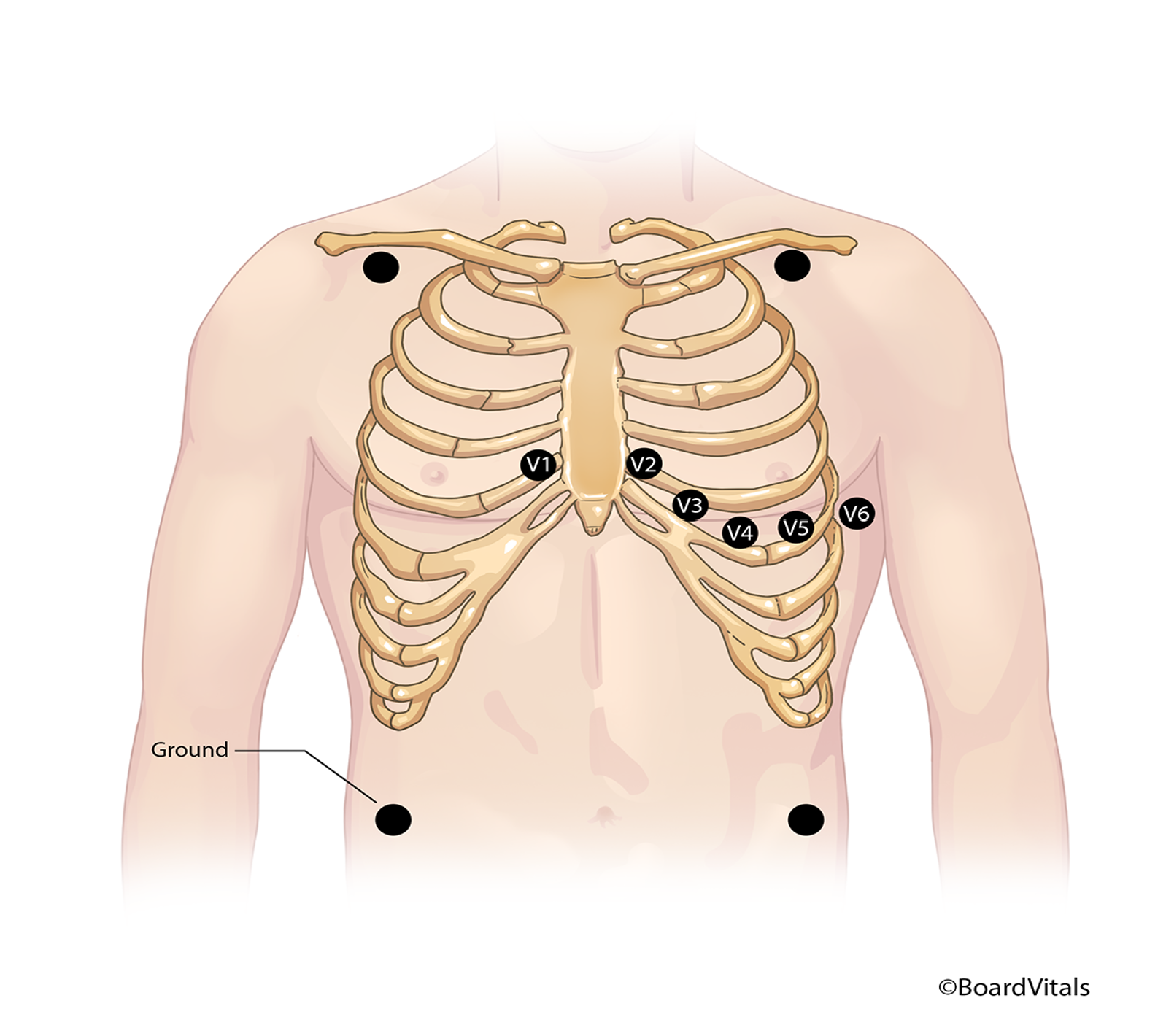
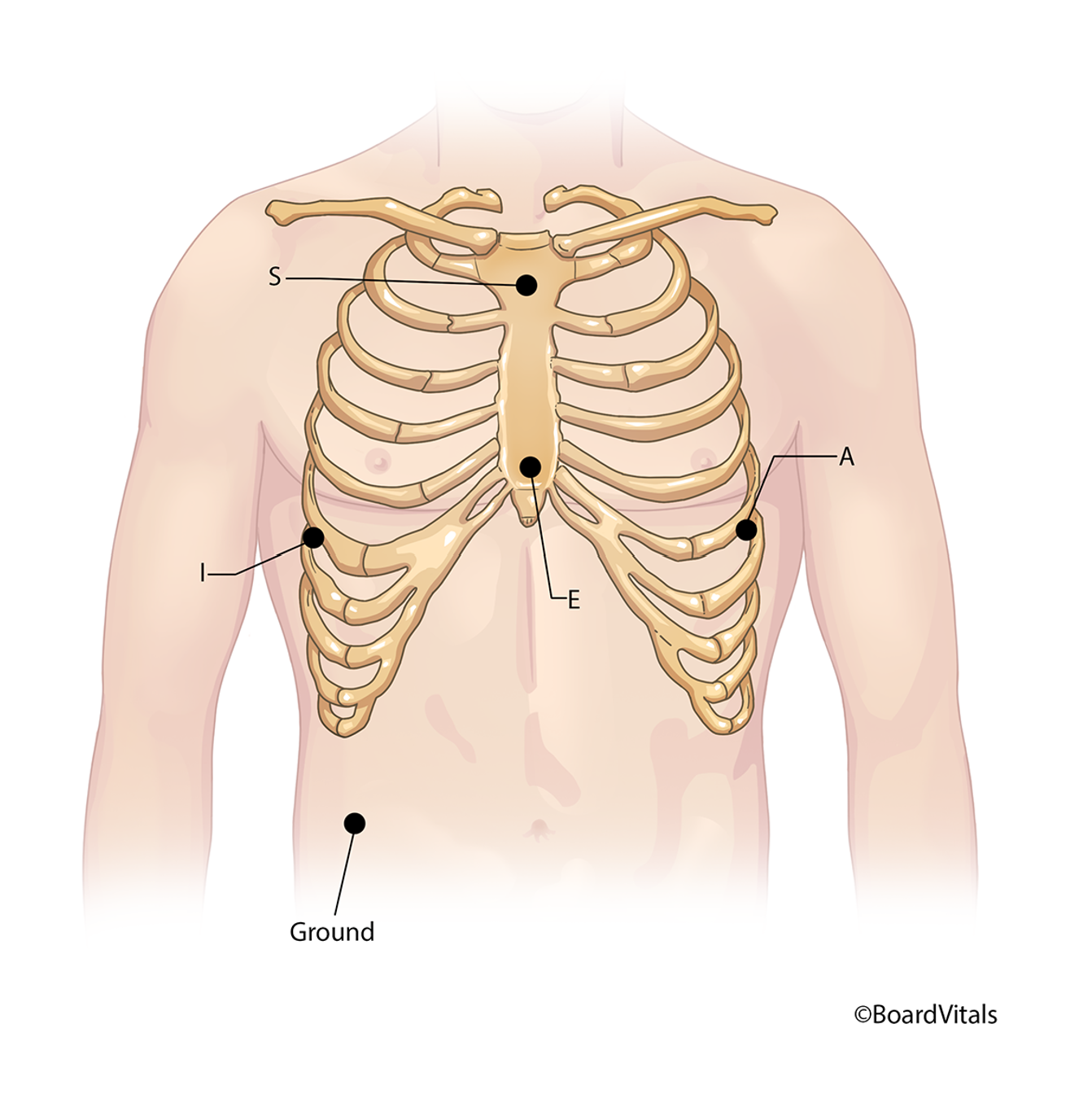
A third alternative is the five-lead wire EASI system (refer to Figure 8), which uses just five lead wires to project a complete three-dimensional view of the heart’s conduction, just as a 12-lead ECG would do (Shank Coviello, 2020). This option places:
- the E lead in the center of the sternum at the 5th intercostal space
- the A lead on the left midaxillary line at the 5th intercostal space
- the S lead in the center of the upper sternum at about the first intercostal space
- the I lead at the right midaxillary line at the 5th intercostal space
- a ground lead placed anywhere on the torso (Shank Coviello, 2020)
Figure 8
The Five-Lead EASI System
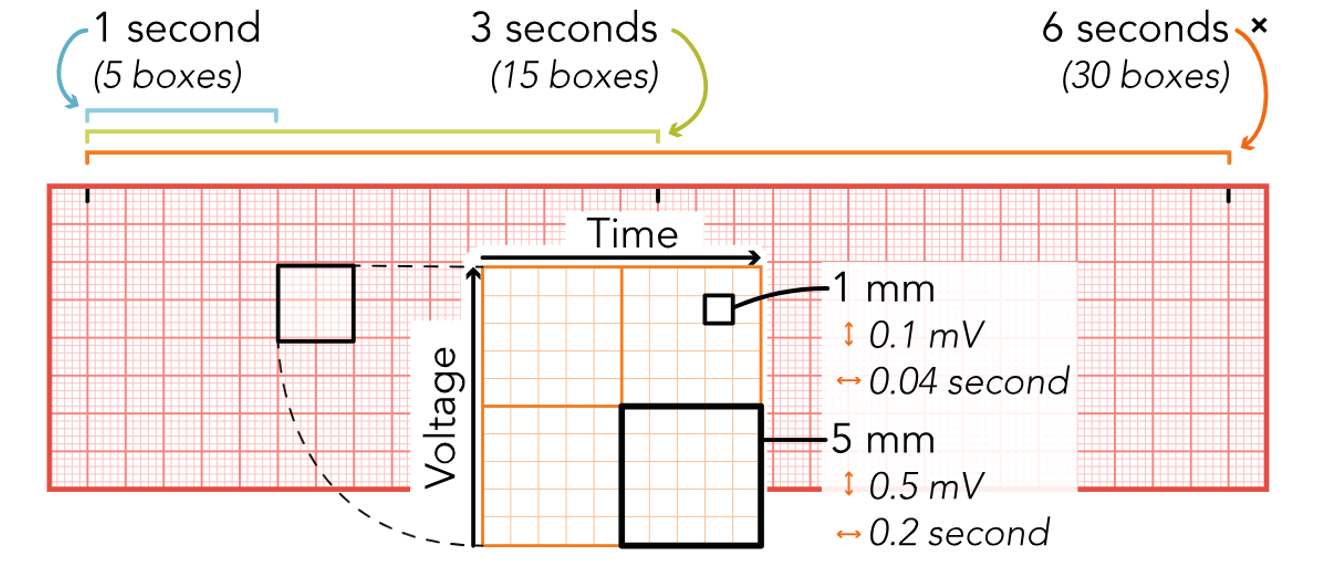
Once a readout has been produced, it should be printed and labeled with the patient’s name and ID number, date, and time (if not already included). The HCP’s interpretation of the patient’s cardiac rhythm may also be noted on the printout, along with any current medications, signs or symptoms, or activities affecting the rhythm. For many health care institutions, this will be documented electronically in the EHR. The measurements are standardized when reading an ECG printout or telemetry strip (refer to Figure 6). The horizontal axis represents time in seconds (s), while the vertical axis represents amplitude in millimeters (mm) or voltage in millivolts (mV). A small box or square represents 0.04s and 1 mm or 0.1 mV. Five small boxes make up one large box, representing 0.2sand 5 mm or 0.5 mV. Based on these numbers, 15 large boxes make up a 3-second strip, and 1500 small squares make up one full minute (refer to Figure 9; McGee, 2024; Shank Coviello, 2020).
Figure 9
ECG Grid
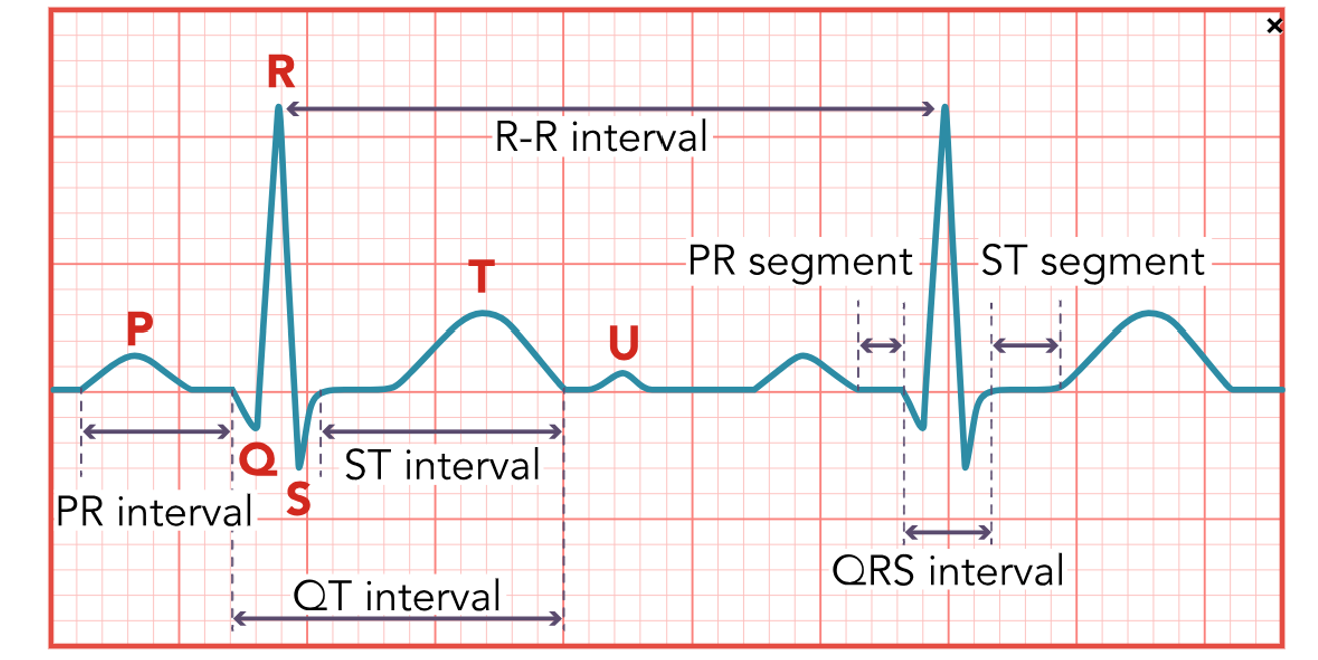
A normal heart rhythm (refer to Figure 10) has a P wave, a QRS complex, a T wave, and occasionally a U wave. Other measurements that should be considered include the PR interval, the ST segment, and the QT interval. Understanding the typical appearance of all these items helps HCPs identify abnormal characteristics. The P wave is the first component and represents atrial depolarization. The normal amplitude is 2–3 mm, and the expected duration is 0.06–0.12s. The P wave should be smooth, rounded, and upright in leads I, II, aVF, and V2-6, negative in lead aVR, and variable in leads III, aVL, and V1. If the P wave meets all these characteristics and precedes each QRS complex, the impulse originated in the SA node as expected (McGee, 2024; Sauer, 2024; Shank Coviello, 2020). Some variables might include a P wave that is:
- peaked, notched, or enlarged, which may indicate atrial hypertrophy secondary to COPD, pulmonary embolism (PE), valvular disease, or CHF
- inverted, which may indicate retrograde or reverse conduction up from the AV node
- variable, which may indicate impulses that are coming from various sites within the heart, such as with a wandering pacemaker, irritable atrial tissue, or damage near the SA node
- absent, indicating conduction by a route other than the SA node, such as a junctional rhythm, AFib, or ventricular arrhythmia (McGee, 2024; Sauer, 2024; Shank Coviello, 2020)
The PR interval is measured from the beginning of the P wave to the beginning of the QRS complex. It represents the time for the conduction impulse to travel from the atria to the AV node, and through the bundle of His and right and left bundle branches. The expected duration for the PR interval is 0.12–0.2s (twice the QRS complex). A lengthened PR interval may indicate a conduction delay, such as an AV block or digoxin (Lanoxin) toxicity. On the other hand, a PR interval that is too short may indicate the impulse did not originate in the SA node, such as with a junctional arrhythmia or preexcitation syndrome (McGee, 2024; Sauer, 2024; Shank Coviello, 2020).
The QRS complex represents the depolarization of the ventricles. The average amplitude is 5-30 mm, and the expected duration is 0.06–0.1s (half of the PR interval) measured from the beginning of the Q wave to the end of the S wave. The Q and S waves will have a negative deflection, while the R wave will have a positive deflection in leads I–III, aVL, aVF, and V4-6. The deflections will be reversed in leads aVR and V1-3 (McGee, 2024; Sauer, 2024; Shank Coviello, 2020). Some possible variations in the QRS complex include:
- a deep Q wave (greater than 25% amplitude of the R wave) or a wide Q wave (greater than 0.04s), which may indicate a possible MI
- a notched R wave, which may indicate a BBB
- a widened QRS complex (greater than 0.12s), which may mean a ventricular conduction delay
- an absent QRS complex, which may indicate an AV block or ventricular standstill (McGee, 2024; Sauer, 2024; Shank Coviello, 2020)
The ST segment represents the end of ventricular conduction and depolarization to the beginning of recovery and repolarization. It is measured from the end of the S wave (called the J point) to the beginning of the T wave. It should be isoelectric (at the established baseline, not above or below) with a positive amplitude of no more than 1mm and a negative amplitude no less than -0.5mm below the baseline. An elevated ST segment (amplitude greater than 1mm) may indicate myocardial injury. In contrast, an ST segment that is depressed (less than -0.5mm below baseline) may predict myocardial ischemia or digoxin (Lanoxin) toxicity. The AHA guidelines suggest that hospitals develop clear interdisciplinary protocols regarding which patients would benefit from ST-segment monitoring, as changes in the ST segment or the adjoining T wave are often the first indicators of myocardial ischemia. Ideally, the AHA recommends computer software that allows for the simultaneous monitoring of the ST segment on all 12 leads in these at-risk patients. They also comment that hyperkalemia, hypothermia, electrolyte abnormalities, pericarditis, BBB, or an expected reaction post-defibrillation may also cause ST-segment changes. Consult the above section for specific indications for ST-segment monitoring recommendations (McGee, 2024; Sandau et al., 2017; Sauer, 2024; Shank Coviello, 2020).
The T wave represents ventricular recovery and repolarization. It should appear round and smooth with an amplitude of 0.5 mm in leads I–III. Its deflection should be upright in leads I, II, and V3-6, negative in lead aVR, and variable in leads III, aVF, aVL, and V1-2. If the T wave is:
- taller, peaked, or tented may indicate myocardial injury or hyperkalemia
- inverted T wave in leads I, II, or V3-6 may indicate myocardial ischemia
- notched or pointed may indicate pericarditis, an inflammation of the pericardium, the double layer of connective tissue that covers the heart (McGee, 2024; Sauer, 2024; Shank Coviello, 2020)
The QT interval is measured from the beginning of the Q wave to the end of the T wave, and represents ventricular depolarization and repolarization. The typical duration is 0.36–0.44s but can vary with age, sex, and HR. It should be less than half the time between consecutive R waves in any patient. If a patient’s QT interval is shortened, this may indicate digoxin (Lanoxin) toxicity or hypercalcemia. On the other hand, a prolonged QT interval puts the patient at increased risk for a serious arrhythmia called Torsades de Pointes (TdP; McGee, 2024; Sauer, 2024; Shank Coviello, 2020). TdP may be caused by medications that prolong the QT interval, including:
- antiarrhythmics (e.g., amiodarone [Cordarone, Pacerone], disopyramide [Norpace], dofetilide [Tikosyn], ibutilide [Corvert], procainamide [Procan], quinidine [Quinora], sotalol [Betapace])
- antidepressants (e.g., amitriptyline [Elavil], desipramine [Norpramin], fluoxetine [Prozac], sertraline [Zoloft])
- antipsychotics (e.g., chlorpromazine [Thorazine], haloperidol [Haldol], thioridazine [Mellaril])
- antibiotics (e.g., clarithromycin [Biaxin], erythromycin [Erythrocin], levofloxacin [Levaquin])
- antiemetics (e.g., dolasetron [Anzemet], droperidol [Inapsine])
- antifungals (e.g., ketoconazole [Nizoral])
- antimigraine medications (e.g., sumatriptan [Imitrex])
- opioid agonists (e.g., methadone [Methadose, Dolophine]; McGee, 2024; Sauer, 2024; Shank Coviello, 2020)
In addition to the above list, the AHA also cautions about the risk of QT prolongation with azithromycin (Zithromax), ciprofloxacin (Cipro), moxifloxacin (Avelox), citalopram (Celexa), escitalopram (Lexapro), ondansetron (Zofran), and fluconazole (Diflucan). The AHA also recommends monitoring and recording the corrected QT (QTc) by correcting the QT interval for HR. Regular ECG monitoring is recommended in patients who have recently started antiarrhythmics such as dofetilide (Tikosyn), ibutilide (Corvert), sotalol (Betapace), disopyramide (Norpace), procainamide (Procan), quinidine (Quinora), amiodarone (Cordarone), dronedarone (Multaq), and flecainide (Tambocor); patients with a history of prolonged QTc or risk factors for TdP; patients undergoing targeted temperature management (therapeutic hypothermia); patients with inherited prolonged QT with unstable ventricular arrhythmias; patients with medically or metabolically induced prolonged QTc; patients with drug overdose or toxicity; and patients with moderate to severe hypokalemia or hypomagnesemia (Sandau et al., 2017).
The U wave is not consistently seen, but when present it represents the recovery and repolarization of the Purkinje fibers. It should be upright and rounded. If it is prominent, it may indicate hypercalcemia, hypokalemia, or digoxin (Lanoxin) toxicity (McGee, 2024; Sauer, 2024; Shank Coviello, 2020).
Figure 10
Normal ECG
Interpreting a telemetry strip will become faster and easier with repetition, but following a stepwise approach can be helpful initially. This process is one such approach (McGee, 2024; Sauer, 2024; Shank Coviello, 2020).
- Determine the rhythm: Measure the distance between consecutive P and R waves using paper and pencil or a caliper. A variation of less than 0.04s is okay. Are they regular (consistently spaced)? If not regular, is there a pattern?
- Determine the rate: This can be done in several ways. First, count the number of P (atrial rate) and then R (ventricular rate) waves in a 6-second strip and multiply by 10. Another option is to count the number of small boxes/squares between consecutive P waves and R waves and divide by 1500 (the number of small squares in one minute). The sequence method estimates HR based on the thicker black lines that outline the big squares/boxes. Locate a P wave that lands on a thicker black line. Locate the next P wave and determine how many big boxes are between them:
- one big box: HR of 300
- two big boxes: HR of 150
- three big boxes: HR of 100
- four big boxes: HR of 75
- five big boxes: HR of 60
- six big boxes: HR of 50
Repeat this process with the R waves to determine the ventricular rate.
- Evaluate the P waves: Are they present with every QRS complex? Are they similar in size and shape? Are they of standard configuration?
- Measure the PR interval duration by counting the number of small squares and multiplying by 0.04s. Measure from the beginning of the P wave to the beginning of the QRS complex. It should be consistent, measuring 0.12–0.2s and about twice the QRS complex.
- Measure the QRS complex duration from the beginning of the Q wave to the end of the S wave by counting the number of small squares and multiplying by 0.04s. The expected duration is 0.06–0.1s, or about half the PR interval. Do they occur with every P wave? Are they consistent in size and shape?
- Evaluate the T waves to see if they are present with every QRS complex and deflect in the same direction on each lead. The deflection should be upright in leads I, II, and V3-6, negative in lead aVR, and variable in leads III, aVF, aVL, and V1-2. Are they regular and consistent in amplitude and shape?
- Measure the QT interval from the beginning of the Q wave to the end of the T wave by counting the number of small squares and multiplying by 0.04s. An average QT interval will be 0.36–0.44s, or less than half the time between consecutive R waves.
- Evaluate for any other characteristics, such as ectopic (extra) beats, ST segment abnormalities (elevation or depression), or the presence of U waves.
Normal Sinus and Not-So-Normal Sinus Rhythms
Normal sinus rhythm (refer to Figure 11) has a consistent regular rhythm, a rate of 60–100 bpm, a P wave preceding every QRS complex (1:1 ratio), typical PR and QT intervals, and standard/upright T waves. In addition, all P waves and QRS complexes are similar in size and shape (McGee, 2024; Prutkin, 2023b; Sauer, 2024; Shank Coviello, 2020).
Figure 11
Normal Sinus Rhythm

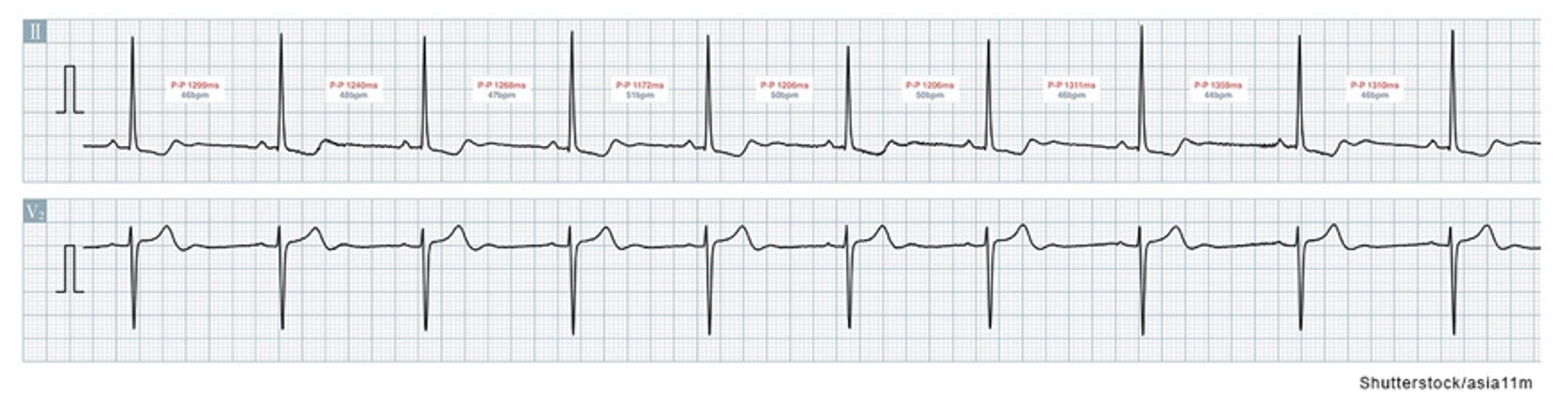
Sinus Arrhythmia
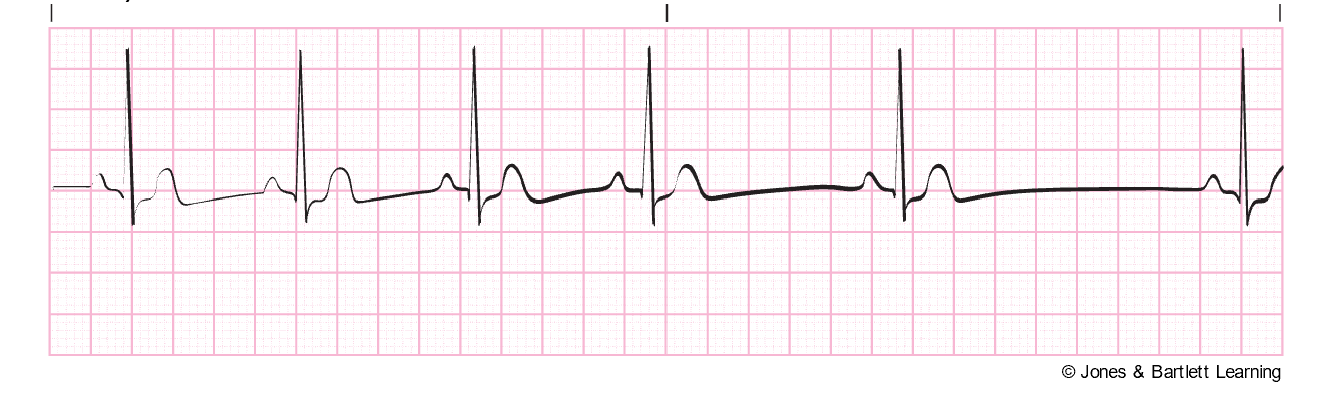
Sinus arrhythmia is a normal variant of a sinus rhythm (refer to Figure 12). The HR is still 60–100, but the rhythm is irregular and cyclic, varying with the patient’s respirations. This variation is related to the reduction in vagal tone and an increase in HR during inspiration, and an increase in vagal tone and a decrease in HR during exhalation. Sinus arrhythmia should not be present when the patient is holding their breath. Although sinus arrhythmia is a normal variation, especially in younger people, it can be mistaken for other arrhythmias. Sinus arrhythmia has been associated with obesity, DM, and HTN. Patients with sinus arrhythmia should be asymptomatic. The difference between P-P and R-R intervals is usually greater than 0.12s, contains a P wave preceding every QRS complex, maintains typical PR and QT intervals, and includes standard/upright T waves. In addition, all P waves and QRS complexes are similar in size and shape (Prutkin, 2023b; Sauer, 2024; Shank Coviello, 2020).
Figure 12
Sinus Arrhythmia
Sinus Bradycardia
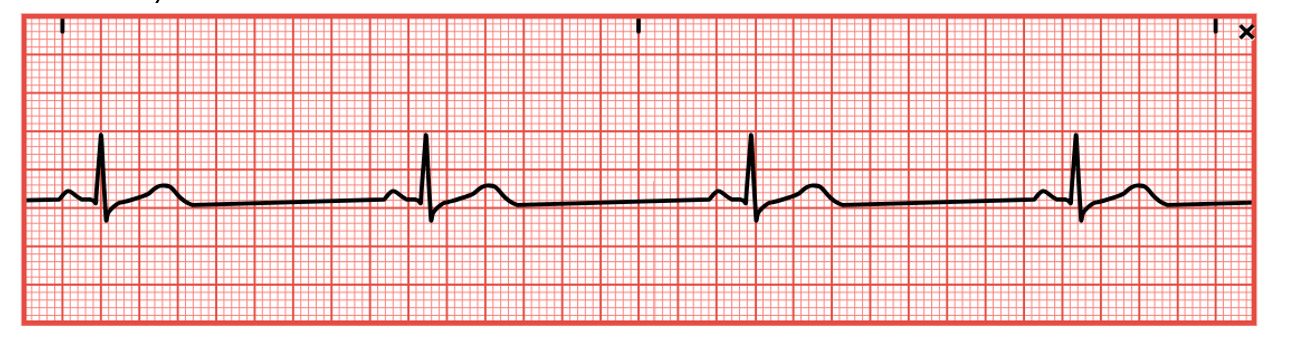
Sinus bradycardia (refer to Figure 13) has a regular rhythm but a rate that is less than 60 bpm. The QT interval may be slightly prolonged, but a P wave precedes every QRS complex, and there is a normal PR interval and standard/upright T waves. In addition, all P waves and QRS complexes are similar in size and shape. Sinus bradycardia typically occurs because of automaticity in the heart’s SA nodes decreasing due to excess vagal stimulation (such as the Valsalva maneuver, carotid sinus massage, or vomiting) or decreased sympathetic stimulation (such as sleep or deep relaxation). It may also be caused by hyperkalemia, increased intracranial pressure, hypothyroidism, hypothermia, glaucoma, SA node disease, cardiomyopathy, myocarditis, or myocardial ischemia (especially after an inferior-wall MI that involves the right coronary artery, which feeds the SA node). In addition, sinus bradycardia can be caused by certain medications (beta-blockers, opioids, sedatives, chemotherapeutic agents, digoxin [Lanoxin], calcium channel blockers, lithium [Lithobid], cimetidine [Tagamet], sotalol [Betapace], amiodarone [Cordarone], propafenone [Rhythmol], and quinidine [Quinora]; Homoud, 2024b; McGee, 2024; Shank Coviello, 2020).
No treatment is required for individuals with asymptomatic sinus bradycardia, such as elite athletes. Instead, carefully monitor the patient’s vital signs, airway, breathing, and HR. Sinus bradycardia may produce dizziness, hypotension, decreased level of consciousness (LOC), confusion, cool/clammy skin, blurred vision, chest pain, or bradycardia-induced syncope (Stokes-Adams attack). Sinus bradycardia in a child is an ominous sign and should be taken seriously and monitored very closely. An underlying cause should first be identified and corrected if treatment is required. In the interim, or if an underlying cause cannot be immediately identified, transcutaneous pacing can be used until a more definitive plan can be established. Alternatively, medications such as atropine (Atreza), epinephrine (Adrenalin), or dopamine (Intropin) can be given to increase the HR. A 3 to 10 mg bolus of IV glucagon should be given over 3 to 5 minutes if a beta-adrenergic or a calcium channel blocker overdose is suspected. This bolus may be repeated once if there is no response. If the glucagon bolus is effective, a continuous infusion should be started at 3 mg to 5 mg per hour and titrated as needed (Homoud, 2024b; McGee, 2024; Shank Coviello, 2020).
Figure 13
Sinus Bradycardia
Sinus Tachycardia
Sinus tachycardia (refer to Figure 14) is a regular rhythm with a rate of 100–160 bpm. A P wave will precede every QRS complex, a normal PR interval, a shortened QT interval, and standard/upright T waves. In addition, all P waves and QRS complexes are similar in size and shape, but P waves may have a higher amplitude and become superimposed on the preceding T wave. Sinus tachycardia may be caused by exercise, increased stress, hypovolemia, pain, hemorrhage, CHF, cardiogenic shock, pericarditis, PE, sepsis, or hyperthyroidism. It can also be prompted by excessive alcohol, caffeine, cocaine, or nicotine intake. In addition, sinus tachycardia can be prompted by certain medications, including amphetamines, atropine (Atreza), dopamine (Intropin), dobutamine (Dobutrex), epinephrine (Adrenalin), isoproterenol (Isuprel), or aminophylline (Phyllocontin). Abrupt withdrawal of beta-blockers can also cause sinus tachycardia. The sudden onset of sinus tachycardia following an MI may indicate an extension of the infarction. The increased HR causes increased myocardial demands and decreased cardiac output due to reduced ventricular filling time, leading to angina, palpitations, decreased peripheral perfusion, hypotension, syncope, nervousness/anxiety, and blurred vision (Homoud, 2023b; McGee, 2024; Shank Coviello, 2020).
HCPs should carefully monitor the patient’s vital signs, airway, breathing, and LOC. The environment should be kept as calm as possible. If untreated, tachycardia can lead to heart failure (as evidenced by respiratory crackles, S3 heart sounds, and jugular venous distention) or cardiogenic shock. An underlying cause should first be identified and corrected if treatment is required. In the interim, or if an underlying cause cannot be identified immediately, medications such as beta-blockers (metoprolol [Lopressor] or atenolol [Tenormin]) and/or calcium channel blockers (verapamil [Calan]) may be given to reduce the HR. If beta-blockers are used, monitor for first-degree heart block development or reports of digestive symptoms, trouble sleeping, or erectile dysfunction. If calcium channel blockers are selected, monitor for signs of AFib, lower extremity swelling, hypotension, and reports of digestive symptoms (Homoud, 2023b; McGee, 2024; Shank Coviello, 2020).
Figure 14
Sinus Tachycardia
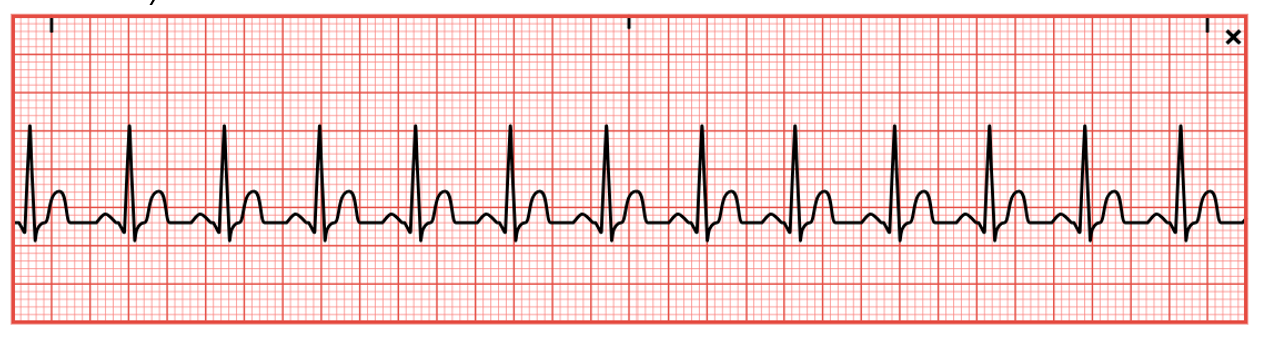
Sinus Arrest
Sinus arrest (refer to Figure 15) or atrial standstill is secondary to a lack of electrical activity from the SA node in the atrium. The patient will have a previously regular rhythm, followed by one or more missing beats. If just one or two beats are missed, it is considered a sinus pause; three or more skipped beats are regarded as a sinus arrest. The length of the pause is not a multiple of the previous R-R intervals and commonly ends with a junctional escape beat. When present, there will be a P wave preceding every QRS complex, typical PR and QT intervals, and standard/upright T waves. In addition, all existing P waves and QRS complexes are similar in size and shape. It closely resembles third-degree SA block and can be caused by SA node disease (fibrosis or idiopathic degeneration), increased vagal tone (such as Valsalva maneuver, carotid sinus massage, or vomiting), acute inferior wall MI, acute infection, chronic coronary artery disease (CAD), acute myocarditis, cardiomyopathy, hypertensive heart disease or sick sinus syndrome (SSS). In addition, various medications can cause sinus arrest, including digoxin (Lanoxin), quinidine (Quinora), procainamide (Procan), salicylates, or excessive dosages of beta-blockers such as metoprolol (Lopressor) or propranolol (Inderal; Homoud, 2024a; McGee, 2024; Shank Coviello, 2020).
If asymptomatic, no treatment may be necessary. Normal adults may have two to three-second pauses during sleep, increased vagal tone, or hypersensitive carotid sinus disease. However, a prolonged pause or arrest (usually seven seconds or more) can cause syncope, leading to falls, injuries, car accidents, or other secondary injuries. Any pause over two to three seconds should be noted and considered significant. The patient may also have hypotension, altered mental status (AMS), dizziness, blurred vision, and cool/clammy skin. HCPs should carefully monitor the patient’s vital signs, airway, breathing, and HR. An underlying cause should first be identified and corrected if treatment is required. In the interim, or if an underlying cause cannot be identified immediately, a transcutaneous pacemaker may be used, as well as medications such as atropine (Atreza) or epinephrine (Adrenalin) to prevent circulatory collapse until more definitive and long-term treatment can be established (Homoud, 2024a; McGee, 2024; Shank Coviello, 2020).
Figure 15
Sinus Arrest
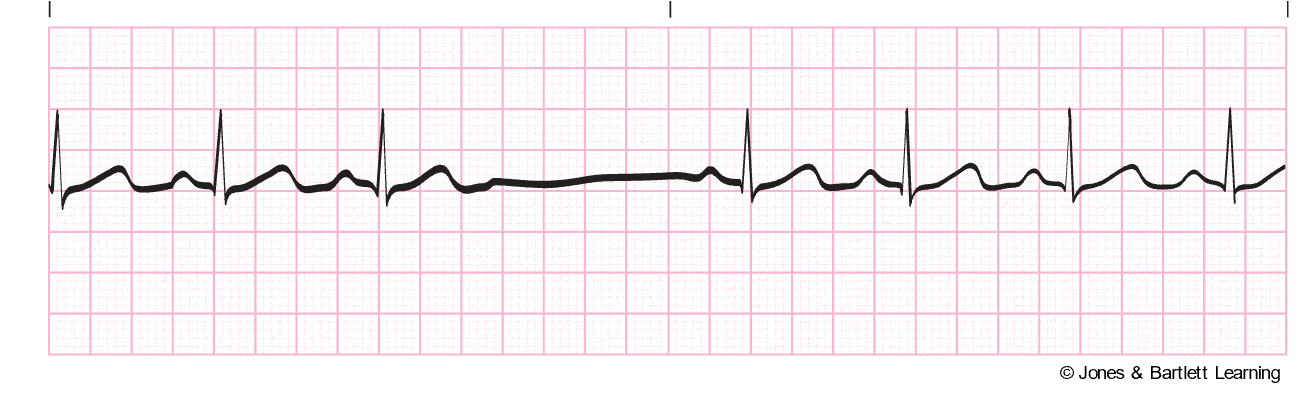
Sick Sinus Syndrome
Sick Sinus Syndrome (SSS) refers to various SA node abnormalities (refer to Figure 16). The syndrome can refer to both disturbances in the way impulses are generated or conducted. SSS is most common in individuals over the age of 60. The rhythm is irregular, and the rate may vary. P waves usually precede each QRS. The QRS complex, T wave, PR interval, and QT interval are generally normal, but vary with rhythm changes. SSS typically presents with an insidious and progressive onset of bradycardia and episodes of sinus arrest or SA block with varying periods of rapid AFib. There can also be periods of atrial tachyarrhythmias, such as atrial flutter or atrial tachycardia, dispersed amongst periods of bradycardia, a condition known as bradycardia-tachycardia (or brady-tachy) syndrome. SSS may also present as an inappropriate response by the SA node to increase the HR during exercise. In addition, SSS can be caused by fibrosis of the SA node (advanced age, atherosclerotic heart disease, HTN, or cardiomyopathy), trauma to the SA node due to surgery, pericarditis, rheumatic heart disease, or autonomic disturbances. Various medications can also provoke SSS, including digoxin (Lanoxin), beta-blockers, and calcium channel blockers. It can develop after an inferior wall MI that involves the right coronary artery, which feeds the SA node. Patients may experience symptoms of cardiomyopathy (such as crackles, an S3 sound, and a dilated/displaced left ventricular apical HR), AMS, hypotension, blurry vision, or syncope (Homoud, 2023a; McGee, 2024; Shank Coviello, 2020).
If asymptomatic, no treatment may be necessary. If symptomatic, carefully monitor the patient’s mental status, vital signs, airway, breathing, and HR. An underlying cause should first be identified and then corrected if treatment is required. In the interim, or if an underlying cause cannot be identified immediately, atropine (Atreza) or epinephrine (Adrenalin) may be administered for symptomatic bradycardia, or metoprolol (Lopressor) or digoxin (Lanoxin) may be given for tachyarrhythmias (although these may worsen underlying SA node disease). If digoxin (Lanoxin) is given, look for reports of nausea and the development of other arrhythmias, such as heart block. Transcutaneous pacing may also be recommended as a short-term solution. Anticoagulants should be given to reduce the risk of blood clots and stroke if the patient develops AFib. If anticoagulants are prescribed, the HCP should educate the patient and family regarding the risks of bleeding and monitor for signs/symptoms of this in the future (Homoud, 2023a; McGee, 2024; Shank Coviello, 2020).
Figure 16
Sick Sinus Syndrome
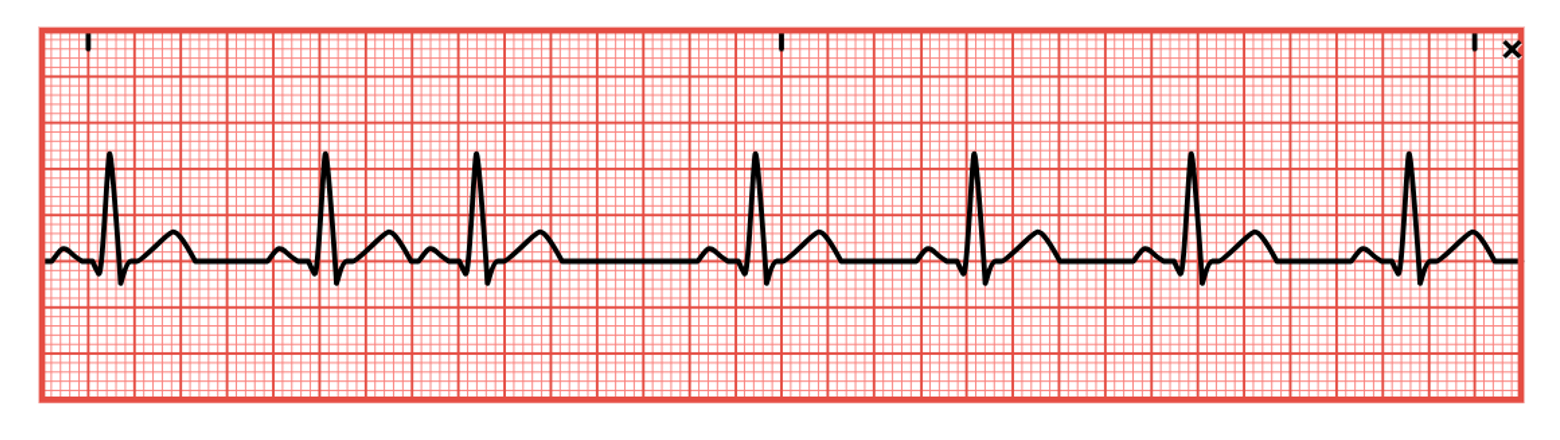
Atrial Arrhythmias
Atrial or supraventricular arrhythmias affect the atrium. According to the AHA, common symptoms of atrial arrhythmias include angina, shortness of breath, dizziness, lightheadedness, syncope, or heart palpitations. Premature atrial contractions (PACs) are ectopic or premature beats that originate from an irritable or ectopic spot elsewhere in the atria instead of the SA node (refer to Figure 17). Unfortunately, this erroneous signal may be conducted through the AV node and the rest of the heart, just as any other impulse. Atrial arrhythmias can be caused by excessive alcohol or nicotine use, anxiety, extreme fear or fatigue, infection, coronary or valvular heart disease, acute renal failure, hypoxia, pulmonary disease, digoxin (Lanoxin) toxicity, or electrolyte imbalances. They are rarely dangerous in patients without heart disease and are typically asymptomatic. The ECG strip will show a premature P wave with an abnormal configuration compared to the other waves and an irregular rate. If conducted, a standard QRS complex will follow. If the signal is not conducted, no QRS complex will appear. The SA node is typically able to reset itself, causing a normal sinus beat to follow slightly earlier. If PACs occur every other beat, this is called bigeminy, and every third beat is trigeminy. Two PACs in a row are called a couplet. If symptomatic, the patient may report palpitations, fluttering, or the feeling of skipped beats; vital signs, especially HR, should be monitored. An underlying cause should first be identified and corrected (AHA, 2024c; McGee, 2024; Prutkin, 2024a; Shank Coviello, 2020).
Figure 17
Premature Atrial Contraction
Atrial Tachycardia
Atrial tachycardia is also known as SVT (refer to Figure 18). According to the AHA, it is more common in pediatric patients and those assigned female at birth. This rhythm has an atrial rate of 150–250 bpm. This rapid rate may lead to shorter diastole, reduced atrial kick and cardiac output, decreased coronary perfusion, and myocardial ischemia. If untreated, this can eventually lead to angina, heart failure, or an MI. Atrial tachycardia can be due to a block (not every impulse is conducted through the AV node, so atrial and ventricular rates will differ), multifocal (multiple atrial foci firing impulses lead to various P wave configurations and an irregular rhythm), or paroxysmal (starts and stops suddenly, with a regular rhythm). In addition, atrial tachycardia can be caused by excessive caffeine or other stimulants, marijuana use, electrolyte imbalances, hypoxia, stress, MI, cardiomyopathy, congenital anomalies, WPW, valvular heart disease, SSS, cor pulmonale, hyperthyroidism, HTN, or digoxin (Lanoxin) toxicity (AHA, 2024c; McGee, 2024; Prutkin, 2024a; Shank Coviello, 2020).
In atrial tachycardias, the QRS complex and QT interval are usually normal but may be shorter with a faster rate. ST-segment changes and T wave inversion may occur if ischemia develops. Symptoms may include palpitations, blurry vision, syncope, and hypotension. If symptomatic, carefully monitor the patient’s mental status, airway/breathing, and vital signs, especially HR. An underlying cause should first be identified and corrected if treatment is required. Carotid sinus massage may be a treatment option for younger patients, but should be avoided in older adult patients. Potential risks include hypotension, bradycardia, vasodilation, ventricular arrhythmias, stroke, and cardiac standstill. The Valsalva maneuver is another option for vagal stimulation, but it carries many of the same risks. Medications such as digoxin (Lanoxin), beta-blockers, adenosine (Adenocard), or calcium channel blockers may be used to reduce HR. If adenosine (Adenocard) is used, watch for the development of chest pain, shortness of breath, and flushing. If medications are unsuccessful or inappropriate, synchronized cardioversion or atrial overdrive pacing may also be used. Synchronized cardioversion works by precisely timing (at the peak of the R wave) a low-energy shock to the heart to restore a normal rhythm. The patient should be premedicated with a sedative, anxiolytic, and/or analgesic medication intravenously. Caution should be advised, as cardioversion can lead to new or worsening arrhythmias or a clot embolism (AHA, 2024c; McGee, 2024; Prutkin, 2024a; Shank Coviello, 2020).
Figure 18
Atrial Tachycardia/Supraventricular Tachycardia
Atrial Flutter
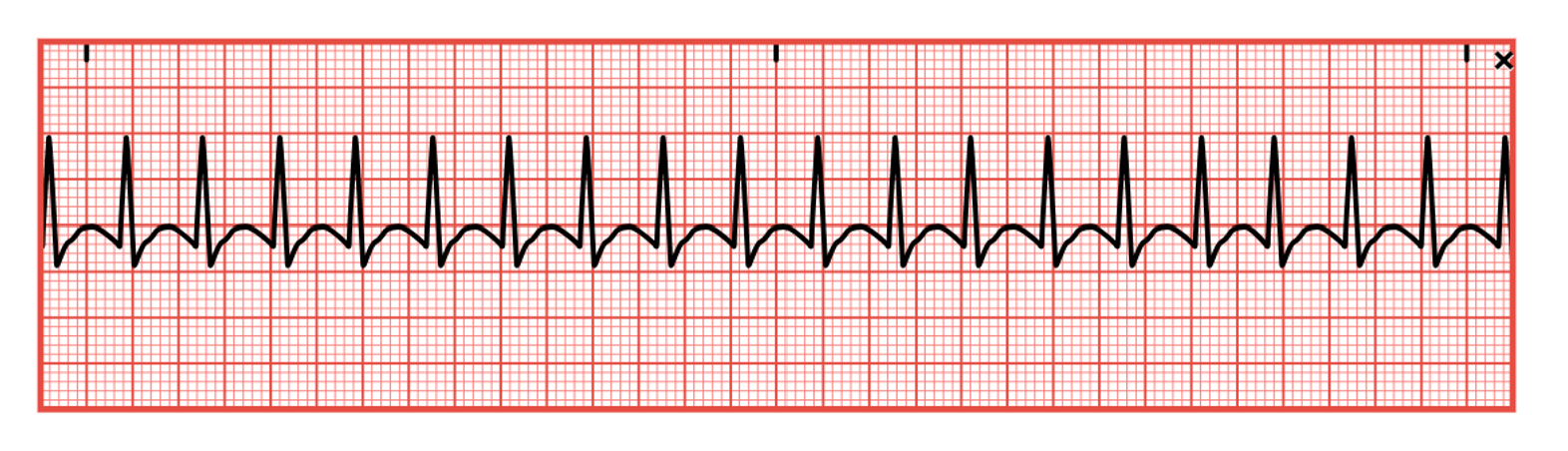
Atrial flutter is an SVT with an atrial rate of 250–350 bpm (refer to Figure 19). The impulse is generated from a single atrial focus and results from circus reentry and increased automaticity. The ECG for this rhythm has a classic and unmistakable sawtooth pattern as the P waves lose their distinction and the waves blend. T waves are not usually discernible, and the QRS complexes may be widened if P waves are superimposed. Atrial flutter is often associated with a second-degree block, which does not allow all impulses through the AV node and results in a slower ventricular rate. If the ventricular rate is below 40 or above 150, cardiac output may be compromised, leading to reduced ventricular filling and coronary perfusion, angina, heart failure, pulmonary edema, hypotension, or syncope. Atrial flutter is often caused by conditions that raise the atrial pressure or cause atrial hypertrophy, such as severe mitral valve disease, hyperthyroidism, pericardial disease, or primary myocardial disease. It can also be seen in patients with recent cardiac surgery, acute MI, COPD, and systemic arterial hypoxia. If symptomatic, HCPs should carefully monitor the patient’s mental status, airway/breathing, and vital signs, especially HR. An underlying cause should be identified first and then corrected if treatment is required. Treatment will focus on rate control and converting the arrhythmia to a normal rhythm. In a stable patient, direct current cardioversion (DCCV) may be considered if the atrial flutter has been present for less than 48 hours. If present for more than 48 hours, the risk of thromboembolism is greater, and cardioversion should not be considered unless the patient is adequately anticoagulated or a transesophageal echocardiogram (TEE) has been completed to rule out the presence of a clot. If the patient is unstable, synchronized cardioversion should be considered. If pharmacologic treatment is necessary, ibutilide (Covert) is the preferred drug, reverting atrial flutter to a sinus rhythm in approximately 60% of patients. Other pharmacologic options that can be used include procainamide (Procan), sotalol (Betapace), or amiodarone (Cordarone; McGee, 2024; Phang & Prutkin, 2025; Shank Coviello, 2020).
Figure 19
Atrial Flutter
Atrial Fibrillation
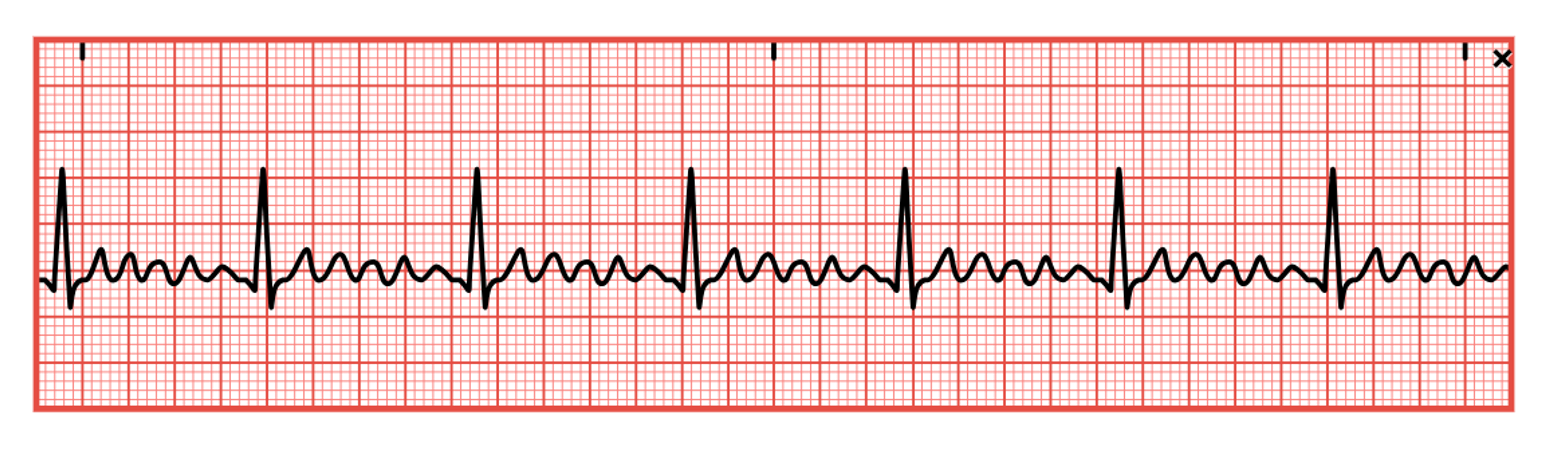
AFib is the most common arrhythmia (about 2 million people are affected in the United States). It is defined as chaotic, random electrical activity in atrial tissue with an impulse rate of 400–600 per minute, resulting in atrial kick loss and quivering instead of coordinated contractions in the atria. The ECG baseline will contain erratic (fibrillatory) waves that may be coarse or fine with no discernible P waves (refer to Figure 20). AFib may be preceded by or caused by PACs. An irregular transfer of impulses through the AV node to the ventricles leads to an irregular ventricular rate and corresponding irregular HR. AFib can be caused by cardiac surgery, hypotension, PE, COPD, electrolyte imbalances, mitral insufficiency or stenosis, hyperthyroidism, infection, CAD, acute MI, pericarditis, hypoxia, or atrial septal defects. It can also be triggered by excessive caffeine, alcohol, or nicotine combined with fatigue and stress. Medications such as aminophylline (Phyllocontin) or digoxin (Lanoxin) may also trigger AFib (AHA, 2024c; Kumar, 2023; McGee, 2024; Shank Coviello, 2020).
If left untreated, AFib can cause heart failure, angina, syncope, cardiovascular collapse, and thrombus or embolism formation. If the ventricular rate is greater than 100, it is termed uncontrolled AFib. The peripheral pulses may differ from the apical rate on the physical exam due to weaker contractions that do not produce a palpable pulse. Acute symptoms may include lightheadedness or hypotension due to reduced cardiac output. HCPs should assess for embolism symptoms, such as pulmonary embolism (PE) or stroke, in patients with chronic AFib. If symptomatic, HCPs should carefully monitor the patient’s mental status, airway/breathing, and vital signs, especially HR. Any underlying cause should first be identified and corrected if possible. Like atrial flutter, treatment is targeted at controlling the rate (below 100) with medications if needed and cardioverting to a normal rhythm if possible. Just as above, synchronized cardioversion is most successful if done within the first 48 hours of AFib development. Patients should be adequately anticoagulated. Vagal stimulation with carotid sinus massage or Valsalva maneuvers may temporarily slow the ventricular rate. Medications such as digoxin (Lanoxin), propranolol (Inderal), quinidine (Quinora), amiodarone (Cordarone), diltiazem (Cardizem), and verapamil (Calan) are often given to maintain normal sinus rhythm and control the ventricular rate after cardioversion. Radiofrequency ablation is sometimes required in patients with refractory ectopic sites in the atria. Ablation procedures use either a radiofrequency (high-energy) catheter, cryoablation (cold), or a laser to create a focal area of scar tissue to prevent extraneous electrical activity from a specific ectopic site. The patient is premedicated with an anxiolytic, and the procedure carries many of the same risks as a cardiac catheterization (AHA, 2024c; Kumar, 2023; McGee, 2024; Shank Coviello, 2020).
Figure 20
Atrial Fibrillation
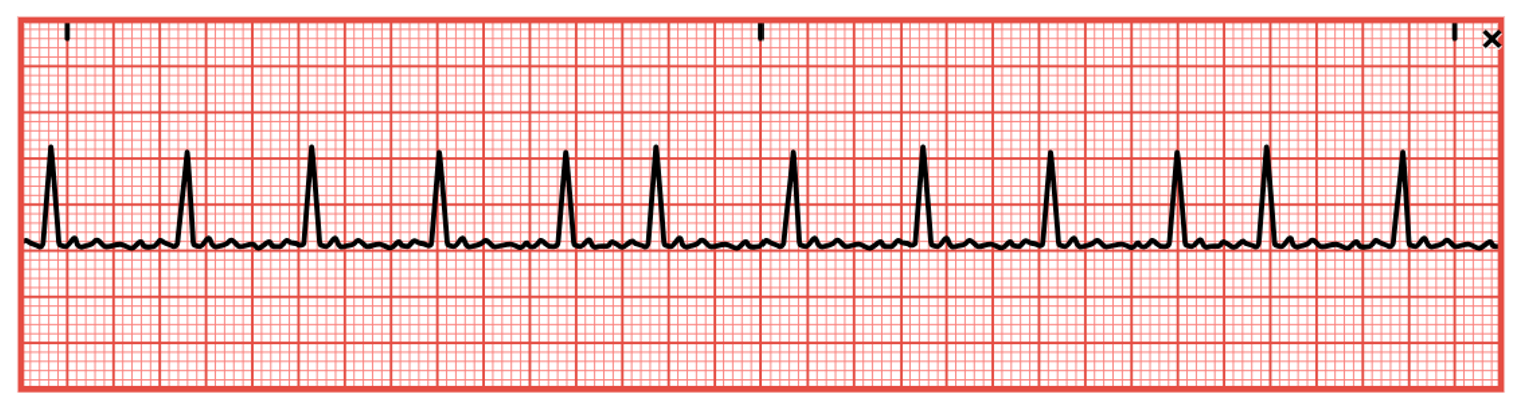
Wandering Pacemaker
A wandering pacemaker is an irregular rhythm caused by the heart's pacemaker changing from the SA node to another area above the ventricles or the AV junction. It is commonly transient and rarely serious. A wandering pacemaker is a rhythm with a standard rate of 60–100 bpm but varying P waves and PR intervals (refer to Figure 21). The QRS complex, T wave, and QT interval are usually standard, but the ventricular rate may be slightly irregular. It may be caused by increased vagal tone, digoxin (Lanoxin) toxicity, or heart disease such as rheumatic carditis. A wandering pacemaker is often, but not always, asymptomatic and requires no treatment other than monitoring. The patient’s mental status, airway/breathing, and vital signs, especially HR, should be monitored if they are symptomatic. If treatment is required, an underlying cause should first be identified and corrected if possible (Prutkin, 2024a; Shank Coviello, 2020).
Figure 21
Wandering Atrial Pacemaker
Junctional Arrhythmias
Five junctional arrhythmias originate in the AV junction: the area around the AV node and the bundle of His. They occur when the SA node fails as the heart’s pacemaker or the impulse fails to conduct properly. This abnormal signal from the AV junction may cause upward or retrograde depolarization of the atria, which appears as inverted P waves in leads II, III, and aVF (which are generally upright). Another key to spotting a junctional arrhythmia is the PR interval. If a rhythm has an inverted P wave but a normal PR interval (0.12–0.2s), it is likely an atrial arrhythmia. If a rhythm has an inverted P wave and a shortened PR interval (less than 0.12s), it likely originated in the AV junction (McGee, 2024; Prutkin, 2024a; Shank Coviello, 2020).
Wolff-Parkinson-White Syndrome
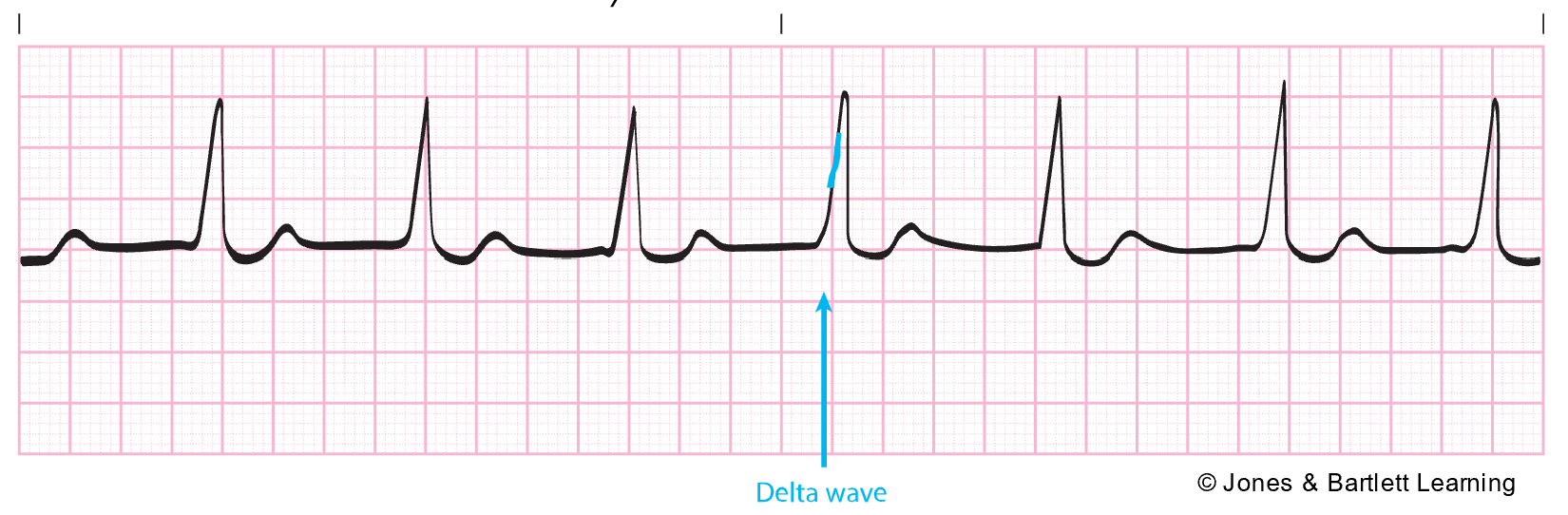
Wolff-Parkinson-White (WPW) syndrome is typically a congenital rhythm disorder seen in young children and young adults aged 20–35. In this condition, impulses bypass the AV node through the bundle of Kent, traveling directly from the atria to the ventricles quickly. WPW can lead to circus reentry, retrograde conduction, or reentrant tachycardia. On ECG, this appears as a shortened PR interval (less than 0.1s) and a widened QRS complex (greater than 0.1s) with a slurred beginning called a delta wave due to abnormal ventricular depolarization (refer to Figure 22). In asymptomatic patients, monitoring may be the only treatment necessary. WPW can also lead to tachyarrhythmias such as AFib or atrial flutter, thus necessitating additional treatment such as radiofrequency ablation and/or rate-controlling medications like amiodarone (Cordarone; Di Biase & Walsh, 2024; Shank Coviello, 2020).
Figure 22
Delta Wave for Wolff-Parkinson White Syndrome
Premature Junctional Contraction
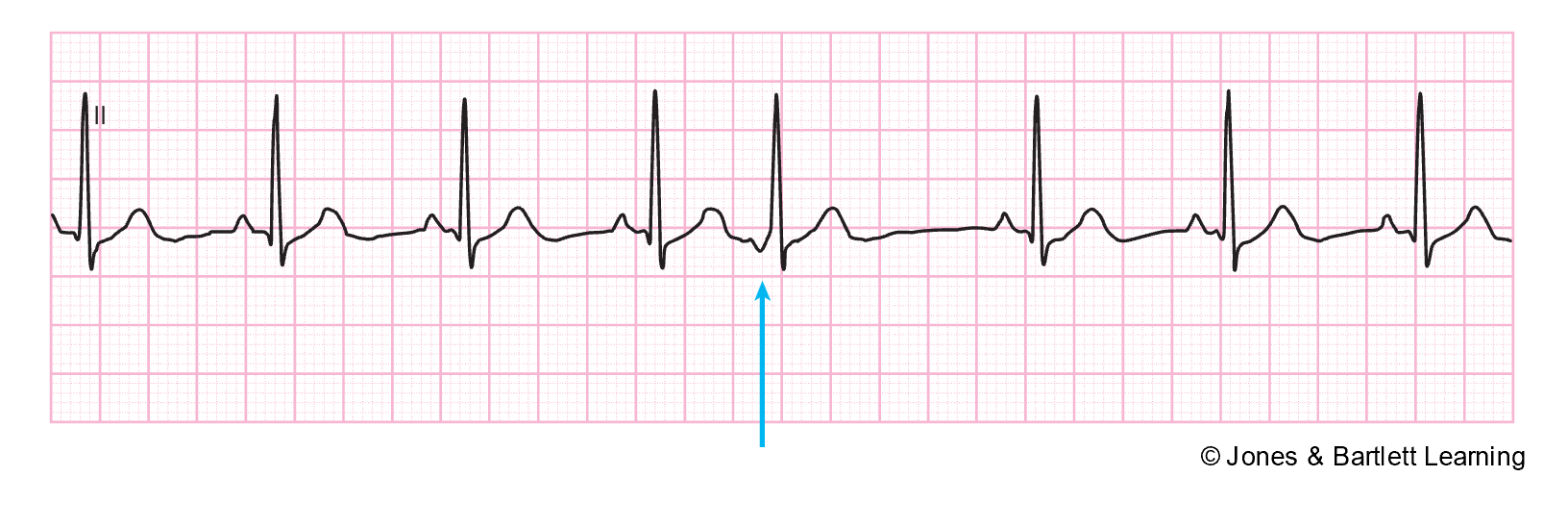
A premature junctional contraction (PJC) is an irregular ectopic beat originating from the AV junction. A PJC appears on ECG with an inverted P wave due to the retrograde depolarization of the atria that may fall before, during, or after the QRS complex. The PR interval is shortened (less than 0.12s) if the P wave occurs before the QRS complex. The QRS complex, T wave, and QT interval are usually normal (refer to Figure 23). A PJC can be caused by digoxin (Lanoxin) toxicity, excessive caffeine intake, inferior wall MI, rheumatic heart disease, valvular disease, hypoxia, heart failure, or swelling of the AV junction following surgery. Patients experiencing PJCs are often asymptomatic and do not require treatment other than monitoring. If symptomatic, the patient may report palpitations or a quickening in their chest and an irregular HR. If cardiac output is compromised, they may be hypotensive or report dizziness, lightheadedness, blurry vision, or syncope. Symptomatic patients require monitoring of their mental status, airway/breathing, and vital signs, especially HR. If treatment is required, an underlying cause should first be identified and corrected if possible (McGee, 2024; Prutkin, 2024a; Shank Coviello, 2020).
Figure 23
Premature Junctional Contraction
Junctional Escape Rhythm
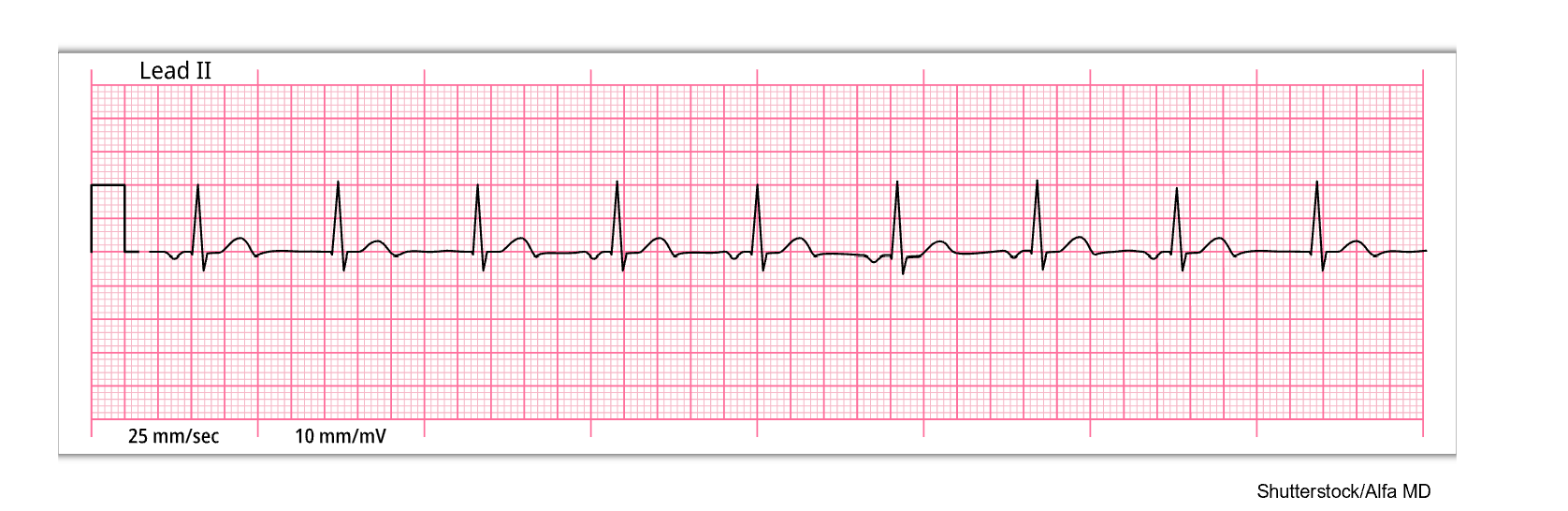
A junctional escape rhythm is a rapid succession of beats after a conduction delay from the atria. It is a compensatory mechanism to prevent ventricular standstill. Junctional escape rhythm appears on ECG at a rate of 40–60 bpm with an inverted P wave due to the retrograde depolarization of the atria that may fall before, during, or after the QRS complex. As described above with PJCs, the PR interval is shortened (less than 0.12s) if the P wave occurs before the QRS complex. The QRS complex, T wave, and QT interval are usually normal (refer to Figure 24). It can be caused by SSS, vagal stimulation, digoxin (Lanoxin) toxicity, inferior wall MI, or rheumatic heart disease. Junctional escape rhythm may be asymptomatic depending on age and cardiovascular fitness level, or individuals may show signs of reduced cardiac output such as hypotension, decreased urine output, or syncope. Symptomatic patients require monitoring, including their mental status, airway/breathing, and vital signs, especially HR. An underlying cause should first be identified and corrected if treatment is required. Atropine (Atreza) may be indicated to increase the patient’s HR. A transcutaneous temporary pacemaker may be used until a permanent one can be implanted (McGee, 2024; Prutkin, 2024a; Shank Coviello, 2020).
Figure 24

Accelerated Junctional Rhythm
An accelerated junctional rhythm is like the junctional escape rhythm but faster. An accelerated junction rhythm is especially problematic if the atria depolarize after the ventricles (the P wave follows the QRS complex), which prevents the atrial kick and limits blood flow into the ventricles from the atria. This rhythm appears on ECG with a rate of 60–100 bpm (or greater than 80 bpm in toddlers up to age three) and an inverted P wave due to the retrograde depolarization of the atria that may fall before, during, or after the QRS complex. As described above, with PJCs and junctional escape rhythms, the PR interval is shortened (less than 0.12s) if the P wave occurs before the QRS complex. The QRS complex, T wave, and QT interval are usually normal (refer to Figure 25). An accelerated junctional rhythm can lead to decreased cardiac output with symptoms of hypotension, dizziness, confusion, syncope, reduced urine output, and weak peripheral pulses. These patients should be carefully monitored, including mental status, airway/breathing, and vital signs, especially HR. An underlying cause should be identified first and then corrected if treatment is required. Pacing may be indicated with a transcutaneous temporary pacemaker until a permanent one can be implanted (McGee, 2024; Prutkin, 2024a; Shank Coviello, 2020).
Figure 25
Accelerated Junctional Rhythm
Junctional Tachycardia
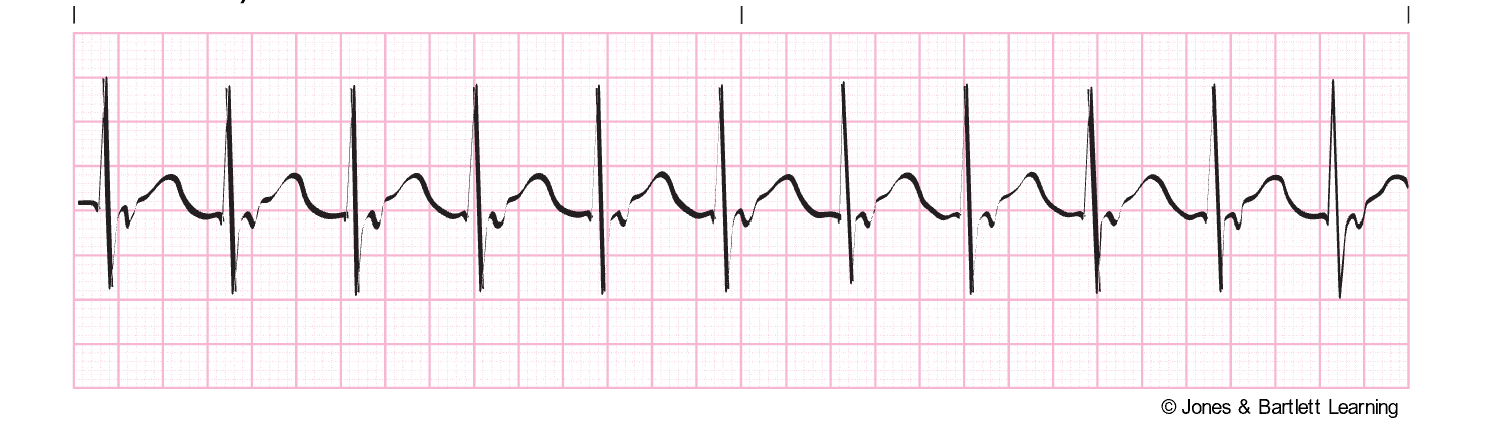
Junctional tachycardia occurs when there are three or more consecutive PJCs. It originates from an irritable focus in the AV junction with increased automaticity, overriding the SA node. The rate on ECG is 100–200 bpm. Features align with the PJC and junctional rhythms described above, although the QRS complex, T wave, and QT interval may not be visible if the rate increases (refer to Figure 26). The significance of this rhythm depends on the rate, the underlying cause, and any accompanying or preexisting heart disease. Junctional tachycardia is most often caused by digoxin (Lanoxin) toxicity. However, it can also be related to hypokalemia, inferior or posterior myocardial ischemia or MI, congenital heart disease, or swelling of the AV junction after surgery. A lack of atrial kick and a high ventricular rate can quickly combine forces to reduce cardiac output. Patients may present with hypotension, dizziness, confusion, syncope, decreased urine output, and weak peripheral pulses. These patients should be carefully monitored, including mental status, airway/breathing, and vital signs, especially HR. An underlying cause should be identified first and then corrected if treatment is required. Vagal maneuvers may temporarily reduce the HR, and medications such as verapamil (Calan) may be indicated to slow the HR down further. Pacemakers, either temporary or permanent, or ablation therapy are sometimes indicated in resistant or recurrent junctional tachycardia (McGee, 2024; Prutkin, 2024a; Shank Coviello, 2020).
Figure 26
Junctional tachycardia
Ventricular Arrhythmias
Ventricular arrhythmias start in the ventricle below the bundle of His and can be very dangerous, requiring immediate medical attention. For that reason, they should be studied, understood, and quickly recognized. The common signs and symptoms of ventricular arrhythmias are dizziness, palpitations, shortness of breath, nausea, altered LOC, or cardiac arrest. Ventricular arrhythmias are often caused by reduced coronary blood flow, cardiomyopathy, inflammation related to sarcoidosis, sepsis, problems with the aorta, or certain medications. The myocardium is depolarized along a different pathway, causing widened QRS complexes on ECG. In addition, characteristic signs of ventricular arrhythmias include an absent P wave (due to lack of atrial depolarization) and a QRS complex and T wave that deflect in opposite directions (due to the difference in the action potential during ventricular depolarization and repolarization). Without the atrial contraction or kick, cardiac output can decrease by 30%, causing hypotension, angina, syncope, and/or respiratory distress (AHA, 2024b; McGee, 2024; Prutkin, 2024c; Shank Coviello, 2020).
Premature Ventricular Contraction
A premature ventricular contraction (PVC) is an ectopic beat caused by electrical irritability in the ventricular conduction system or muscle tissue. PVCs may be caused by electrolyte imbalances, metabolic acidosis, hypoxia, myocardial ischemia or infarction, illicit drugs (cocaine, amphetamines), tricyclic antidepressants, ventricular hypertrophy, increased sympathetic stimulation, myocarditis, excessive alcohol or caffeine intake, antiarrhythmics medication, or tobacco use. If frequent or sustained, PVCs can reduce cardiac output. They can also be a precursor to more severe arrhythmias. PVCs can occur in couplets, bigeminy, or trigeminy. On ECG, PVCs appear wide and abnormally shaped at irregular intervals. There is no P wave, or a retrograde P wave may interfere with the ST segment. The QRS complex is greater than 0.12s with a deflection opposite the T wave (refer to Figure 27). There may or may not be a compensatory pause following the PVC, which should equal two regular P-P intervals. PVCs that are couplets, bigeminy, or variable in shape may indicate a more serious condition and require immediate medical evaluation. Patients with frequent PVCs may describe palpitations or experience hypotension or syncope. If asymptomatic, treatment beyond monitoring may not be necessary. If symptomatic, the HCP should carefully monitor the patient’s mental status, airway/breathing, and vital signs, especially HR, and place them on continuous ECG monitoring. An underlying cause should be identified first and then corrected if treatment is required. Antiarrhythmic medications such as procainamide (Procan), amiodarone (Cordarone), or IV lidocaine (Xylocaine) may be indicated (McGee, 2024; Prutkin, 2024c; Shank Coviello, 2020).
Figure 27
Premature Ventricular Complex
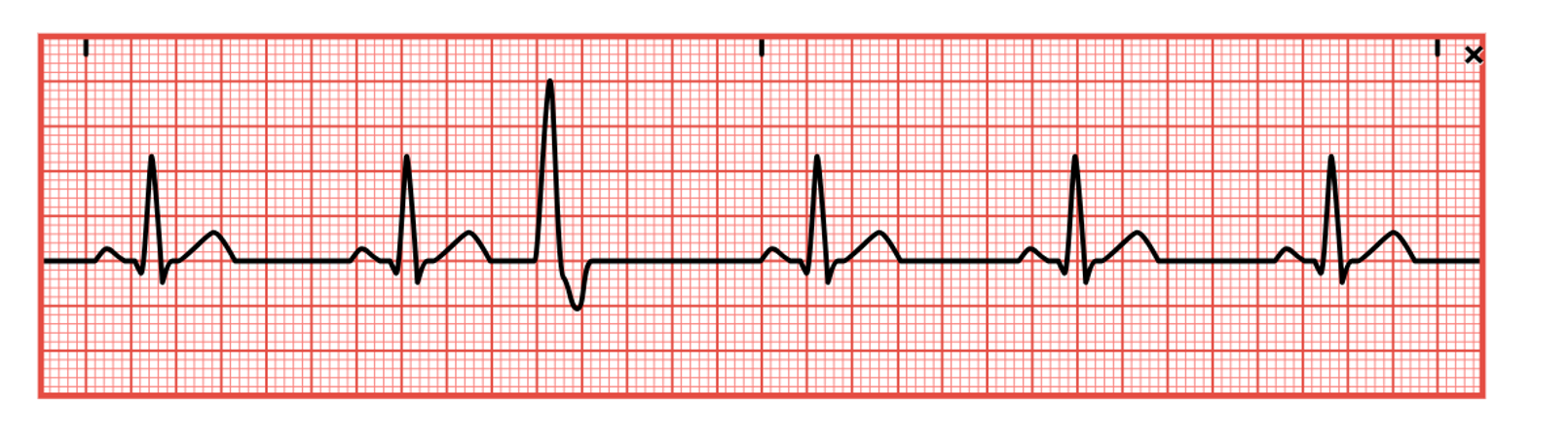
Idioventricular Rhythms
Idioventricular rhythms are the heart's safety mechanism to avoid a ventricular standstill if no impulse is received by the SA node or the AV node above the bundle of His. The cells of the His-Purkinje system will generate electrical impulses at 20–40 bpm. As a result, cardiac output is markedly reduced with a slow rate and no atrial kick. If just a single beat is seen, it is called a ventricular escape beat. If multiple beats occur consecutively at a regular 20–40 bpm rate, it is called an idioventricular rhythm (refer to Figure 28). If the rate is above 40, it is called an accelerated idioventricular rhythm. On ECG, the P wave will be absent, the QRS complex will widen (greater than 0.12s), the QT interval will be prolonged, and the T wave will deflect opposite the QRS complex. Signs and symptoms include palpitations, dizziness, lightheadedness, hypotension, weak peripheral pulses, syncope, decreased urine output, or confusion. Idioventricular rhythms may be caused by myocardial ischemia or infarction, digoxin (Lanoxin) toxicity, beta-blockers, pacemaker failure, or metabolic imbalances. It may accompany a third-degree heart block. The underlying cause should be identified and corrected in symptomatic patients if possible. The patient’s mental status, airway/breathing, and vital signs, especially HR, should be monitored along with continuous ECG monitoring. Treatment should focus on increasing cardiac output by increasing the HR and establishing a normal rhythm. Atropine (Atreza) may be given, or a transcutaneous pacemaker may need to be used until a permanent treatment plan can be established. Lidocaine (Xylocaine) or other antiarrhythmic medications should not be administered to someone with idioventricular rhythm, as this may suppress their last safety mechanism (McGee, 2024; Prutkin, 2024c; Shank Coviello, 2020).
Figure 28
Idioventricular Rhythm
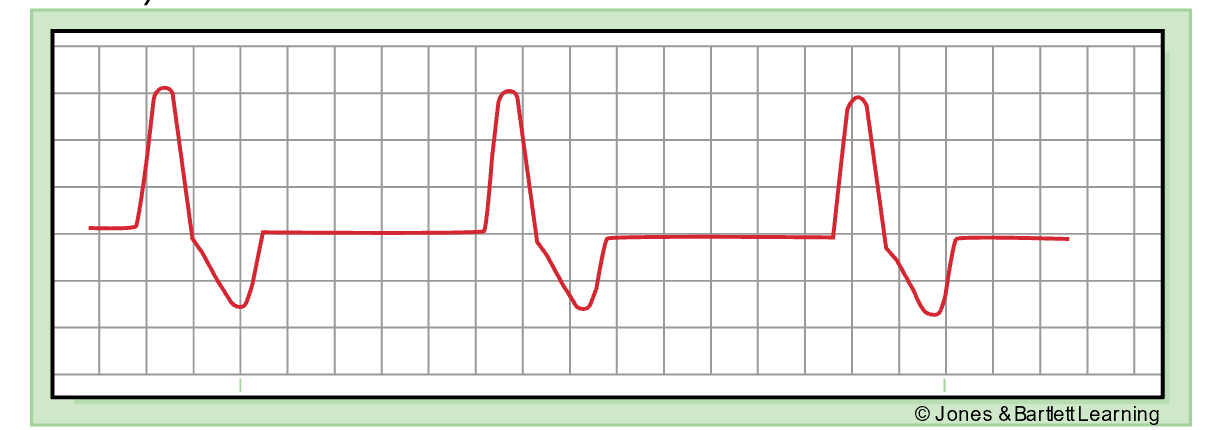
Ventricular Tachycardia
Ventricular Tachycardia (V-tach) is a consecutive series of three or more PVCs. The ventricular rate exceeds 100 bpm. It may be a precursor to ventricular fibrillation (Fib) and sudden cardiac death and should be identified and treated without delay. V-tach may occur in short bursts without symptoms or be sustained. On ECG, the atrial rate cannot be determined, and the ventricular rhythm is usually regular with a rate of 100–250 bpm. The QRS complex is widened (greater than 0.12s) and typically with an increased amplitude. The QRS complex may be uniform (monomorphic) or variable (polymorphic). If the T wave is present, it deflects opposite the QRS complex, and the QT interval is not measurable (refer to Figure 29). V-tach may result from myocardial ischemia or infarction, CAD, valvular heart disease, heart failure, cardiomyopathy, electrolyte imbalances, digoxin (Lanoxin) toxicity, procainamide (Procan) toxicity, quinidine (Quinora) toxicity, cocaine use, or the proarrhythmic effects of some antiarrhythmic medications. Even stable patients need close monitoring as they can decompensate quickly. Poor cardiac output may lead to decreased LOC, dizziness, lightheadedness, hypotension, weak peripheral pulses, syncope, reduced urine output, or confusion. The patient’s vital signs, LOC, mental status, and airway/breathing should be assessed, and they should be placed on continuous ECG monitoring. If no HR is present, defibrillation and cardiopulmonary resuscitation (CPR) should be started immediately. Synchronized cardioversion may be indicated if a pulse is present and the patient is hemodynamically unstable. If the patient is hemodynamically stable and the rhythm is monomorphic, amiodarone (Cordarone) can be administered first. If medication is ineffective, then cardioversion is recommended. In a stable patient with polymorphic V-tach, treatments with beta-blockers, lidocaine (Xylocaine), amiodarone (Cordarone), or procainamide (Procan) should be attempted before cardioversion therapy. An implantable cardioverter-defibrillator may be indicated for those with chronic V-tach (McGee, 2024; Prutkin, 2024c; Shank Coviello, 2020).
Figure 29
Ventricular Tachycardia
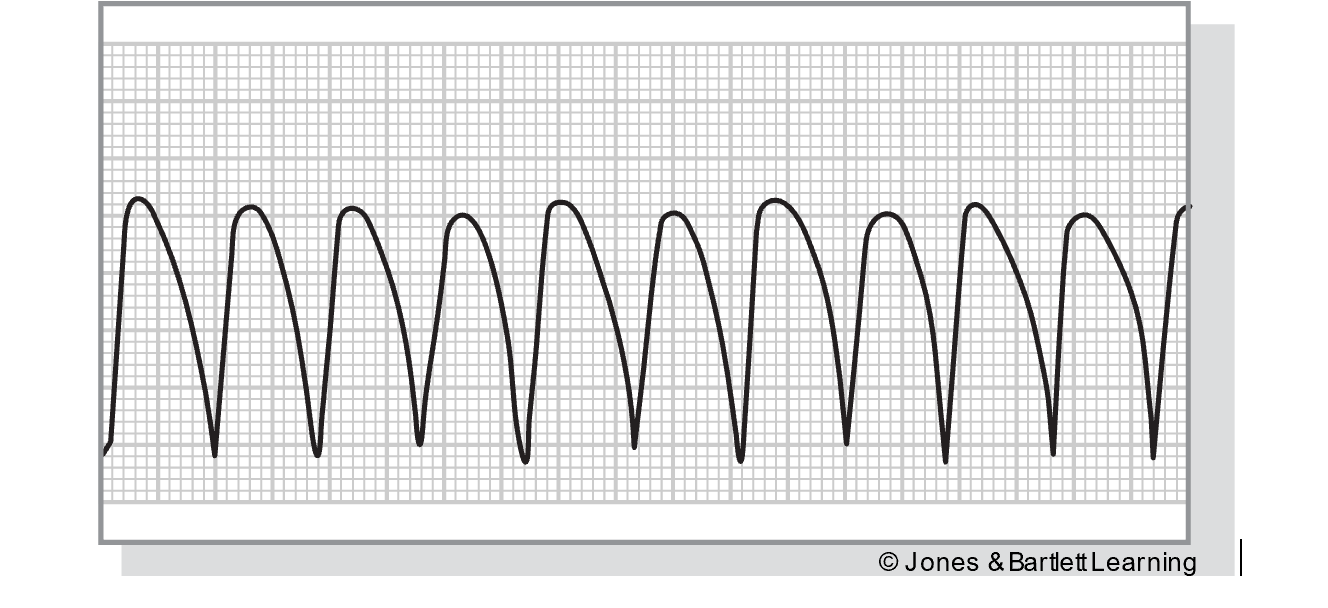
Torsades de Pointes
Torsades de Pointes (TdP), which translates to “twisting around the points,” is a polymorphic ventricular arrhythmia. It shares many of the same characteristics of V-tach, plus a rotation of the QRS complexes deflecting above and below the baseline. The rate is 150–250 bpm, the rhythm is irregular, and the QRS complexes are wide with varying amplitude (refer to Figure 30). TdP may be paroxysmal and appear or cease suddenly. In addition, TdP may deteriorate into ventricular fibrillation (VFib). It is often caused by something reversible, such as medications (consult the list of medications that prolong the QT interval located above; McGee, 2024; Prutkin, 2024c; Shank Coviello, 2020). The AHA also lists the following risk factors for QT interval prolongation and potential TdP:
- older age
- individuals assigned female at birth
- heart disease (e.g., left ventricular hypertrophy, low left ventricular ejection fraction, myocardial ischemia)
- bradyarrhythmia (e.g., sinus pauses, compensatory pauses after PVCs, pauses after conversion from AFib or flutter to sinus rhythm, complete heart block, or Mobitz II)
- electrolyte abnormalities (e.g., hypomagnesemia, hypokalemia, malnutritional electrolyte disorders)
- genetic or acquired metabolic impairment (e.g., renal failure, hepatic failure)
- genetic predisposition to QT interval prolongation (e.g., family history of syncope, sudden death; Sandau et al., 2017)
Treatment for TdP includes correcting the underlying cause. In addition, mechanical overdrive pacing may be utilized to override the ventricular rate. Magnesium sulfate or electrical cardioversion are also options that should be considered if other treatment options do not work (McGee, 2024; Prutkin, 2024c; Shank Coviello, 2020).
Figure 30
Torsades de Pointes
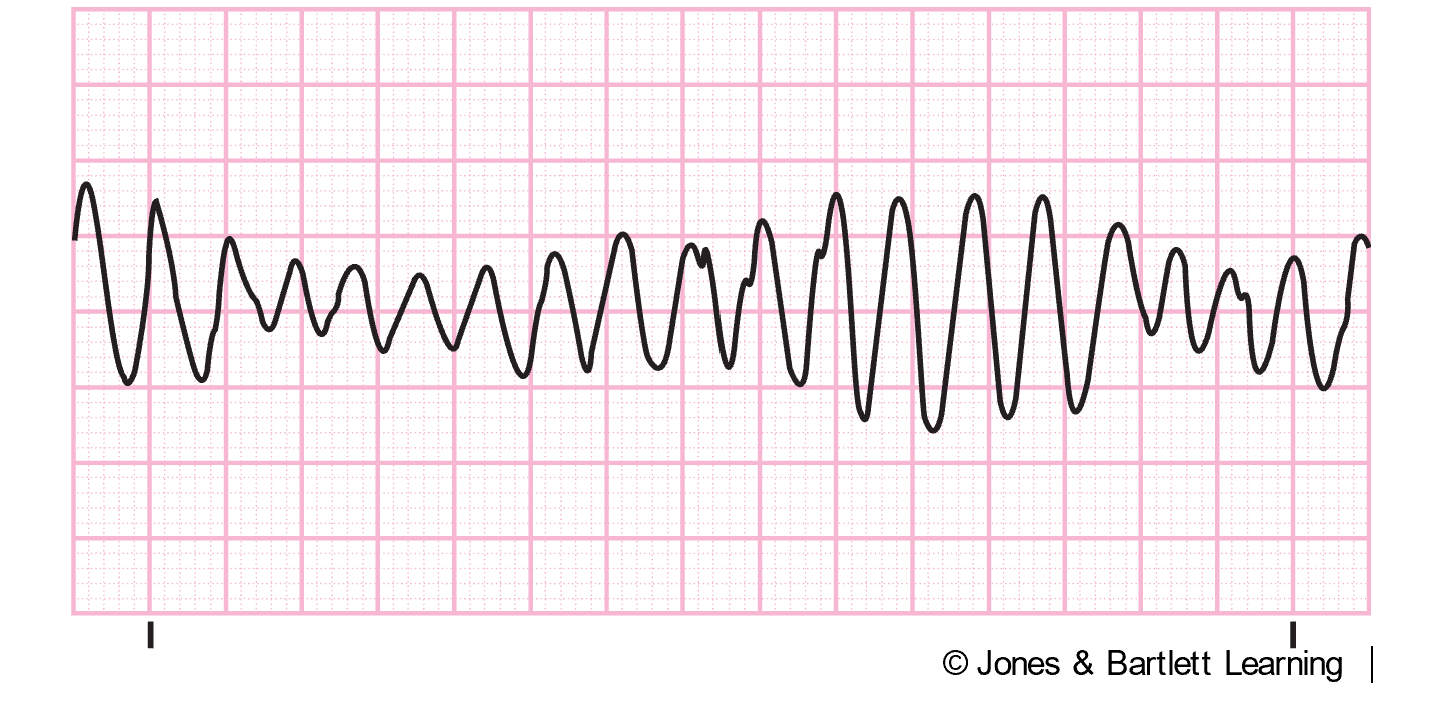
Ventricular Fibrillation
Ventricular Fibrillation (VFib) is a chaotic rhythm of electrical activity. The impulses arise from multiple different foci in the ventricles. VFib produces significant contraction and no cardiac output. If untreated, sudden cardiac death will occur. VFib may be caused by myocardial ischemia or infarction, untreated V-tach, underlying heart disease, acid-base imbalance, electric shock, severe hypothermia, electrolyte imbalances, drug toxicity (including digoxin [Lanoxin]), or severe hypoxia. On ECG, VFib appears as fibrillatory waves with no discernible P waves, QRS complexes, or T waves. The larger the waves, the higher the electrical activity in the heart, and the easier it is to convert it back to a usable and acceptable rhythm (refer to Figure 31). Patients with this rhythm are in full cardiac arrest, with no detectable pulse or blood pressure, and are unresponsive. Shivering or electric razors can interfere with ECG monitors and even mimic VFib, so patients should be assessed first. Treatment for VFib is defibrillation, but CPR can help improve survival until the defibrillator is set up. Defibrillation causes the myocardium to depolarize and allows the SA node to resume pacemaker duties. Intubation to facilitate gas exchange and medications such as epinephrine (Adrenalin) or vasopressin (Pitressin) are vital components to help the heart respond to defibrillation and recover afterward. Other medications that may be given during resuscitation include amiodarone (Cordarone), lidocaine (Xylocaine), procainamide (Procan), or magnesium sulfate (McGee, 2024; Prutkin, 2024c; Shank Coviello, 2020).
Figure 31
Ventricular Fibrillation
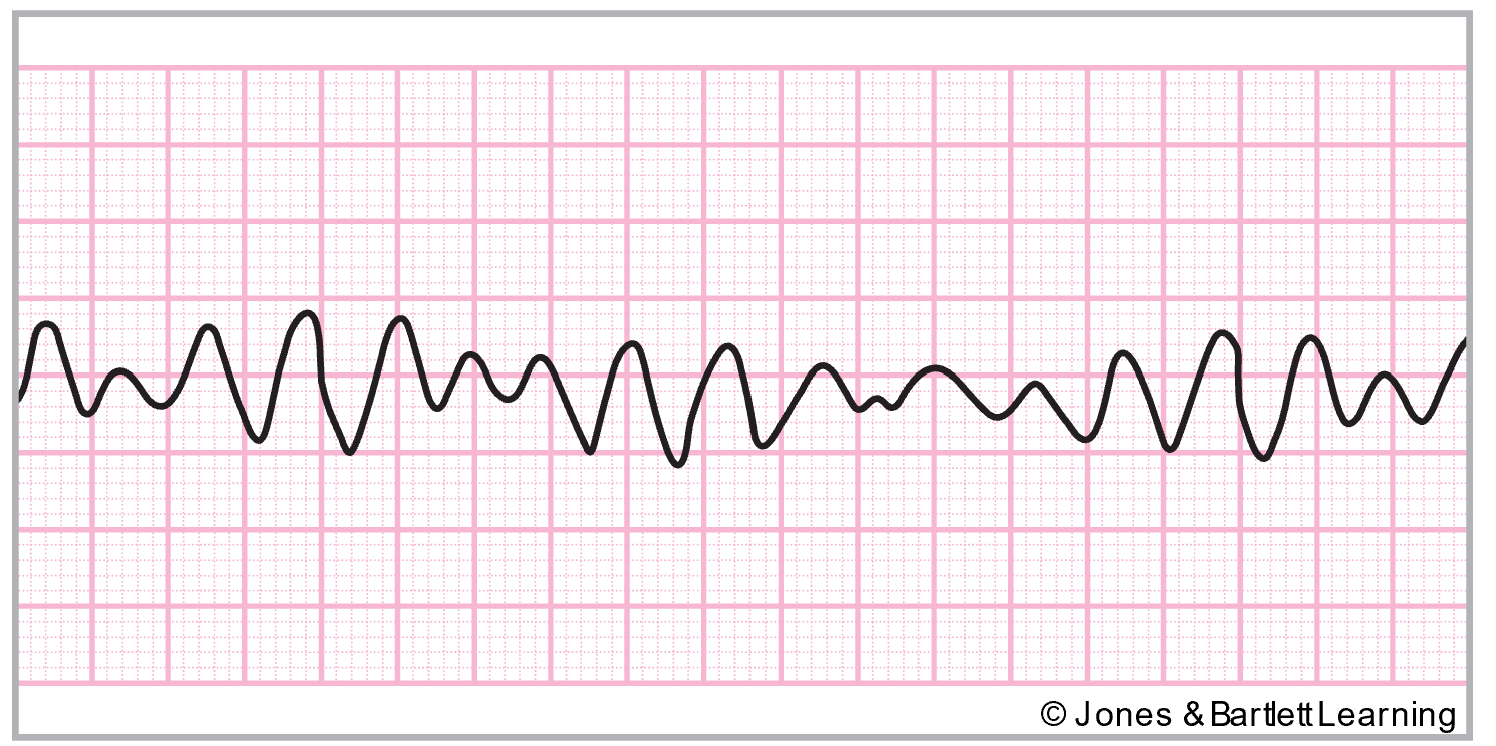
Asystole
Asystole, or ventricular standstill, corresponds with no electrical activity or cardiac output; it should be confirmed in multiple ECG leads. Asystole is predominantly related to inadequate blood flow to the heart, such as MI, severe electrolyte disturbances, massive PE, prolonged hypoxemia, severe acid-base disturbances, electric shock, drug overdose, cardiac tamponade, or hypothermia. On ECG, asystole looks like a flat line, although there may be fluctuations from chest compressions during CPR (refer to Figure 32). Pacer spikes may still be present in a patient with an implanted pacemaker. The patient should be assessed rapidly yet carefully; they will be unresponsive and have no pulse or blood pressure if truly in asystole. Treatment includes CPR followed by repeated doses of epinephrine (Adrenalin). Then, transcutaneous pacing should be initiated, and steps should be taken to correct the underlying cause (Elmer, 2025; McGee, 2024; Shank Coviello, 2020).
Figure 32
Asystole
Pulseless Electrical Activity
Pulseless electrical activity (PEA) is like asystole in that the heart is not contracting, and there is no cardiac output. The primary difference is that the heart’s electrical impulses are preserved in PEA. Therefore, the ECG will show organized electrical activity, yet the patient will be unresponsive without a pulse or measurable blood pressure. PEA can be caused by hypovolemia, hypoxia, acidosis, tension pneumothorax, cardiac tamponade, massive PE, hypothermia, electrolyte imbalances, massive acute MI, or drug overdose. As above with asystole, CPR in conjunction with epinephrine (Adrenalin) is the recommended treatment of choice, followed by correcting the underlying cause. PEA can lead to asystole if left untreated (Elmer, 2025; McGee, 2024; Shank Coviello, 2020).
Conduction Blocks
SA blocks occur when the SA node discharges regularly, but some or all of those discharges are delayed or blocked in transit through the atria. There are three degrees of SA block (Hamoud, 2024a; McGee, 2024; Shank Coviello, 2020):
- A first-degree SA block is a delay between the SA node firing and depolarization of the atria. This type of SA block cannot be seen on the ECG as there is no specific indicator for SA node activity within the electrocardiograph. Typically, first-degree SA block is asymptomatic, and only monitoring is required. Underlying causes should be treated if possible.
- Second-degree SA block is further broken down into types I and II:
- Type I (refer to Figure 33) is characterized by a progressively longer conduction time with each beat (causing an irregular rhythm and a progressively longer P-P interval) until an entire PQRST cycle is missed or dropped. The resulting pause is less than two times the shortest P-P interval.
Figure 33
SA Block Type I
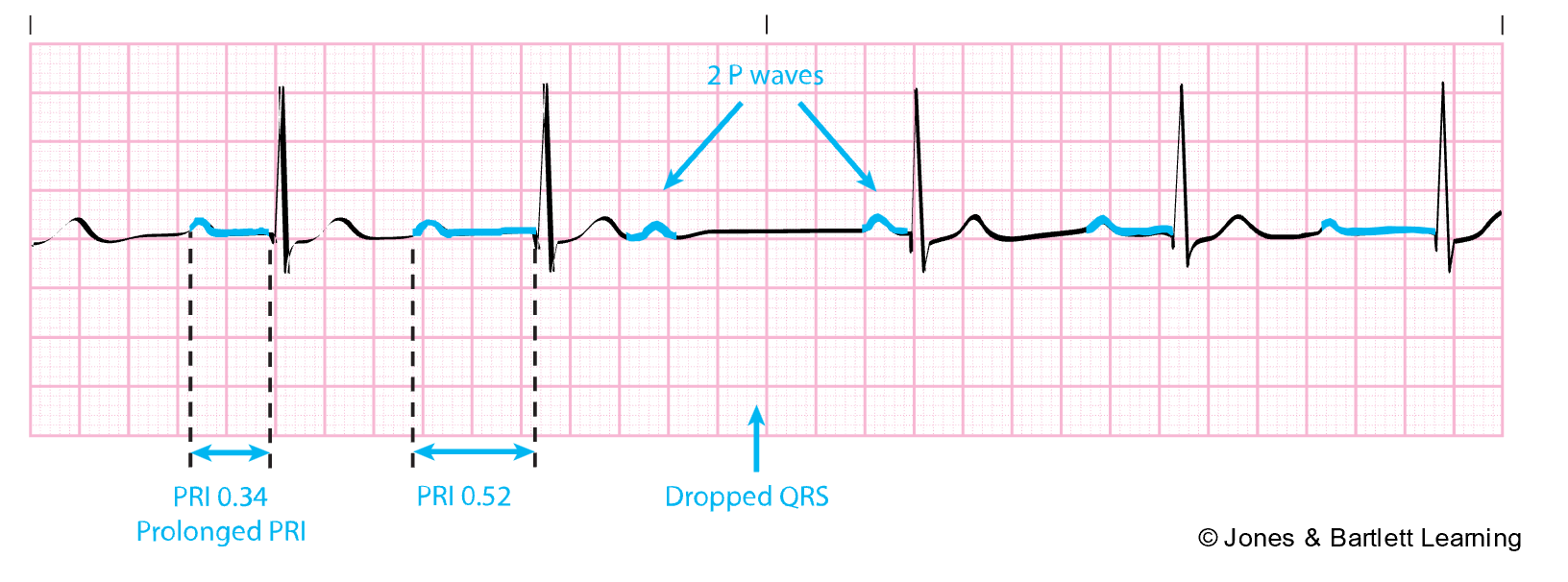
- Type II (refer to Figure 34) is characterized by a standard conduction time and a regular rhythm until a single impulse is blocked. The resulting pause is a multiple of the previous P-P interval.
Figure 34
SA Block Type II
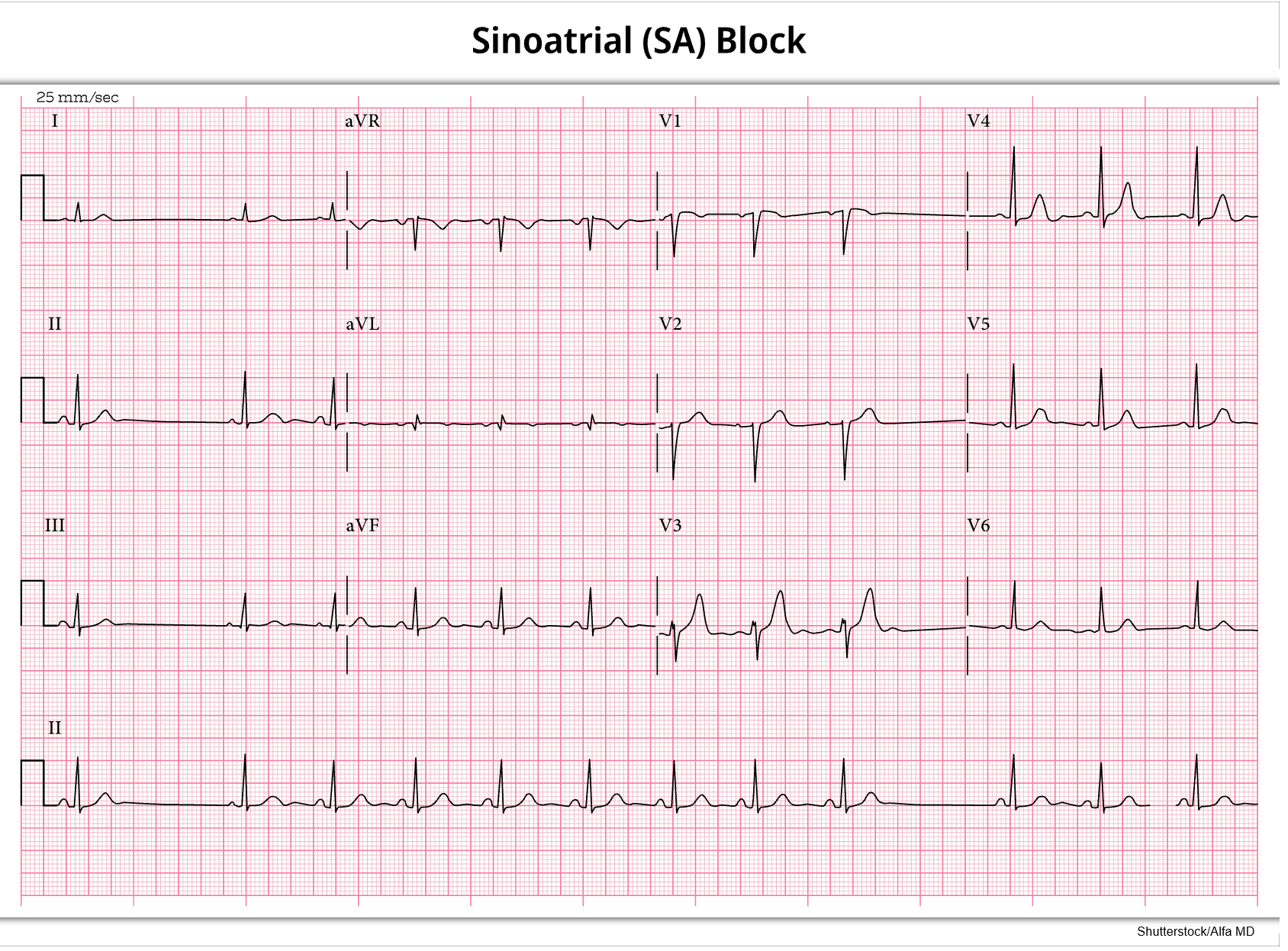
- Some impulses from the SA node are entirely blocked in third-degree SA block, causing sinus pauses. The pause is not a multiple of the previous R-R intervals. Third-degree SA block appears similar to sinus arrest on ECG but is caused by a lack of impulse conduction rather than lack of impulse formation (refer to Figure 13). The pause can be indefinite and typically ends with a sinus beat (in contrast to sinus arrest, which typically ends with a junctional escape beat; Homoud, 2024a; McGee, 2024; Shank Coviello, 2020).
Atrioventricular Blocks
Atrioventricular (AV) blocks involve blocking the electrical impulses from the SA node at the AV node. In AV blocks, atrial rates are regular, with a rate of 60–100 bpm. However, ventricular rates vary depending on the type and severity of the block. If slowed significantly, the reduced ventricular rate can reduce cardiac output and produce signs and symptoms of lightheadedness, hypotension, and confusion. AV blocks can be caused by an inferior wall or anteroseptal MI, digoxin (Lanoxin) toxicity, acute myocarditis, calcium channel blockers, beta-blockers, cardiac surgery or radiofrequency ablation procedure, congenital abnormalities, or cardiomyopathy. There are three degrees of AV block (McGee, 2024; Prutkin, 2024b; Shank Coviello, 2020):
- First-degree AV block is characterized by a consistent delay of the impulses passing through the AV node, but every impulse eventually passes through. It may be temporary or permanent. On ECG, first-degree AV block will present with a longer than average PR interval (greater than 0.2s) and a regular rate (refer to Figure 35). Typically, it is asymptomatic, with only monitoring required. If possible, underlying causes should be treated.
Figure 35
First Degree AV Heart Block
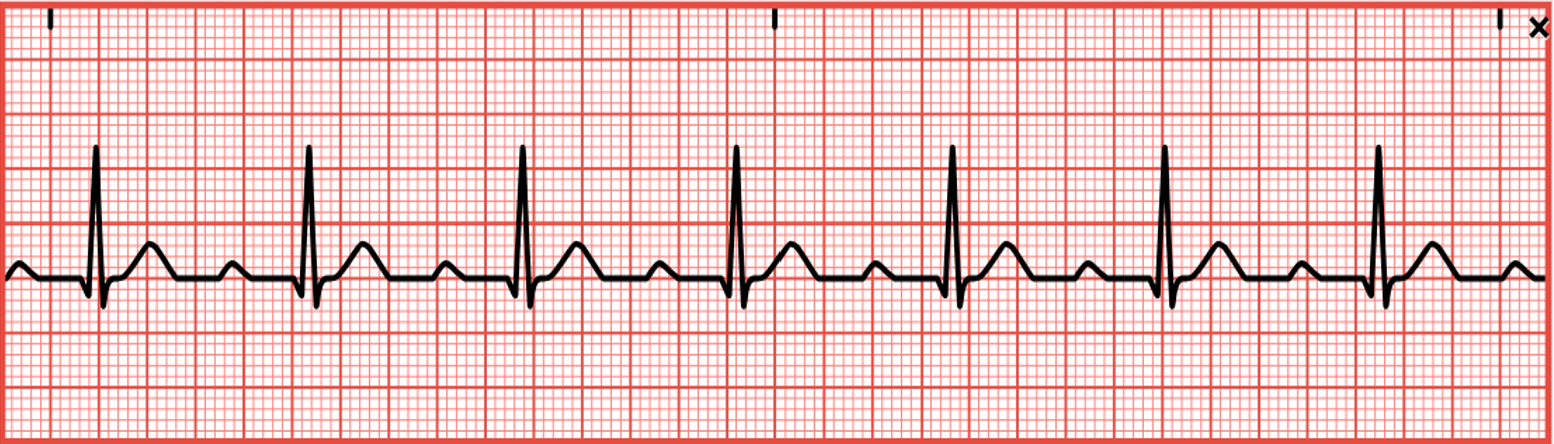
- Second-degree AV block is further broken down into type I and type II.
- Type I, or Wenckebach/Mobitz type I, block is characterized by progressively longer delays with each heartbeat until a beat is finally missed, and the cycle then restarts. An MI, CAD, rheumatic fever, or certain medications may cause it. In addition, type I AV block can also be caused by increased vagal stimulation. Treatment focuses on resolving the underlying condition and, thus, the consequent block. Type I may progress to a more severe form if it occurs during an MI. The atrial rhythm is regular (P-P interval) on ECG, yet the ventricular rhythm is regularly irregular (R-R interval). The PR interval progressively lengthens on each beat until a P wave fails to conduct and a QRS complex is missing (refer to Figure 36). Patients are usually asymptomatic, but may present with signs of reduced cardiac output, such as hypotension and dizziness/lightheadedness if the ventricular rate is slow. In symptomatic patients, atropine (Atreza) and/or a temporary pacemaker are indicated to help AV conduction. Any underlying cause should be assessed and corrected.
Figure 36
Second Degree AV Heart Block Type I
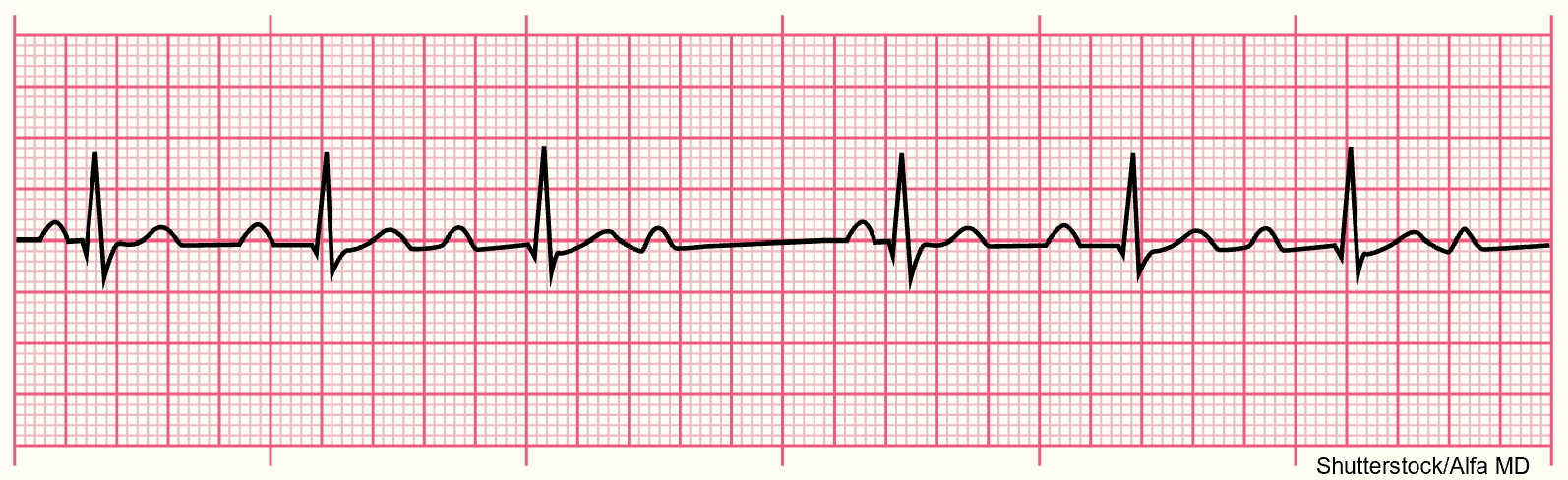
- Type II, or Mobitz type II, block is less common, yet more dangerous than type I. In type II AV block, impulses pass through the AV node regularly until a single beat fails to conduct. It can be caused by an anterior wall MI, severe CAD, or degenerative changes. The ventricular rate tends to be slower, thus decreasing cardiac output and causing symptoms such as hypotension and dizziness. The ratio of conducted to dropped beats can be as low as 2:1. On ECG, the atrial rate will be regular, but the ventricular rate may not be. There will be missing QRS complexes. The QRS complexes may be wide, and the PR interval may be prolonged but consistent (refer to Figure 37). As dropped beats increase, patients may report fatigue, dyspnea, chest pain, or lightheadedness. HR may be slow and blood pressure low. If symptomatic, atropine (Atreza), dopamine (Intropin), or epinephrine (Adrenalin) may be given to increase HR and improve cardiac output and oxygen to treat hypoxia. Transcutaneous pacing may also be indicated until a permanent pacemaker can be safely placed.
Figure 37
Second Degree AV Heart Block Type II
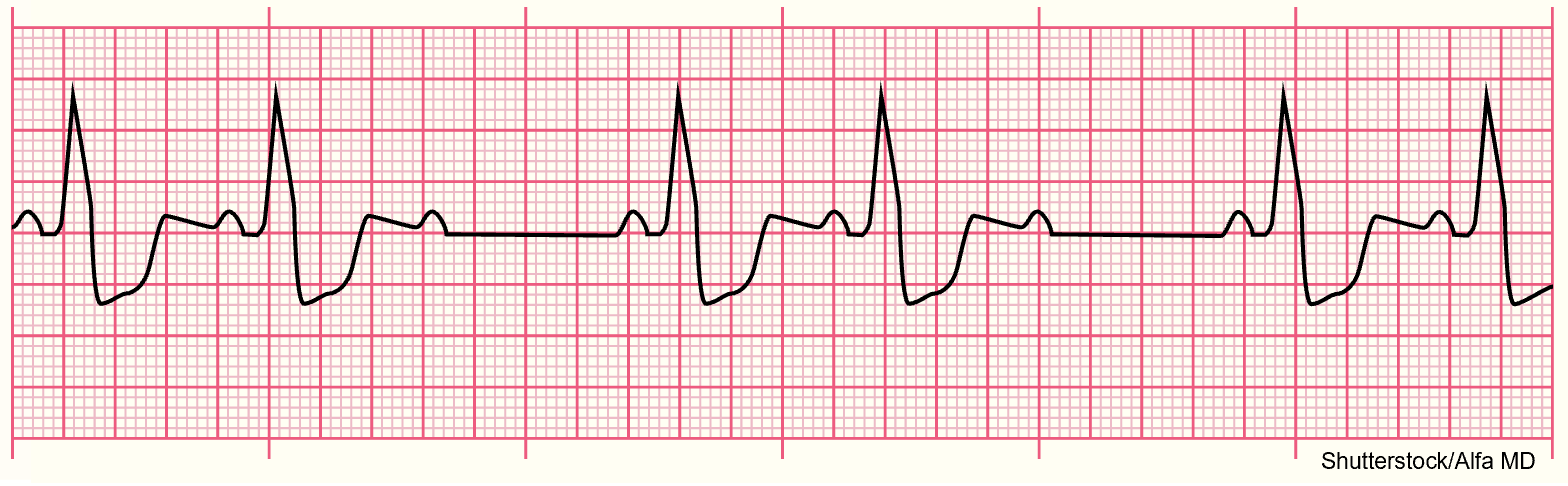
- Third-degree AV block, or complete heart block, occurs when all impulses are permanently or temporarily blocked at the AV node. The atrial rate continues at 60–100 bpm, with regular P waves on ECG. However, the ventricular rate is 40–60 bpm (if originating from the AV node) or 20–40 bpm (if arising from the Purkinje system), with no connection or coordination between the two (refer to Figure 38). It is most commonly congenital but may be caused by CAD, an anterior or inferior wall MI, degenerative changes, digoxin (Lanoxin) toxicity, calcium channel blockers, beta-blockers, or a surgical injury. With a decreased ventricular rate and no atrial kick, cardiac output can drop significantly, causing severe fatigue, dyspnea, chest pain, lightheadedness, altered mental status, loss of consciousness, hypotension, pallor, diaphoresis, bradycardia, and variable pulses. Atropine (Atreza), dopamine (Intropin), epinephrine (Adrenalin), and/or a temporary pacemaker may be utilized to increase the ventricular rate and improve cardiac output. The underlying cause may be resolved, or a permanent pacemaker may be inserted.
Figure 38
Third Degree AV Heart Block
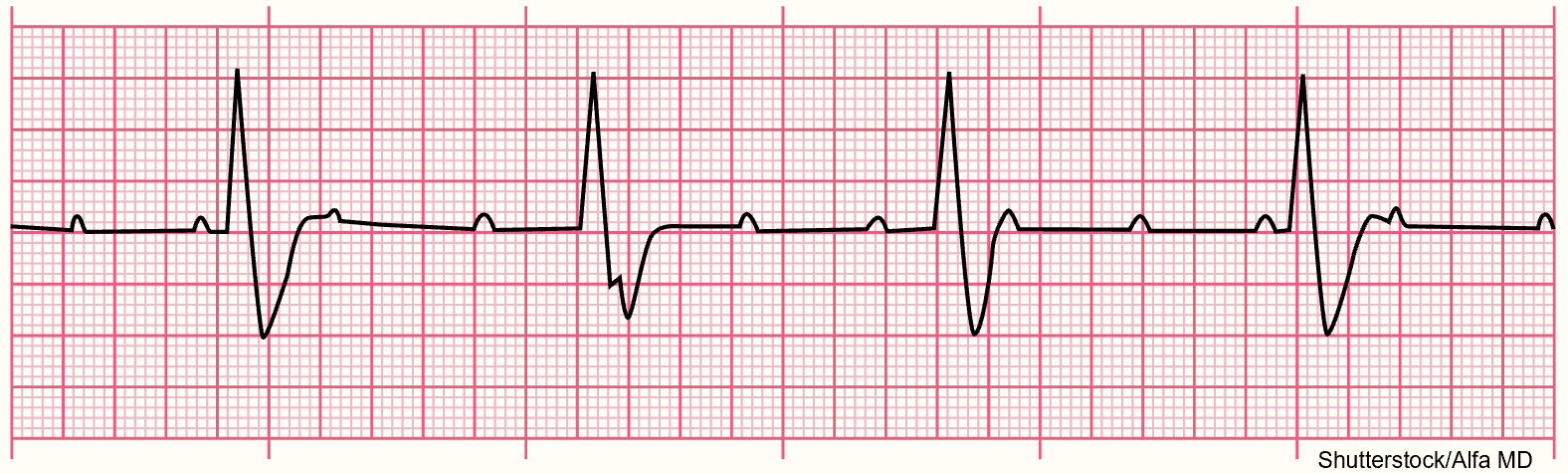
Bundle Branch Block
Bundle branch block (BBB) is a potential complication of an MI. Either the left or the right bundle branch does not conduct impulses. If the block happens further down the left bundle branch, it is called a hemiblock. If the block occurs as HR increases, it is called rate-related. Ventricular depolarization is prolonged because the signal travels down one bundle branch and then across to the opposite ventricle via myocardial cell-to-cell conduction. This leads to a widened QRS complex (greater than 0.12s) on the strip. Leads V1 and V6 help determine which side of the heart is affected. A right BBB (refer to Figure 39) can be caused by an anterior wall MI, CAD, cardiomyopathy, cor pulmonale, or PE. The QRS complex may be double-notched in lead V1, which is an rSR (small R wave, followed by an S wave, followed by a large R wave) due to late right ventricular depolarization and a negative T wave. In lead V6, a widened S wave may be seen. A left BBB (refer to Figure 39) may be caused by hypertension, aortic stenosis, degenerative changes in the conduction system, or CAD. If left BBB occurs with an anterior wall MI, it may indicate a complete heart block and require pacemaker placement. A wide S wave will be seen after the widened QRS complex in lead V1 with a positive T wave. In lead V6, a tall, notched R wave with an inverted T wave will be seen. Most patients are asymptomatic, although BBBs may cause syncope if symptomatic and require pacing or cardiac resynchronization therapy to help the ventricles get back in sync (McGee, 2024; Prutkin, 2023a; Shank Coviello, 2020).
Figure 39
Left and Right Bundle Branch Block
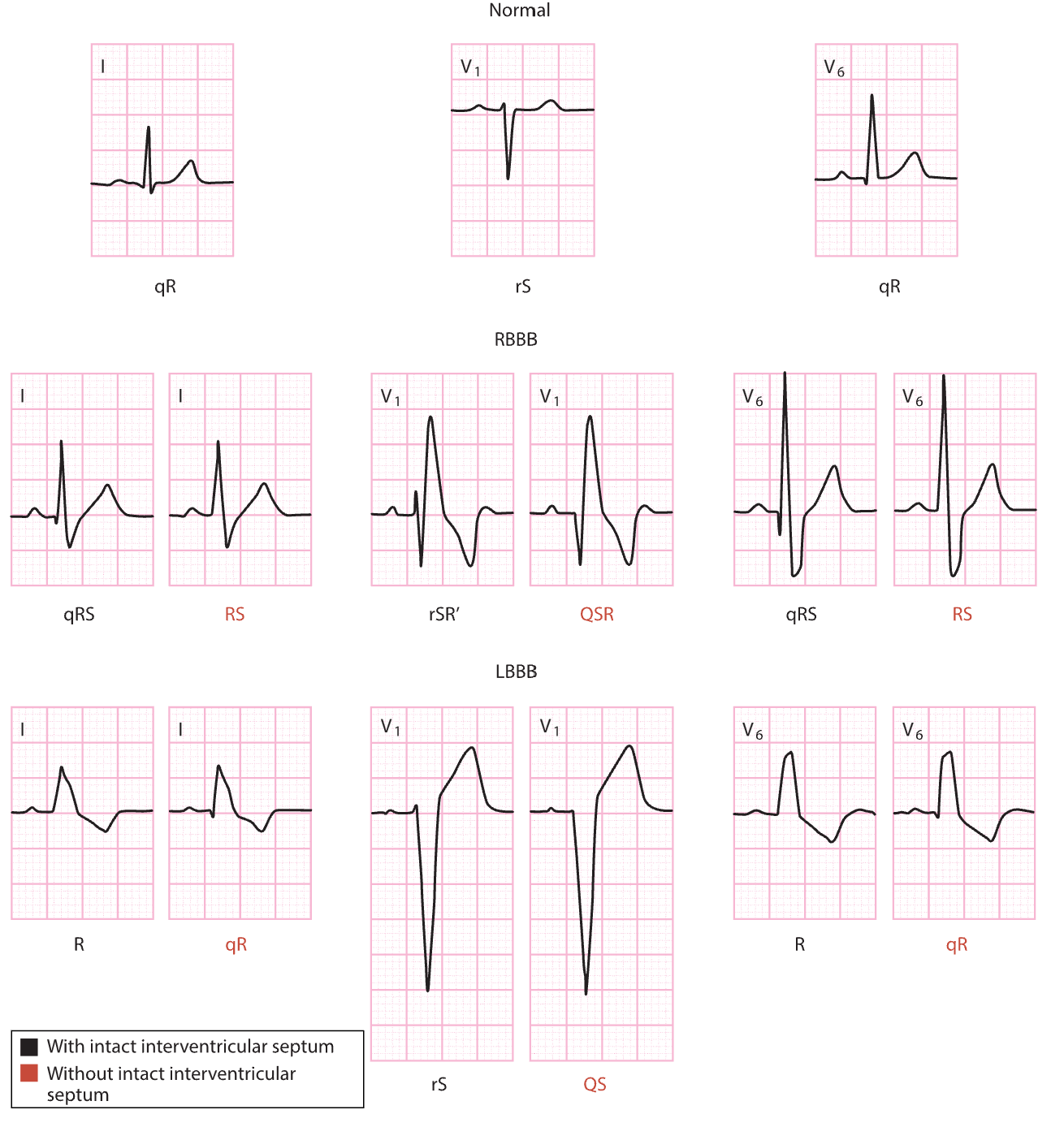
Pacemakers
A pacemaker is a device that generates electrical stimuli to the heart muscle when a patient has a slower-than-normal impulse formation or a symptomatic AV or ventricular conduction disturbance. Common indications for pacemaker placement include sinus node dysfunction, acquired AV block, and being post-MI. Other less common indications may include long QT syndrome, congenital complete heart block, and hypertrophic cardiomyopathy. Biventricular (i.e., both ventricles) pacing may be used to treat advanced heart failure. Most pacemakers are permanent; however, temporary pacemakers can be used in the hospital setting after an acute MI or open-heart surgery. The temporary pacemaker can be removed when the patient’s condition improves or a permanent pacemaker is implanted (Hinkle et al., 2021; Lak & Goyal, 2022).
Pacemakers have two components: an electrical pulse generator and the pacemaker electrodes (refer to Figure 40). The electrical pulse generator determines the rate (in beats per minute) and strength (in milliamperes [mA]) of the stimulus delivered to the heart. The generator can detect intracardiac electrical activity to respond appropriately (e.g., sensitivity measured in millivolts [mV]). The sensitivity is set, and the intracardiac electrical activity must exceed this threshold to be sensed by the device. The pulse generator is most commonly placed in the infraclavicular region of the anterior chest wall. The pacemaker's leads carry the electrical impulse created by the generator to the heart. Most pacing systems use transvenous electrodes that transmit impulses from the generator to the heart musculature. These endocardial leads are threaded through a central vein into the heart (right atrium and ventricle). Less commonly, temporary epicardial wires may directly stimulate the surface of the heart (Hinkle et al., 2021; Lak & Goyal, 2022).
Figure 40
Pacemaker Generator and Lead Placement
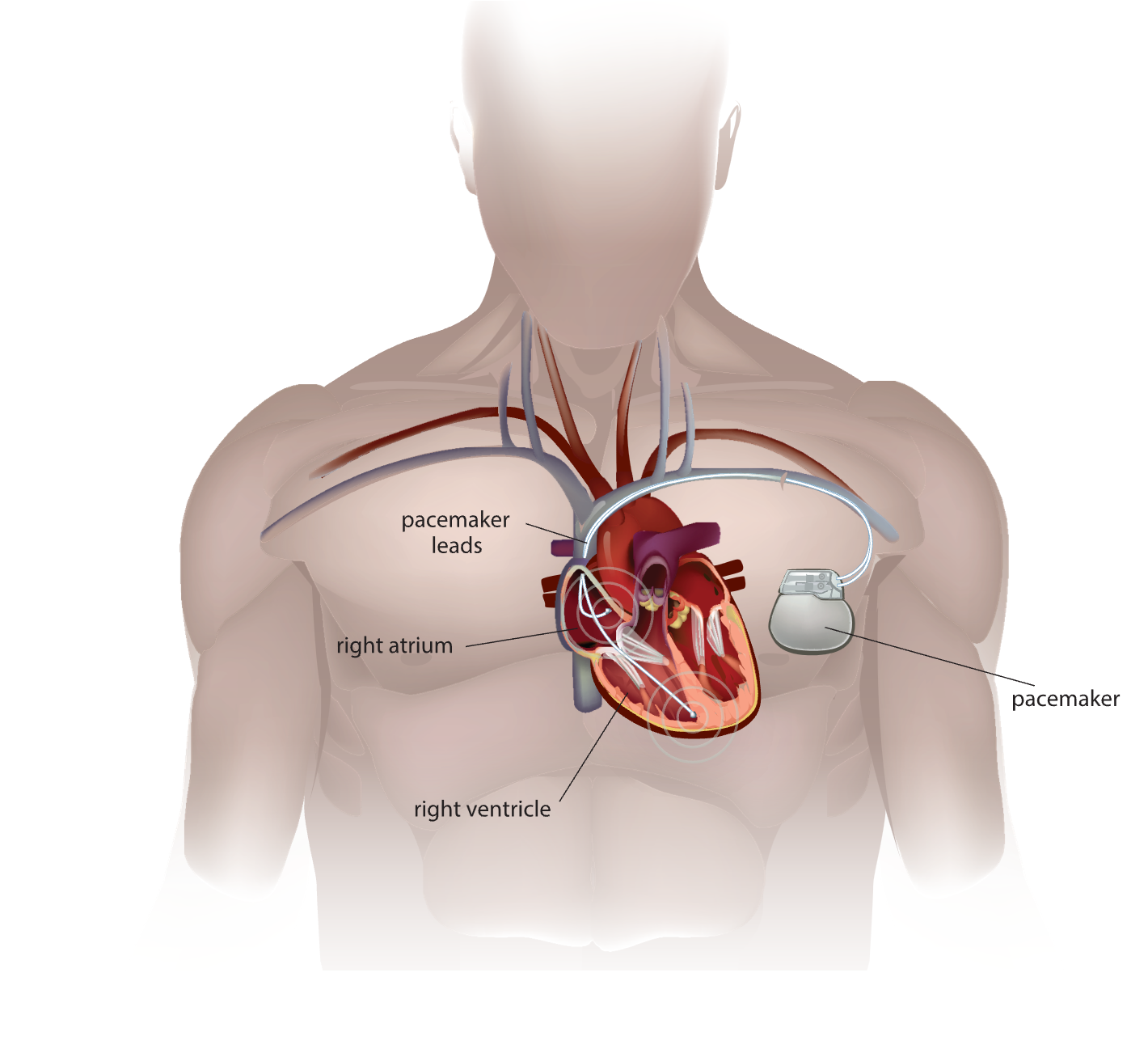
A pacemaker can pace the ventricle (refer to Figure 41), atrium (refer to Figure 42), or both. A pacemaker spike (i.e., a vertical line) is seen on an ECG when pacing is initiated. The appropriate ECG complex (termed capture) should immediately follow the pacemaker spike (i.e., a P wave when atrial pacing or a QRS complex when ventricular pacing). When pacing occurs, the impulse starts differently from the patient’s normal rhythm; therefore, the P wave and QRS complex look different from the typical ECG complex. There are three basic types of pacemakers: single chamber (i.e., one lead attaches to the upper or lower heart chamber), dual-chamber (i.e., one lead in the upper and one in the lower chamber), and biventricular (i.e., used for cardiac resynchronization therapy). The type of pacemaker and the settings selected are dependent on the patient’s dysrhythmia, cardiac function, and age. Generally, pacemakers are set to sense and respond to intrinsic activity (i.e., on-demand pacing). A fixed or asynchronous pacemaker is set to pace but not sense (i.e., paces at a constant rate regardless of intrinsic activity; Hinkle et al., 2021; Lak & Goyal, 2022).
Figure 41
Ventricular Paced Rhythm
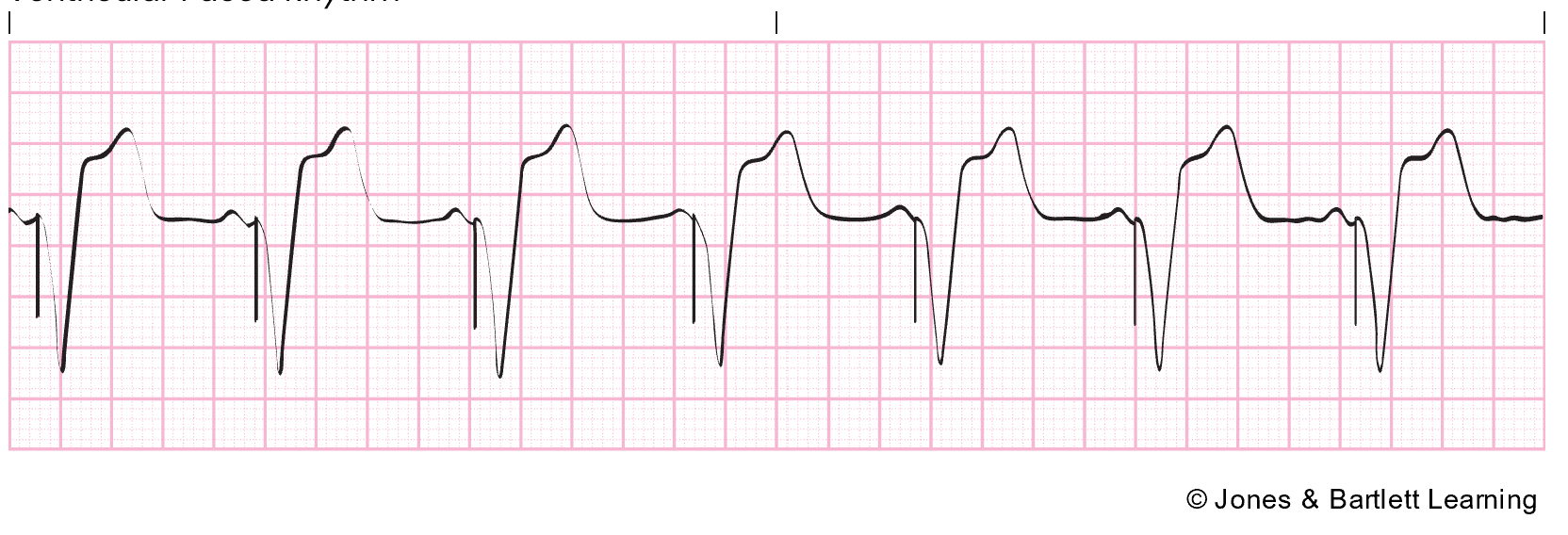
Figure 42
Atrial Paced Rhythm
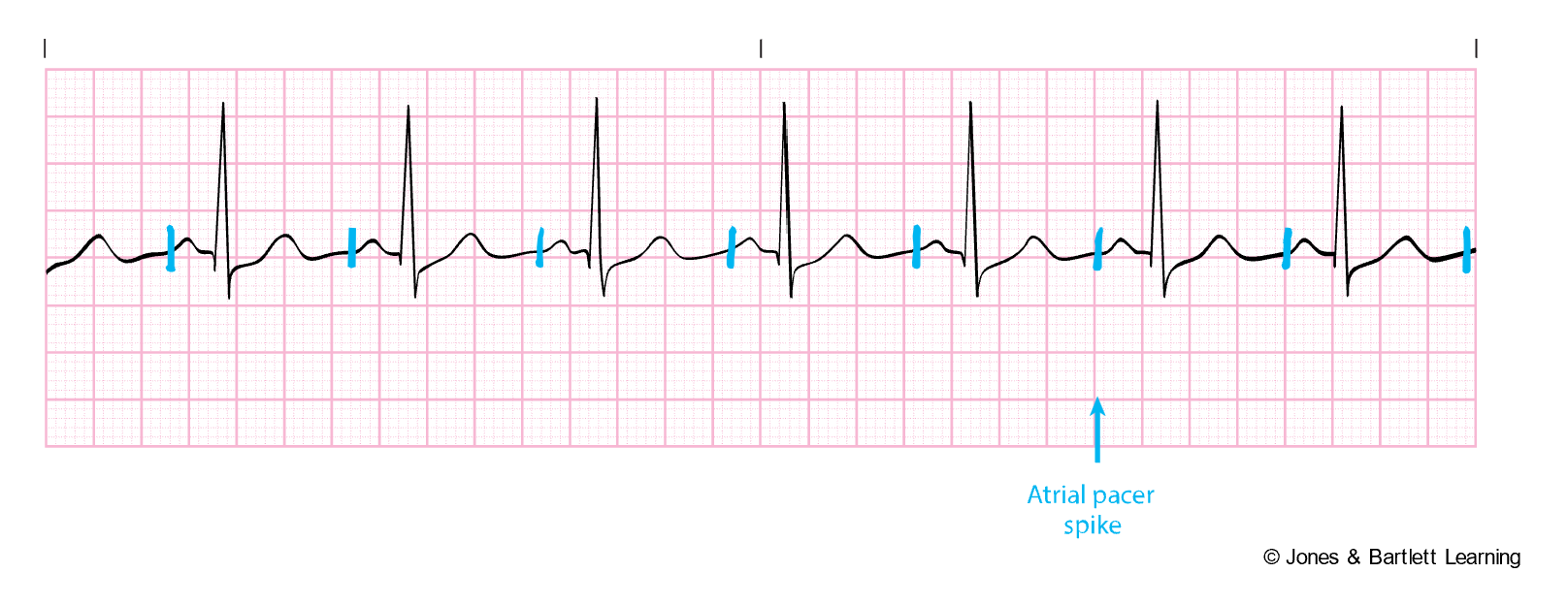
Since pacemakers are widely used, universal codes are used to communicate the modes of each pacemaker. The coding system is known as NASPE-BPEG because it is sanctioned by the North American Society of Pacing and Electrophysiology and the British Pacing and Electrophysiology Group. The codes consist of five letters, with the fourth and fifth letters only used for permanent pacemakers. Most commonly, only the first three letters are used in the code (Hinkle et al., 2021; Lak & Goyal, 2022). The letters refer to:
- Letter 1 refers to the area being paced (e.g., A designates the atria, V designates the ventricle, D designates dual, and O designates none).
- Letter 2 refers to the area which is sensed (e.g., A designates the atria, V designates the ventricle, D designates dual, and O designates none).
- Letter 3 refers to the response of the pacemaker to sensing (e.g., O designates non, I designates inhibiting, T designates triggering, and D designates dual).
- Letter 4 refers to rate adaptiveness (e.g., O designates none and R designates rate adaptiveness).
- Letter 5 indicates one of two things:
- The permanent generator has multi-site pacing capability (A designates the atria, V designates the ventricle, D designates dual, and O designates none).
- That the pacemaker has an anti-tachycardia function (Hinkle et al., 2021; Lak & Goyal, 2022).
The modes of a pacemaker can easily be identified by dividing them into single- and dual-chamber categories. Single-chamber modes include (Hinkle et al., 2021; Lak & Goyal, 2022):
- VOO (V designates pacing in the ventricle, O designates sensing is OFF, O means that the response to sensing is OFF) mode paces at a programmed rate regardless of intrinsic activity.
- VVI (V designates pacing in the ventricle, V designates sensing in the ventricle, I designates inhibit) mode indicates that the pacemaker can sense electrical activity and withhold when not needed.
- AOO (A designates pacing in the atrium, O designates sensing is OFF, O means that the response to sensing is OFF) mode paces at a programmed rate regardless of intrinsic activity.
- AAI (A designates pacing in the atrium, A designates sensing in the atrium, I designates inhibit) mode indicates that the pacemaker can adapt to the intrinsic atrial rate and pace or inhibit if needed.
Dual-chamber modes can be divided into tracking and nontracking and include (Hinkle et al., 2021; Lak & Goyal, 2022):
- Tracking Modes
- DDD (the first D designates pacing in the atrium and ventricle, the second designates sensing in the atrium and ventricle, and the third designates inhibit and/or trigger) mode can adapt to the intrinsic heart rhythm and mimic normal conduction. DDD has four distinct pacing patterns.
- The AsVs (atrial sensed ventricular sensed) pattern is used when the sinus and AV node functions are good.
- The AsVp (atrial sensed ventricular paced) pattern is used when the sinus node function is good, but the AV node function is poor.
- The ApVs (atrial paced ventricular sensed) pattern is used when the AV node function is good, but the sinus node function is poor.
- ApVp (atrial paced ventricular paced) pattern is used when sinus and AV node functions are poor.
- VDD (V designates pacing in the ventricle, the first D designates sensing in the atrium and ventricle, and the second D designates inhibit and/or trigger) mode cannot pace the atrium. However, intrinsic atrial activity can trigger an AV delay, leading to P wave tracking and AV synchrony. VDD should only be used for patients with good SA node function because AV dissociation can occur if the sinus node function drops below the pacemaker rate.
- DDD (the first D designates pacing in the atrium and ventricle, the second designates sensing in the atrium and ventricle, and the third designates inhibit and/or trigger) mode can adapt to the intrinsic heart rhythm and mimic normal conduction. DDD has four distinct pacing patterns.
- Non-Tracking Modes
- DDI (the first D designates pacing in the atrium and ventricle, the second designates sensing in the atrium and ventricle, and the I designates response to sensing will be either pace or inhibit) is primarily used in patients with atrial tachyarrhythmias.
- DOO (D designates pacing in the atrium and ventricle, the first O designates sensing is OFF, and the second O establishes that the response to sensing is OFF) is an asynchronous pacing mode with AV sequential pacing at a lower rate regardless of intrinsic activity.
- R (rate response or rate-adaptive pacing) is used for patients with chronotropic incompetence (i.e., the inability of the heart to increase the rate with increased activity).
References
American Heart Association. (2024a). Common tests for arrhythmias. https://www.heart.org/en/health-topics/arrhythmia/symptoms-diagnosis--monitoring-of-arrhythmia/common-tests-for-arrhythmia
American Heart Association. (2024b). Symptoms, diagnosis and monitoring of arrhythmia. https://www.heart.org/en/health-topics/arrhythmia/understand-your-risk-for-arrhythmia
American Heart Association. (2024c). Tachycardia: Fast heart rate. https://www.heart.org/en/health-topics/arrhythmia/about-arrhythmia/tachycardia--fast-heart-rate
American Heart Association. (2024d). Understand your risk for arrhythmia. https://www.heart.org/en/health-topics/arrhythmia/understand-your-risk-for-arrhythmia
American Heart Association. (2024e). What is an arrhythmia? https://www.heart.org/en/health-topics/arrhythmia/about-arrhythmia
Chakravarthy, R., Goggins, K., Leverenz, D., Trumbo, S. P., Kripalani, S., & Limper, H. M. (2020). Lessons learned from efforts to reduce overuse of cardiac telemetry monitoring. The Joint Commission Journal on Quality and Patient Safety, 46(8), 464–470. https://doi.org/10.1016/j.jcjq.2020.05.001
Desai, D. S., & Hajouli, S. (2023). Arrhythmias. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK558923
Di Biase, L., & Walsh, E. P. (2024). Wolff-Parkinson-White syndrome: Anatomy, epidemiology, clinical manifestations, and diagnosis. UpToDate. Retrieved March 13, 2025, from https://www.uptodate.com/contents/wolff-parkinson-white-syndrome-anatomy-epidemiology-clinical-manifestations-and-diagnosis
Elmer, J. (2025). Advanced cardiac life support (ACLS) in adults. UpToDate. Retrieved March 15, 2025, from https://www.uptodate.com/contents/advanced-cardiac-life-support-acls-in-adults
Hinkle, J. L., Cheever, K. H., & Overbaugh, K. (2021). Brunner & Suddarth’s textbook of medical-surgical nursing (15th ed.). Wolters Kluwer.
Homoud, M. K. (2023a). Sinus node dysfunction: Clinical manifestations, diagnosis, and evaluation. UpToDate. Retrieved March 11, 2025, from https://www.uptodate.com/contents/sinus-node-dysfunction-clinical-manifestations-diagnosis-and-evaluation
Homoud, M. K. (2023b). Sinus tachycardia: Evaluation and management. UpToDate. Retrieved March 8, 2025, from https://www.uptodate.com/contents/sinus-tachycardia-evaluation-and-management
Homoud, M. K. (2024a). Sinoatrial nodal pause, arrest, and exit block. UpToDate. Retrieved March 11, 2025, from https://www.uptodate.com/contents/sinoatrial-nodal-pause-arrest-and-exit-block
Homoud, M. K. (2024b). Sinus bradycardia. UpToDate. Retrieved March 8, 2025, from https://www.uptodate.com/contents/sinus-bradycardia
Kim, E-J., Hoffman, T. J., Nah, G., Vittinghoff, E., Delling, F., Marcus, G. M. (2021). Coffee consumption and incident tachyarrhythmias: Reported behavior, Mendelian randomization, and their interactions. JAMA Internal Medicine, 181(9), 1185–1193. https://doi.org/10.1001/jamainternmed.2021.3616
Kumar, K. (2023). Atrial fibrillation: Overview and management of new-onset atrial fibrillation. UpToDate. Retrieved March 12, 2025, from https://www.uptodate.com/contents/atrial-fibrillation-overview-and-management-of-new-onset-atrial-fibrillation
Lak, H. M., & Goyal, A. (2022). Pacemaker types and selection. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK556011
Levy, S., & Olshansky, B. (2024). Arrhythmia management for the primary care clinician. UpToDate. Retrieved March 1, 2025, from https://www.uptodate.com/contents/arrhythmia-management-for-the-primary-care-clinician
McGee, J. E. (2024). EKG essentials: A student handbook. University of Arkansas. https://uark.pressbooks.pub/ekgmanual/chapter/basic-cardiac-concepts
Mechanic, O. J., Gavin, M., Grossman, S. A., & Ziegler, K. (2023). Acute myocardial infarction (nursing). StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK568759
Mitchell, L. B. (2025). Overview of arrhythmias. https://www.merckmanuals.com/professional/cardiovascular-disorders/overview-of-arrhythmias-and-conduction-disorders/overview-of-arrhythmias
National Cancer Institute. (n.d.). Structure of the heart [image]. Retrieved March 1, 2025, from https://training.seer.cancer.gov/anatomy/cardiovascular/heart/structure.html
National Heart, Lung, and Blood Institute. (2022a). Arrhythmias: Causes and triggers. https://www.nhlbi.nih.gov/health/arrhythmias/causes
National Heart, Lung, and Blood Institute. (2022b). Arrhythmias: Diagnosis. https://www.nhlbi.nih.gov/health/arrhythmias/diagnosis
National Institute of Health. (2022). What is an arrhythmia? https://www.nhlbi.nih.gov/health/arrhythmias
National Library of Medicine. (2024). Arrhythmias. https://medlineplus.gov/ency/article/001101.htm
Oberman, R., Shumway, K. R., & Bhardwaj, A. (2023). Physiology, cardiac. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK526089
Pendharkar, S. S., Barry, I. B., Patibandla, S., Leung, T. I., Gupta, A., Lin, A. N., & Gasperino, J. A. (2020). AHA telemetry guidelines improve telemetry utilization in the inpatient setting. The American Journal of Managed Care, 26(11), 476–481. https://doi.org/10.37765/ajmc.2020.88525
Phang, R., & Prutkin, J. M. (2025). Atrial flutter: Overview of diagnosis and management. UpToDate. Retrieved March 11, 2025, from https://www.uptodate.com/contents/overview-of-atrial-flutter
Prutkin, J. M. (2023a). ECG tutorial: Intravetricular block. UpToDate. Retrieved March 15, 2025, from https://www.uptodate.com/contents/ecg-tutorial-intraventricular-block
Prutkin, J. M. (2023b). ECG tutorial: Rhythms and arrhythmias of the sinus node. UpToDate. Retrieved March 8, 2025, from https://www.uptodate.com/contents/ecg-tutorial-rhythms-and-arrhythmias-of-the-sinus-node
Prutkin, J. M. (2024a). ECG tutorial: Atrial and atrioventricular nodal (supraventricular) arrhythmias. UpToDate. Retrieved March 11, 2025, from https://www.uptodate.com/contents/ecg-tutorial-atrial-and-atrioventricular-nodal-supraventricular-arrhythmias
Prutkin, J. M. (2024b). ECG tutorial: Atrioventricular block. UpToDate. Retrieved March 14, 2025, from https://www.uptodate.com/contents/ecg-tutorial-atrioventricular-block
Prutkin, J. M. (2024c). ECG tutorial: Ventricular arrhythmias. UpToDate. Retrieved March 15, 2025, from https://www.uptodate.com/contents/ecg-tutorial-ventricular-arrhythmias
Rehman, I., & Rehman, A. (2023). Anatomy, thorax, heart. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK470256
Sandau, K. E., Funk, M., Auerbach, A., Blum, K., Cvach, M., Lampert, R., May, J. L., McDaniel, G. M., Perez, M. V., Sendelbach, S., Sommargren, C. E., & Wang, P. J. (2017). Update to practice standards for electrocardiographic monitoring in hospital settings: A scientific statement from the American Heart Association. Circulation, 136, e273–e344. https://doi.org/10.1161/CIR.0000000000000527
Sattar, Y., & Chhabra, L. (2023). Electrocardiogram. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK549803
Sauer, W. H. (2024). Normal sinus rhythm and sinus arrhythmia. UpToDate. Retrieved March 4, 2025, from https://www.uptodate.com/contents/normal-sinus-rhythm-and-sinus-arrhythmia
Shank Coviello, J. (Ed.). (2020). ECG interpretation made incredibly easy (7th ed.). Lippincott Williams & Wilkins.
Zimetbaum, P. J. (2023). Evaluation of palpitations in adults. UpToDate. Retrieved March 2, 2025, from https://www.uptodate.com/contents/evaluation-of-palpitations-in-adults
Powered by Froala Editor
Single Course Cost: $25.00
Add to CartSave while Futhering your Education
Introduction to Telemetry Monitoring for Arrhythmias Nursing CE Course is offered in the packages below.- 27 ANCC Hours
- 10 Courses
- 530 ANCC Hours
- 190 Courses