About this course:
This course reviews the fundamental aspects of nutritional support in patients with critical illness, including the components of a nutritional assessment, types of enteral and parenteral nutrition access routes, nursing care of nutritional access devices, and clinical considerations to enhance nursing practice and improve patient outcomes.
Course preview
Nutritional Support in Critical Illness: Enteral and Parenteral Nutrition
This course reviews the fundamental aspects of nutritional support in patients with critical illness, including the components of a nutritional assessment, types of enteral and parenteral nutrition access routes, nursing care of nutritional access devices, and clinical considerations to enhance nursing practice and improve patient outcomes.
By the completion of this course, nurses should be able to:
- Discuss the vital role of nutrition in patients with critical illness and the components of a nutrition assessment.
- Provide an overview of enteral nutrition (EN), indications, access routes, benefits, nursing care, and common complications.
- Provide an overview of parenteral nutrition (PN), indications, access routes, benefits, nursing care, and common complications.
- Discuss the most common complications associated with short-term and long-term use of EN and PN.
- Understand ethical issues and considerations related to supporting critically ill patients nutritionally.
Nutrition is a crucial aspect of health and is of paramount significance in patients with critical illness, especially in an intensive care unit (ICU). Critical conditions, such as sepsis, trauma, or burns, are typically accompanied by a metabolic stress response generated by the body's survival mechanisms. Activation of this stress response is often accompanied by a systemic inflammatory response that threatens intestinal integrity and immune function, leading to complications such as infection, multiple organ dysfunction (or failure), prolonged hospitalization, and death. These processes induce complex metabolic and physiologic adaptations in energy production and metabolism, leading to alterations in body composition and heightening malnutrition risk. Advancements in life-sustaining support measures have improved survival rates for previously fatal critical conditions. While nutritional support was historically regarded as an adjunctive measure to preserve lean body mass and support a critically ill patient throughout the stress response, this understanding has evolved over the last few decades with advances in science and technology. These innovations have led to a more comprehensive understanding of how adequate nutritional support during the acute phase of illness promotes long-term recovery (Lambell et al., 2020; Seres, 2024; van Zanten et al., 2019).
According to the Society of Critical Care Medicine (SCCM) and the American Society for Parenteral and Enteral Nutrition (ASPEN) guidelines (SCCM/ASPEN; Compher et al., 2022), the early initiation of nutritional therapy, through either the parenteral or enteral routes, favorably impacts patient outcomes. Adequate nutritional support initiated during the acute phase of critical illness is a proactive therapeutic strategy that can diminish metabolic deterioration, reduce infection rates, decrease ICU length of stay, and improve clinical outcomes. While nutritional support should begin during the acute phase of illness in the ICU, it often extends beyond hospital discharge through long-term recovery. There has been an increase in health care systems offering dedicated dietary services (registered dietitians, clinical nutritionists, and dietary aides) to care for patients with nutritional deficits. Nurses must have the appropriate skills to care for critically ill patients with nutritional deficiencies and malnutrition requiring nutritional support. Nurses are an integral part of the medical team and collaborate with dietary professionals, providers, and other interdisciplinary team members to ensure the patient's nutritional needs are met throughout the trajectory of illness (Compher et al., 2022; Lambell et al., 2020; Mueller et al., 2017; van Zanten et al., 2019).
Metabolic Responses in Critical Illness
Metabolism during critical illness is associated with a significant increase in energy expenditure, ranging between 20% and 60% above basal (baseline) energy requirements. SCCM/ASPEN recommends an intake of 12 to 25 kcal/kg during the first 7 to 10 days of an ICU admission. These increased energy requirements are associated with amplified carbohydrate and protein metabolism and impaired lipid metabolism. An overview of each metabolic adaptation will be described in this section (Compher et al., 2022; McClave et al., 2016; Mueller et al., 2017).
Carbohydrate Metabolism
In critically ill patients, stress-induced hyperglycemia results from alterations in glucose production, decreased glucose uptake by tissues, and low insulin sensitivity referred to as insulin resistance (muscles, fat, and liver do not respond as well to insulin and cannot easily absorb and metabolize glucose from the blood). Proinflammatory cytokines potentiate the release of catabolic hormones (glucagon, catecholamines, cortisol). These hormones stimulate glycogenolysis and gluconeogenesis to mobilize glucose. Through glycogenolysis, glycogen (the primary carbohydrate stored in the liver and muscle) is broken down into glucose to provide immediate energy and maintain blood glucose levels during periods of low glucose (fasting, hypoglycemia) or increased glucose utilization (critical illness). Gluconeogenesis involves glucose synthesis via metabolic pathways, primarily in the liver, to meet the body's needs during catabolic stress periods. Moderate or severe infections are associated with a 200% increase in glucose production rate, leading to elevated circulating glucose levels (hyperglycemia). Once glycogen stores are depleted (often within hours), lipids and proteins become the primary energy sources for survival (Dudek, 2022; McClave et al., 2016; Mueller et al., 2017; Yahia et al., 2022).
Protein Metabolism
The catabolic response to critical illness is primarily characterized by whole-body protein loss, although protein metabolism and breakdown occur at accelerated rates. Since humans do not have protein stores, any protein utilized comes at the expense of other, more labile tissues. Most notably, there is an accelerated breakdown of peripheral muscle proteins, accompanied by decreased amino acid uptake by muscles and increased uptake in the liver. Correspondingly, the synthesis of creatinine, uric acid, and ammonia is accelerated and excreted in increased amounts in the urine. Excess amino acids pool in the skeletal muscles, connective tissues, and gut, leading to a process known as hepatic reprioritization. Increased hepatic uptake of amino acids augments hepatic protein synthesis, fueling gluconeogenesis and producing positive acute-phase proteins (haptoglobin, C-reactive protein). In response, there is a corresponding decline in negative-phase proteins (albumin, prealbumin). Patients who receive adequate exogenous amino acids to maintain acute-phase protein production are more likely to survive critical illness than those who do not. In unfed, stressed, critically ill patients, up to 250 g of lean body mass is broken down every 24 hours (Dudek, 2022; McClave et al., 2016; Mueller et al., 2017).
Lipid Metabolism
Three catabolic hormones (epinephrine, norepinephrine, and glucagon) serve a vital role in stimulating lipolysis (the breakdown of stored triglycerides) into glycerol and free fatty acids (FFAs) for energy. However, the intracellular transport of FFAs is impaired, causing FFA to accumulate within the cell. This impairment prompts intracellular acidosis and the accumulation of lactate and pyruvate. Poor FFA utilization leads to decreased aerobic respiration and impairs the cells' ability to use the Krebs cycle for efficient energy production. It also limits ketogenesis...
...purchase below to continue the course
Pathophysiology of the Digestive System
A fundamental understanding of the digestive tract helps healthcare providers appreciate the core components of nutritional support. The gastrointestinal (GI) tract extends from the mouth to the anus. This organ system is responsible for nutrient intake, digestion, absorption, and excretion. It carries out various digestive processes to break down food, assimilate nutrients, and eliminate waste (McCance & Huether, 2019).
Food is processed and mixed with salivary amylase enzyme in the mouth before being propelled by the esophagus into the stomach. The stomach is a muscular, J-shaped organ that connects to the esophagus at the gastroesophageal junction. The uppermost part of the stomach—the cardia—contains the cardiac sphincter. This thin ring of muscles helps prevent acidic stomach contents from backflowing into the esophagus. The fundus is the rounded region that facilitates the stomach's expansion and contraction in response to food volume. The body (middle), the largest portion of the stomach, is where food is broken down and combined with enzymes, mucus, gastric acids, and other secretions (McCance & Huether, 2019; Ogobuiro et al., 2023).
The food is stored in the stomach's antrum until phasic contractions propel it into the small intestine, where most nutrient absorption occurs. The pylorus is the part of the stomach that connects to the small intestine and includes the pyloric sphincter, a thick ring of muscle that functions as a valve to control the stomach contents as they empty into the duodenum (the first part of the small intestine). Here, biochemicals and enzymes secreted by the pancreas and liver break down the food particles into smaller, absorbable nutrients. The nutrients pass through the small intestine walls into blood vessels and lymphatics before reaching the large intestine, where fluid absorption continues. Liquid waste is transported to the kidneys, where it is excreted from the body in urine. Solid waste travels into the rectum and is eliminated through the anus. The large intestine is the final passageway of undigested food particles and is subdivided into four regions: cecum, colon, rectum, and anus. Its primary function is to complete the absorption of any residual nutrients and water, synthesize specific vitamins, form feces (waste), and eliminate it (McCance & Huether, 2019).
The cecum is the cul-de-sac or small pouch at the beginning of the large intestine. It receives partially digested food (known as chyme) from the small intestine, mixes it with bacteria to continue digestion, and forms feces before transporting it into the colon. The colon is about 5 feet long and consists of four connecting regions: ascending colon, transverse colon, descending colon, and sigmoid colon. The ascending colon receives feces from the cecum. Then, bacteria digest the waste, which is transported to the transverse colon. The splenic flexure connects the transverse and descending colon, forming a sharp, right-angle bend on the abdomen's left side. The descending colon walls absorb water and any remaining nutrients from feces, dehydrating the stool in preparation for elimination. The sigmoid colon is the curved, S-shaped section of the colon that transports and stores the residual fecal matter until it is transported to the rectum. The rectum is the final straight portion of the large intestine; it stores fecal matter until the body is ready to eliminate the waste through defecation. The rectum connects the colon to the anus, the GI tract's final segment. The anus is a short tube at the end of the rectum that allows feces to pass outside the body during defecation (Ignatavicius et al., 2021; McCance & Huether, 2019).
Defining Malnutrition
Defining malnutrition is not straightforward, as definitions vary in terminology and often lack clear diagnostic criteria. Malnutrition is most simply defined as a physical state of unbalanced nutrition. ASPEN broadly defines malnutrition as "an inadequacy of nutrients to maintain a person's health that is caused by one or more of the following factors: insufficient intake, impaired absorption, increased nutrient requirements, and altered nutrient transport and utilization" (Mueller et al., 2017). The Academy of Nutrition and Dietetics (AND, 2021) compares the patient's recent nutrient intake with estimated requirements as the primary criterion for defining malnutrition. Specifically, they define malnutrition as "the inadequate intake of nutrients, particularly protein over time, [that] may contribute to chronic illness and acute disease or illness and infection" (AND, 2021). Malnutrition can occur with under- and over-nutrition since increased intake does not equate to balanced intake (Grodner et al., 2023). Regardless of the variable definitions, malnutrition is an independent risk factor that negatively influences a person's quality of life, autonomy, and clinical outcomes across all illness phases. Malnutrition often develops gradually and is associated with several metabolic and physiologic consequences, including immunosuppression, infection, impaired wound healing, and increased mortality. Patients who are malnourished while in the hospital have an increased risk of complications, longer lengths of stay, higher readmission rates, and increased cost burden (Grodner et al., 2023; Keller, 2019; Mueller et al., 2017).
Nutritional Support and Screening
Nutritional support refers to the enteral or parenteral provision of calories, protein, vitamins, minerals, electrolytes, and fluids. The body's primary fuel is generated from the intake of carbohydrates, protein, and lipids. The acquisition and transformation of energy from the environment are essential for life. Energy transformation involves complex biochemical processes through which the body converts energy into the necessary components to power cellular functions and sustain homeostasis. Nutrition screening identifies patients who may be malnourished or at risk for malnutrition and who would benefit from a comprehensive nutrition assessment. Early identification of at-risk patients is crucial for the timely intervention of adequate nutritional support. Screening for the risk of malnutrition in care settings supports early and effective interventions (Mueller et al., 2017; Seres, 2024). Evidence-based practice guidelines collectively recommend nutrition screening for all hospitalized patients within 24 hours of hospital admission using a validated screening tool (McClave et al., 2016). Furthermore, nutrition screening and rescreening should occur in response to a clinical concern for nutritional deficits or malnutrition risk. According to The Joint Commission (2022), a nutrition screening should be performed if the patient's needs or condition warrants it. When appropriate for the patient's condition, screenings must be completed within 24 hours of hospital admission. Under The Joint Commission standards, the hospital is permitted to determine what criteria are used to determine if a screening or more in-depth assessment is warranted (Dudek, 2022; Grodner et al., 2023; The Joint Commission, 2022).
Subjective Global Assessment
While there are numerous nutrition screening tools, ASPEN endorses using the Subjective Global Assessment (SGA) when assessing for nutrition deficits in hospitalized patients. The SGA emerged in 1982 for preoperative patients. Over the last three decades, it has become a well-cited and universally recognized tool for diagnosing malnutrition. The Canadian Malnutrition Task Force (2022) designated the SGA as the gold standard for diagnosing malnutrition. The SGA evaluates whether a patient is appropriately nourished (whether nutrient intake and absorption meet an individual's requirements) to determine whether nutrition deficits relate to their illness and if nutritional support is warranted. The SGA is a quick, simple, validated, and reliable tool across diverse patient populations. It is highly predictive of the outcome (it accurately diagnoses malnutrition). This simple bedside tool takes under 10 minutes to complete and can be administered by dietitians, clinicians, and other trained professionals. The SGA includes assessing recent food and fluid intake, weight changes, GI symptoms, other reasons for low intake, and a focused physical evaluation (evaluating muscle wasting and body fat). It does not require additional laboratory testing and strongly correlates with other subjective and objective measures of nutrition. As demonstrated in Table 1, the SGA classifies patients into three categories: well-nourished, mildly/moderately malnourished, and severely malnourished. Correcting nutritional abnormalities to help normalize muscle and fat stores is a gradual process (Canadian Malnutrition Task Force, 2022; Mueller et al., 2017).
Table 1
Determining the Severity of Malnutrition Using the SGA
Score | Category | Description |
A | Well-nourished |
|
B | Mildly/moderately malnourished |
|
C | Severely malnourished |
|
(Canadian Malnutrition Task Force, 2017, 2022; Mueller et al., 2017; Reber et al., 2019)
Nutritional Risk Screening
The SCCM/ASPEN guidelines also endorse the Nutritional Risk Screening (NRS-2002) for patients admitted to the ICU. The NRS-2002 is more complex than the SGA, but it helps determine a patient's nutritional risk based on nutritional impairment (mild, moderate, or severe) and the severity of the disease. The NRS lists four initial "yes or no" screening questions. If all four screening questions are answered "no," then the patient is low-risk. Any "yes" responses require the user to proceed to the final screening. The risk level is calculated by tallying the total score. An NRS-2002 score of greater than 3 defines patients at risk, and those at high risk have a score of greater than or equal to 5 (Kroc et al., 2021; McClave et al., 2016; Reber et al., 2019).
Malnutrition Screening Tool
The AND endorses using the Malnutrition Screening Tool (MST) to screen adults for malnutrition regardless of age, medical history, or setting. Ferguson and colleagues developed the MST in 1999 to detect malnutrition risks and manifestations. Since then, the MST has been validated across acute, long-term, rehabilitation, and ambulatory care settings in several countries. In 2020, the AND released an updated position statement endorsing the use of the MST "to screen all adults for malnutrition, regardless of their age, medical history, or setting" (Skipper et al., 2020). The MST is supported by Grade I evidence (strong evidence with good generalizability). Compared to other screening tools, the MST ranked the highest in its degree of validity and inter-rater reliability in identifying malnutrition risk in adults. The MST is a two-question metric that gives a score out of 5 to indicate a patient's malnutrition risk (Dudek, 2022; Mueller et al., 2017; Skipper et al., 2020).
Regardless of the screening tool used, any patient with an identified nutritional risk should subsequently undergo a thorough nutrition assessment to identify the specific risks and confirm the presence and severity of a nutritional deficit (Dudek, 2022; Reber et al., 2019).
Comprehensive Nutrition Assessment
A comprehensive nutrition assessment defines the patient's nutritional state using a combination of subjective and objective findings. A thorough assessment includes both subjective and objective measures. Subjective measures consist of a complete dietary review, current medical diagnoses, past medical history, medications, lifestyle habits (physical activity level), socioeconomic status/food security, support systems, and substance use (alcohol consumption, tobacco use, drug misuse). Components of a dietary assessment include:
- dietary intake (past, recent [last 2 weeks], and current), eating patterns including 24-hour food recall, portions, and frequency of meals
- ability to chew, including the use and fit of dentures
- use of medications or herbal supplements
- presence of substance misuse
- swallowing difficulties
- taste changes
- GI symptoms (nausea, vomiting, bowel patterns [constipation, diarrhea, stool patterns], heartburn, bloating, anorexia, early satiety)
- any other symptoms interfering with the ability to ingest a regular diet
- ability to obtain or purchase food, cook, and prepare meals
- food allergies, intolerances, preferences, and methods of preparing meals
- dietary restrictions, including ethnic, cultural, and religious influences (Kesari & Noel, 2023; Siobal et al., 2021)
Objective findings are gleaned from a physical examination, anthropometric measurements (height, usual body weight, current body weight, recent weight change, and body mass index [BMI]; see Table 2), functional and mental health assessment, and biochemical assessment (laboratory values, see Table 3; McClave et al., 2016; Mueller et al., 2017; White et al., 2012). Physical examination signs suggesting malnutrition include:
- loss of subcutaneous fat; more pronounced in the orbital region and upper arms (biceps and triceps)
- anasarca (generalized edema)
- decreased strength
- atrophy (muscle wasting)
- poor wound healing
- loss of balance or coordination
- dry, peeling, and rough skin
- dull and brittle hair
- hair loss
- abdominal distention
- hepatomegaly
- splenomegaly
- ridged or fissured areas on nails
- cheilosis (swelling and cracking of the lips)
- smooth tongue
- diminished strength
- weight loss of over 10% (Dudek, 2022; Kesari & Noel, 2023; Shashidhar, 2022)
Table 2
Centers for Disease Control and Prevention BMI Categories
BMI is an index of weight-for-height that assigns weight categories. BMI is defined as a person's weight in kilograms (kg) divided by the square of their height in meters (m2). It is used in conjunction with other patient data to support a diagnosis, condition, or recommended interventions. The classification of BMI is as follows. | |
Category | BMI (kg/m2) |
Adult | |
Underweight | less than or equal to 18.4 |
Normal weight | 18.5 to 24.9 |
Overweight | 25 to 29.9 |
Obesity Class I | 30 to 34.9 |
Obesity Class II | 35 to 39.9 |
Extreme Obesity Class III/Severe Obesity | greater than 40 |
Child and teen (ages 2 to 20) | |
Category | Weight percentile |
Underweight | less than 5th percentile |
Healthy weight | 5th to 85th percentile |
Overweight | 85th to 95th percentile |
Obese | greater than or equal to 95th percentile |
(Centers for Disease Control and Prevention [CDC], 2022; Zierle-Ghosh & Jan, 2023)
Laboratory Assays of Malnutrition
Serum proteins such as albumin, prealbumin, and transferrin have traditionally been used as laboratory markers of nutritional status. The prealbumin level is preferred over albumin due to its shorter half-life, reflecting more rapid changes in nutritional status (Keller, 2019). Table 3 provides an overview of these serum proteins and their clinical interpretations. Other laboratory markers used to determine nutritional status are retinol-binding protein and C-reactive protein. Despite their widespread presence in the nutritional support literature, there are no universally supported laboratory indicators for malnutrition. Changes in these values can result from other processes, such as fluid and electrolyte imbalance, hepatic and renal impairment, pregnancy, or infection (Siobal et al., 2021). Albumin is not specific for malnutrition, and changes are more indicative of inflammation than malnutrition (Dudek, 2022). AND (2021) and the SCCM/ASPEN guidelines (McClave et al., 2016) collectively advise against using these laboratory assays when managing critically ill patients. In critical care settings, these serum protein markers reflect the acute-phase response to illness driven by increased vascular permeability and hepatic protein reprioritization. They do not accurately represent nutrition status in the ICU setting and offer little value for clinical decision-making for these patients. Recent evidence has demonstrated that serum levels of these proteins do not change in response to nutrient intake changes, rendering them an inaccurate assessment of malnutrition status (AND, 2021; McClave et al., 2016; Mueller et al., 2017).
Table 3
Serum Protein Assays
Laboratory marker | Normal range | Description and interpretation |
Albumin | 3.5 to 5.5 g/dL |
|
Prealbumin | 16 to 30 mg/dL |
|
Transferrin | Male: 215 to 365 mg/dL Female: 250 to 380 mg/dL |
|
(American Board of Internal Medicine, 2024; Keller, 2019; Mueller et al., 2017; Pagana et al., 2022)
Assess for Malnutrition
The definitions of malnutrition lack clearly defined diagnostic criteria. In 2009, ASPEN and the AND recognized the need to coordinate their efforts and standardize the diagnostic approach to adult malnutrition. Acknowledging that no single parameter is definitive for adult malnutrition, they generated specific criteria to diagnose malnutrition. As cited in their updated consensus statement (White et al., 2012), a diagnosis of non-severe (moderate) or severe malnutrition requires at least two of the following six characteristics:
- insufficient energy intake
- weight loss
- loss of muscle mass
- loss of subcutaneous fat
- localized or generalized edema
- diminished functional status as measured by handgrip strength (Dudek, 2022; White et al., 2012)
More recently, in an attempt to establish a global consensus on diagnosing malnutrition, newer criteria were introduced in 2018 by the Global Leadership Initiative on Malnutrition. According to these criteria, at least one phenotypic criterion and one etiologic criterion, defined as follows, should be present to diagnose malnutrition:
- Phenotype criteria: non-volitional weight loss, low BMI, and reduced muscle mass
- Etiologic criteria: reduced food intake or absorption/assimilation and inflammation or disease burden (Jensen et al., 2019)
Table 4
Muscle Wasting: Physical Examination Findings
Physical exam | Normal | Mild/moderate | Severe |
Temple | Well-defined muscle | Slight depression | Hollowing, depression |
Clavicle | Not visible in males, may not be visible in females | Some protrusion | Protruding/prominent bone |
Shoulder | Rounded | No square look, acromion process may protrude slightly | Square appearance, prominent bones |
Scapula/ribs | Bones not prominent, no significant depressions | Mild depressions or bone may show slightly, but not all areas | Bones prominent, significant depressions |
Quadriceps | Well-defined | Depression/atrophy medially | Prominent knee, severe depression medially |
(AND; 2021; White et al., 2012)
Enteral Nutrition vs. Parenteral Nutrition
EN incorporates any feeding method that uses the GI tract to deliver all or part of a person's caloric requirements. EN may include a regular or altered oral diet, liquid supplements, or delivery of part or all of the daily requirements via a catheter, stoma, or tube (tube feeding). EN offers several benefits, such as decreased cost, maintenance of gut integrity and function and gut-associated lymphoid tissue (which is vital for immune support), and the prevention of bacterial translocation and reduced infections. While EN is the preferred route of nutritional support for critically ill patients, it can be supplemented with PN if nutritional needs are not fully met. However, some patients have complex medical issues that preclude the safe and effective use of the GI tract. PN is appropriate for patients with impaired GI function since it bypasses the typical ingestion, digestion, and absorption processes, delivering all calories and nutrients through a vein. PN is defined as the intravenous (IV) infusion of nutritional needs for patients who cannot take appropriate amounts of nutrition enterally (Doley, 2022; Dudek, 2022; Seres, 2024; Siobal et al., 2021). The core components of EN and PN will be described throughout this learning activity.
Overview of EN
EN is the preferred route of nutritional therapy since it helps maintain the integrity of the GI tract. EN is only indicated for patients with at least a partially functioning GI tract with sufficient length and absorptive capabilities. Most critically ill patients cannot intake anything by mouth, and they rely entirely on nutritional support to satisfy their caloric needs. In the ICU, EN has two primary purposes: to meet the patient's nutrient requirements (particularly the significantly increased protein requirement) and to infuse nutrients into the intestines at a rate that limits metabolic changes due to stress and upholds the normal intestinal barrier and immunological functions. Protein is the most critical nutrient for healing wounds, supporting immune function, and maintaining lean body mass. Since protein requirements are proportionately higher than energy requirements and thus are not easily met in critical illness settings, the role of early intervention with EN is vital. SCCM/ASPEN guidelines indicate that EN should commence within 24 to 48 hours following the onset of critical illness and admission to the ICU or as soon as possible after a critically ill patient has been fluid-resuscitated and stabilized as hemodynamic instability can result in gut ischemia due to decreased perfusion (Doley, 2022; Dudek, 2022; McClave et al., 2016; Siobal et al., 2021).
EN Indications
The most common indications for EN include major trauma, burns, sepsis, mechanical ventilation expected to last longer than 36 hours, and neurologic conditions that result in dysphagia with a high risk of aspiration (stroke, amyotrophic lateral sclerosis, Parkinson's disease). Patients with head and neck cancers often require EN due to obstructing tumors, allowing them to sustain calories during multimodal and complex treatment modalities (Doley, 2022; Siobal et al., 2021).
EN Contraindications
Contraindications to EN therapy include any condition in which the use of the GI tract is not feasible. Contraindications to EN include the following:
- nonfunctional GI tract (severe malabsorptive conditions)
- inaccessible GI tract (upper GI obstruction precluding feeding tube placement)
- severe short bowel syndrome (less than 39.4 in [100 cm] of remaining small bowel in the absence of the colon or 19.7 to 29.5 in [50 to 75 cm] of remaining small bowel in the presence of the colon)
- severe GI bleed or GI hemorrhage
- distal high-output GI fistula (an abnormal opening in the GI tract that leaks fluids, secretions, and nutrients)
- paralytic ileus
- peritonitis
- intestinal ischemia
- inoperable mechanical bowel obstruction
- intractable vomiting or diarrhea that does not respond to medical management
- abdominal compartment syndrome
- hemodynamic instability (Dudek, 2022; Seres, 2024; Siobal et al., 2021)
EN Access Devices
There are several options for EN access devices. When selecting the anatomical site for enteral access, HCPs must consider several factors, including but not limited to the following:
- expected duration of therapy (short-term or long-term/permanent)
- underlying medical diagnoses, including gastric and small bowel function
- risk for aspiration
- desired feeding location (stomach or small intestine)
- nutrient administration mode (bolus or continuous feeding)
- patient or family preference
- institution-specific resources (equipment) and expertise of clinicians available for placement (Doley, 2022; Grodner et al., 2023)
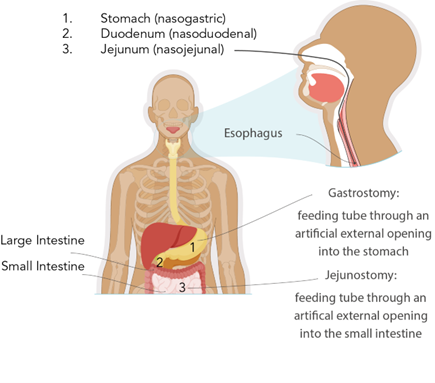
Access devices for short-term enteral access are recommended if the estimated need for feeding will be under 4 to 6 weeks. Short-term enteral access is most commonly obtained by nasoenteric or nasogastric tubes inserted through the nose (or, less commonly, the mouth in patients sedated and receiving mechanical ventilation) into the stomach, duodenum, or jejunum. Long-term enteral access is recommended when enteral feedings may be required for more than 4 to 6 weeks. Long-term EN access is typically obtained through the creation of percutaneous gastric or intestinal access. Examples of long-term access devices include gastrostomy and jejunostomy tubes (Doley, 2022; Grodner et al., 2023). When deciding between gastric or intestinal access in critically ill patients, consideration must be given to the patient's risk of aspiration or altered gastric emptying. Post-pyloric (intestine) feeding decreases the risk of pulmonary aspiration (Doley, 2022). Figure 1 depicts the most common types of enteral access devices.
Figure 1
Enteral Access Devices
Feeding Tube Sizes
Feeding tubes come in various sizes, lengths, and diameters. Flow through the tube and susceptibility to clogging are based on its inner diameter. The universal external diameter measurement for all feeding tubes is the Charrière scale or the French gauge (Fr). The larger the Fr, the bigger the external diameter of the tube. According to the Fr scale, 1 Fr equals 1/3 millimeter (mm). The diameter (D) around the feeding tube is determined by dividing the Fr by 3, as outlined in the example at the bottom of Figure 2 (Compat, n.d.).
Figure 2
Fr Sizes of Feeding Tubes
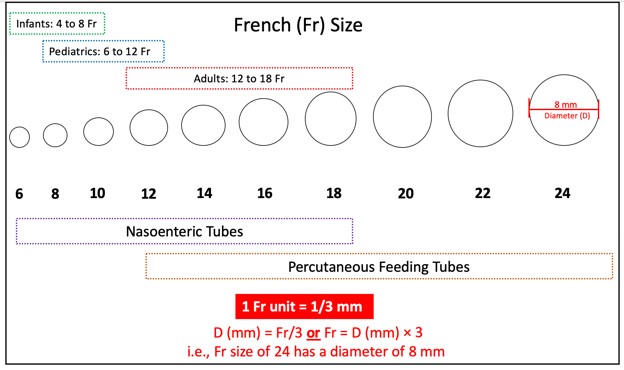
(Compat, n.d.; Hodin & Bordeianou, 2023; Selchick, 2021)
Nasoenteric Tubes
Nasoenteric tubes are ideal for short-term EN and are typically made of polyurethane, offering a larger inner tube diameter for each size. Among adults, the most commonly placed nasoenteric tube size in adults is 16 Fr. However, they can clog easily because they are narrow. Since these tubes are floppy and flexible, stylets typically accompany them to offer structure and guidance during placement. The stylet is shorter than the tube's length and has a flexible distal tip to prevent accidental perforation of the GI wall. Due to this risk, it is crucial not to allow the stylet to protrude beyond the end of the nasoenteric tube being inserted. Nasoenteric tubes may have either a port for feeding or two ports in a "Y" configuration, allowing for both feeding and medication administration (Hodin & Bordeianou, 2023; Malik, 2023; Seres, 2024).
There are three major types of nasoenteric tubes, which are named according to their termination site, as shown in Figure 1:
- A nasogastric tube is inserted into the nasal passage, passes through the throat and esophagus, and terminates in the stomach.
- A nasoduodenal tube is inserted into the nasal passage, passes through the throat, esophagus, and stomach, and terminates in the duodenum.
- A nasojejunal tube is inserted into the nasal passage, passes through the throat, esophagus, and stomach, and extends to the second portion of the small intestine (jejunum; Grodner et al., 2023; Hodin & Bordeianou, 2023; Seres, 2024).
Nasoduodenal and nasojejunal tubes are called post-pyloric feeding tubes because they bypass the stomach. All nasoenteric tubes can be placed non-surgically and are temporary. Placement may be performed at a patient's bedside, with the assistance of specialized tracking devices, or under endoscopy or fluoroscopic guidance by an interventional radiologist. Patients who are awake and alert should be positioned in an upright position, whereas unconscious patients should be placed in a supine position. The tube is placed into a nare, and the advancement of the tip is aided by having the patient swallow. Once the tube is in its proper position, it should be taped securely to the nose, but the tube should not push against the nares due to the risk of pressure ulcers, heightened in unconscious patients who cannot communicate discomfort. The tube can be fixed to the patient's gown with a safety pin (Hodin & Bordeianou, 2023; Malik, 2023; Seres, 2024).
Nasoenteric tubes should never be used until their proper position is confirmed. Placement should be documented with a plain radiograph (x-ray) of the lower chest or upper abdomen. X-ray has been the gold standard for verifying placement for decades. However, more recent studies have suggested that radiographic confirmation is unnecessary when the insertion is guided by imaging technology such as fluoroscopy. Disadvantages of x-ray confirmation include availability in specific settings, such as long-term care facilities and private homes, increased x-ray usage, and wait times. Auscultation, pH testing, and capnography are unreliable methods and cannot confirm the placement of nasoenteric tubes. Once the position of the tube is established, the stylet is removed. The stylet should never be replaced or reinserted because it can lead to GI perforation (Boeykens et al., 2023; Hodin & Bordeianou, 2023).
After the appropriate nasoenteric tube has been placed and its position verified, the head of the patient's bed should be raised to at least 30° (45° is preferred) to reduce the risk of regurgitation and aspiration. Ensuring the patency of nasoenteric tubes is essential since they are easily prone to clogging. Most guidelines recommend that the tubes be irrigated with 30 to 50 mL of water every 4 to 8 hours during continuous feeding and immediately before and after each intermittent feeding bolus. While many patients may experience oropharyngeal discomfort, it typically resolves within 24 to 48 hours of placement. Local anesthetic spray (lidocaine) may be applied to the oropharynx to alleviate pain and symptoms associated with the gag reflex from a tube's presence. However, any new or sudden onset of gagging, emesis, or respiratory issues should raise clinical suspicion for possible tube migration. In this case, the feeding should be withheld, and the tube positioning should be reevaluated with an x-ray. All nasoenteric tubes impair the esophageal sphincter's normal functioning, thereby heightening the risk of reflux of gastric content Into the esophagus, leading to esophagitis, esophageal stricture, GI bleeding, or pulmonary aspiration. Improperly securing the tube or placing too large a tube in an unconscious patient can lead to erosion, pressure ulcers, or skin necrosis. Although rare, nasoenteric tubes can perforate the GI tract organs and the respiratory system. These risks are highest in patients who have undergone prior esophageal or gastric surgeries. Removing nasoenteric tubes is typically straightforward and rapid. However, the procedure should be aborted if any resistance is met upon attempted removal as the tube should never be forced. In these instances, radiographs should be obtained to identify any underlying pathology that may be causing resistance. Although uncommon, the tube can become knotted or coiled and may require endoscopy or fluoroscopy for safe removal (Adeyinka et al., 2022; Hodin & Bordeianou, 2023; Malik, 2023; Potter et al., 2023; Seres, 2024).
Gastrostomy Tubes
Long-term EN requires permanent access to the stomach or small bowel. Gastrostomy tubes (G-tubes) are the most common long-term EN devices placed when long-term access (more than 4 weeks) is required. G-tubes offer the most significant benefits for critically ill patients requiring intubation, patients who experience aspiration with swallowing, and those with moderate to severe malnutrition. Multiple studies have cited G-tubes as a superior feeding method compared to nasoenteric tubes in patients at high risk for aspiration. G-tubes can be placed by endoscopic, fluoroscopic, or open/laparoscopic surgical techniques directly into the stomach (gastric region). Endoscopic and fluoroscopic procedures are preferred over surgical intervention because they are associated with lower morbidity and cost. A gastroenterologist or surgeon typically selects the most optimal device based on the patient's needs, anatomy, and institutional resources. Most G-tubes are made of silicone for longevity and enhanced flexibility. Percutaneous endoscopic gastrostomy (PEG) tubes are the most common type of long-term EN access. However, no established guideIines indicate which ICU patients should receive a PEG over other G-tubes. G-tubes are preferred as the first-line long-term EN access over small bowel tubes such as jejunostomy tubes (Adeyinko et al., 2022; DeLegge, 2024; Jamieson & Tadi, 2023). Table 5 provides an overview of the most common types of G-tubes.
Table 5
Types of G-Tubes
Type | Description |
PEG and long tubes |
|
Low-profile tubes or buttons |
|
(Applied Medical Technology, n.d.-a, n.d.-b; DeLegge, 2024; Grodner et al., 2023)
Gastrojejunal Tubes
A gastrojejunal (GJ) tube may be considered when the stomach needs to be bypassed for feeding. However, it is rare for a GJ tube to be placed initially, as most patients start with a G-tube. Like G-tubes, GJ-tubes are inserted into the stomach. However, a thin, long tube is threaded into the jejunum inside the stomach. Most GJ tubes have a Y configuration with separate ports to access the stomach (G-port) and the small intestine (J-port). Less commonly, transjejunal tubes can offer access to the small intestine (DeLegge, 2024). GJ tubes are available as buttons or long tubes, as described in Table 5.
Jejunostomy Tubes
According to the SCCM/ASPEN guidelines, feeding tubes in the lower GI tract should be reserved for critically ill patients with a high risk of aspiration or those who have shown intolerance to gastric EN. Jejunostomy tubes (J-tubes) are less commonly used than G-tubes and are reserved for cases of gastroparesis, chronic vomiting, a history of regurgitation, or severe acute pancreatitis, or in patients that have a functioning GI tract but a proximal obstruction or anatomical change prohibiting the use of a G-tube. J-tubes are also appropriate for those with a risk of aspiration to decrease the risk of aspiration pneumonia. Jejunal feeds minimize pancreatic exocrine secretions by bypassing the upper GI tract, serving as a core aspect of treating pancreatitis. J-tubes decrease the risk of food and fluids passing into the lungs, allowing for early postoperative feeding. These permanent feeding tubes are indicated for long-term EN access and are placed directly into the jejunum. Several guidelines recommend considering a J-tube when EN access is needed permanently. The jejunum has a smaller width than the stomach and does not have an expandable region to accommodate increased volumes. Therefore, J-tubes require slow, continuous feeding via a feeding pump. Feeding through a J-tube typically takes 16 to 24 hours per day. Like G-tubes, J-tubes include long tubes and buttons (D'Cruz & Cascella, 2023; Dudek, 2022; McClave et al., 2016; Seres, 2024). There are three primary modalities for J-tube placement (van Braak et al., 2022; D'Cruz & Cascella, 2023):
- Percutaneous endoscopic jejunostomy: The tube is directly placed into the jejunum using an endoscope.
- Laparoscopic or open surgery: The J-tube is placed directly into the small intestine through an incision in the abdominal wall.
- Gastric bypass procedure or Roux-en-Y: In this more complex procedure, a small "limb" is constructed in a portion of the jejunum and attached to the abdominal wall. The J-tube is placed in the newly created limb. While this option is more complicated, it allows for a more stable tract and easier tube changes, which can be done at home. J-tubes are more likely to leak than G-tubes, causing irritation, granulation tissue development, and peritonitis (Grodner et al., 2023). Table 6 compares G-tubes and J-tubes.
Table 6
Comparison of G-Tubes and J-Tubes
G-tube | J-tube |
Inserted into the stomach | Inserted into the jejunum |
Placed endoscopically, laparoscopically, or surgically (open incision) | Placed endoscopically, laparoscopically, or via gastric bypass surgery (Roux-en-Y) |
Can often be exchanged at home | Rarely can be exchanged at home |
Intermittent or bolus feedings, which are faster and do not require an infusion pump | Slow, continuous feeding; requires an infusion pump |
Higher aspiration risk | Lower aspiration risk |
Less skin irritation and granulation tissue | More skin irritation and granulation tissue |
Larger tube; less likely to clog | Smaller tube; may clog easily |
(D'Cruz & Cascella, 2023; Grodner et al., 2023; Jamieson & Tadi, 2023)
EN Feeding and Formulas
EN formulas contain several types of nutrients and water in varying quantities to meet the patient's needs. Amino acids, probiotics, trace minerals, and fiber may be added to the formulas based on the patient's diagnoses and nutritional needs. While there are numerous commercially prepared formulas, this learning activity focuses on caring for patients requiring nutritional support, not formula selection. Nutrition therapy typically starts at a slower initial rate and increases as tolerated toward the patient's nutritional goals. Current guidelines recommend starting EN as soon as a critically ill patient has been resuscitated, stabilized, and provided with enteral access. EN is then advanced toward the patient's nutritional goal over the following days. There are currently no guidelines or recommendations regarding the optimal starting rate and the frequency of rate increases to meet the goal rate. However, in patients who are severely malnourished or demonstrate high nutritional risk, the goal rate should be reached within 24 to 48 hours. Feeding through a PEG or G-tube mimics oral intake, as the stomach expands when full. Studies have demonstrated a significantly lower regurgitation frequency with PEG than nasogastric feeding tubes. Furthermore, critically ill patients are more likely to achieve nutritional goals when a PEG is used over nasoenteric access. According to the SCCM/ASPEN guidelines, bowel sounds and evidence of bowel function (such as passing flatus or stool) are not required to initiate enteral feedings. The literature supports EN's feasibility and safety within a patient's initial 36 to 48 hours of admission to the ICU regardless of the extent of audible bowel sounds, as bowel sounds only indicate that contractility is present, not the absorption capability. According to Parrish and McCray (2019a), the presence of bowel sounds has never been validated as a valuable indication of peristalsis. Gastric residual volume (GRV) has also been used as an indicator of the tolerance to the administration of EN. A survey of 582 nurses over five hospitals showed that 89% of nurses would hold EN for a GRV greater than 300 mL. However, GRV includes more than EN formula alone, and due to the anatomy and physiology of the stomach, the amount of GRV does not equate to intolerance of EN. Monitoring GRV takes up nursing time and decreases the amount of EN administered, potentially affecting patient outcomes. The SCCM/ASPEN guidelines also state that GRVs should not be part of the routine care of critically ill patients receiving EN. If GRVs are monitored, EN does not have to be held for GRVs under 500 mL in the absence of other signs of GI intolerance, such as nausea, vomiting, abdominal pain, or distention (Dudek, 2022; McClave et al., 2016; Parrish & McCray, 2019a; Seres, 2024).
EN Complications and Management
EN's most common complications include aspiration of regurgitated formula, fluid and electrolyte abnormalities, hyperglycemia, diarrhea, abdominal pain, and failure to achieve nutritional goals. ICU patients with delayed gastric emptying, an impaired gag reflex, and ineffective coughing have a high risk of aspiration pneumonia. Aspiration is common among mechanically ventilated patients. The use of EN is classified as a modifiable risk factor for VAP. Researchers have found that a J-tube is recommended over a G-tube if a critically ill patient is mechanically ventilated and requires EN. This is due to enhanced gut bacteria and decreased gastric reflux and aspiration. However, other benefits—reducing the number of days on mechanical ventilation, costs of care, and mortality—have not been demonstrated. Continuous EN is tolerated better than bolus feeding and is associated with a lower risk of aspiration. VAP is mainly caused by the aspiration of infectious pathogens in the oral cavity and throat. Essential measures to prevent VAP include elevating the head of the bed to 30° to 45°; performing regular oral hygiene, including the use of chlorhexidine mouthwash twice daily; routine irrigation practices; and confirming tube placement (Dudek, 2022; Hu et al., 2022; McClave et al., 2016).
Abnormally high GRVs, abdominal discomfort, distention, diarrhea, and nausea create distress for patients, increase the nursing workload, and offset nutritional goals. These issues can be minimized by ensuring normal fluid and electrolyte balance, maintaining strict glucose control, and responding rapidly to GI distress (administering anti-emetics, checking for constipation). EN formulas typically contain standard amounts of fluid, electrolytes, minerals, and nutrients. However, they are not designed to manage abnormal fluid volume or electrolyte and mineral requirements, which vary considerably and can change rapidly due to underlying disease processes. Additionally, most formulas are high in glucose. In the context of underlying metabolic adaptations to critical illness (impaired carbohydrate metabolism, hyperglycemia), blood glucose must be monitored regularly. Hyperosmolarity as a result of hyperglycemia may lead to hyperkalemia and hyponatremia. Measures must be taken to maintain homeostasis as indicated (IV fluid, electrolyte replacement, insulin therapy). Diarrhea is a common complication when bowel function is compromised by illness or medication (antibiotics or those that contain sorbitol). Infectious (such as Clostridium difficile) and inflammatory etiologies must be ruled out first for critically ill patients. EN-associated diarrhea may be controlled by supplementing feeds with fiber-containing formula or adding antidiarrheal agents. Finally, all feeding tubes are prone to clogging and obstruction. Inadequate tube flushing, dense formulas, and the introduction of inadequately dissolved solid medications, bulking agents, or resins are common causes of clogged tubes. If a clog occurs within the tube, a syringe or administration of pancreatic enzymes can be used to attempt to dislodge the clog. If unable to restore patency, the tube may need to be replaced (Compher et al., 2022; DeLegge, 2024; Ignatavicius et al., 2021; McClave et al., 2016; Parrish & McCray, 2019b).
The SCCM/ASPEN guidelines advise that EN be withheld from hemodynamically unstable patients until they are fully resuscitated or stabilized. Specifically, EN should be withheld in the following clinical scenarios:
- hypotensive patients (mean arterial blood pressure less than 50 mm Hg)
- patients who require the initiation of catecholamine agents (norepinephrine [Levophed], phenylephrine [Biorphen], epinephrine [Adrenalin], or dopamine [Intropin])
- patients for whom escalating doses of medications are needed to maintain hemodynamic stability (McClave et al., 2016)
The initiation or re-initiation of EN may be considered with caution in patients receiving stable doses of vasopressor support, decreasing lactate levels, and a mean arterial blood pressure greater than or equal to 60 mm Hg. EN should be withheld in patients on vasopressor therapy with signs or symptoms of intolerance (abdominal distention, decreased passage of stool or flatus, increased aspiration) due to the potential for gastric ischemia (McClave et al., 2016).
Overview of PN
The successful infusion of hypertonic PN in the 1960s signified a significant advancement in providing nutrition to patients with nonfunctioning GI tracts. PN is typically reserved for patients with contraindications to EN therapy or those whose nutritional goals are unmet by EN. PN is costlier, more resource-intensive, and carries a higher risk of infection and other complications than EN (Dudek, 2022; Seres, 2024). In addition to the patient having one of the contraindications to EN listed above, other indications for PN therapy include the following:
- inability to meet estimated energy needs after 7 to 10 days of EN
- severe short bowel syndrome
- paralytic ileus
- mesenteric ischemia
- small bowel obstruction
- intestinal anastomosis leak
- severe diarrhea or vomiting
- GI fistula preventing the placement and use of tube feeding distal to the fistula (Dudek, 2022; Hamdan & Puckett, 2023)
Routes of Administration
The components of parenteral feeding formulas determine whether the feeding can be administered via peripheral infusion (peripheral parenteral nutrition [PPN]) or require a central venous catheter (CVC; central parenteral nutrition [CPN]). PN formulas are hypertonic to body fluids and, if administered inappropriately, may result in complications such as venous thrombosis, thrombophlebitis, or extravasation. The osmolarity of parenteral feeding depends on the quantity of dextrose, amino acids, and electrolyte concentration and should be limited to 900 mOsm/L. Due to the risks and restrictions, PPN is rarely used. It is reserved for patients who require PN for under 7 to 10 days and is rarely administered to critically ill patients. Furthermore, patients receiving PPN must have excellent peripheral venous access and tolerate large fluid volumes (2.5 to 3 L/day). Therefore, this section focuses on CPN, also called total parenteral nutrition. CPN can fulfill a patient's entire nutrient needs via a reasonable fluid volume tailored to their energy and protein requirements. CPN is administered directly into a patient's central venous blood supply via a CVC (Dudek, 2022; Grodner et al., 2023; Inayat-Hussain et al., 2023).
CVC
A CVC or central line is a long-term indwelling device that can remain in place for extended periods (months to years). This thin, flexible tube is inserted via puncture directly through the skin into a large vein, often in the neck, chest, arm, or groin. The catheter is threaded through the vein until the tip reaches the right atrium or distal portion of the superior vena cava (SVC). The area where the central line leaves the skin is called the exit site. CVCs used for CPN include the following types: peripherally inserted central catheter (PICC), tunneled IV catheter, non-tunneled IV catheter, and implanted port (Dudek, 2022; Leib et al., 2023). Standards of practice published by the Infusion Nurses Society (INS; Gorski et al., 2021) favor tunneled IV catheters for CPN. CVCs may be inserted by specially trained and certified healthcare providers (registered nurses, advanced practice registered nurses, or physicians). Regardless of the device selected, CVC insertion is a sterile procedure and requires formal training, as successful placement depends highly on the technique of the individual inserting the line. Some devices require surgical placement in the operating room or interventional radiology department under fluoroscopy guidance, whereas others allow for bedside insertion (CDC, 2019; Gorski et al., 2021; Leib et al., 2023). The following section will provide a brief overview of the four CVC devices used for CPN.
PICC
A PICC is indicated for long-term access, and the dwell time varies from weeks to 6 months but can be more if proper care is provided. A PICC is inserted in the basilic, cephalic brachial, or median cubital veins in the upper arm; the right basilic vein is preferred due to its size and location. The line is threaded through the vein so that the catheter tip is located in the lower segment of the SVC. A chest x-ray is required to confirm the catheter tip's placement in the lower SVC before the PICC line is used. Catheter sizes range from 1.9 to 6 Fr, and the catheter length is approximately 19.7 to 23.6 in (50 to 60 cm) long but is cut to the patient's size before insertion. PICC lines are available as single-, double-, and triple-lumen catheters. However, multi-lumen PICC lines have a more significant infection rate than single-lumen catheters (First Medical Company, n.d.; Gonzalez & Cassaro, 2023; Leib et al., 2023).
Tunneled CVC
A tunneled CVC is used when CPN is indicated for several months or longer. It is surgically implanted in a central vein in the neck or chest and then subcutaneously tunneled to an exit site in the chest wall. The vein entry site is located on the upper chest, and the exit site is located between the third or fourth intercostal space. The tip of the catheter rests in the SVC. A Dacron cuff device is positioned within the tunneled portion of the catheter, approximately 0.8 to 1.2 in (2 to 3 cm) from the exit site. Tissue grows around the cuff, creating a mechanical barrier against microorganisms and anchoring the catheter in place. The separation between where the catheter enters the vein and exits the skin is intended to reduce infection risk by preventing organisms from reaching the bloodstream (Flick & Winters, 2023; Leib et al., 2023; McCance & Huether, 2019).
Before using a tunneled CVC, the placement of the catheter must be confirmed by an x-ray. The nurse must have an active order from a licensed provider stating that the line may be used, although this policy varies by organization. The INS guidelines recommend placing an initial sterile dressing on the site following insertion. The dressing should be changed 24 hours following line insertion and every 7 days after that or when visibly dirty, wet, or soiled. Once the insertion site has completely healed over, usually about 21 days following insertion, the line may be left open to the air and uncovered. The nurse must assess the catheter's patency before use by aspirating for blood return, which should be brisk. Patency should be checked before administering CPN, and the line should also flush without resistance. Syringes smaller than 10 mL should never be used with a tunneled CVC (Gorski et al., 2021).
Non-Tunneled CVCs
Non-tunneled CVCs are small-bore catheters inserted percutaneously through the subclavian vein of the upper chest or the jugular veins. With these devices, the catheter exits the skin near the venous cannulation site. Non-tunneled CVCs are usually 5.9 to 9.8 in (15 to 25 cm) long based on the insertion site and can have single, double, triple, or quadruple lumen(s). The catheter is sutured in place to prevent it from migrating or being inadvertently dislodged. The tip of the catheter resides in the SVC, and a chest x-ray must confirm proper placement before use. Non-tunneled CVCs are intended for short-term and temporary use, usually 5 to 10 days, and are not appropriate for use in home or ambulatory care settings (Ignatavicius et al., 2021; Kolikof et al., 2023; Tse & Schick, 2022).
Implanted Port
An implanted port is surgically placed into a subcutaneous pocket of the anterior chest wall, about 1 in (2.5 cm) beneath the collarbone. The insertion of the device is performed under local anesthesia by a surgeon or interventional radiologist. The port consists of a thin, flexible catheter attached to a reservoir. The catheter is threaded into the central venous system via the subclavian or jugular vein, and the tip of the catheter resides within the SVC. The reservoir can be made of plastic, stainless steel, or titanium and is about the size of a quarter. It is covered with a self-sealing silicone septum designed to withstand multiple needle punctures. An implanted port is similar to a tunneled catheter but is not visible since it resides beneath the subcutaneous tissue. Ports do not require as much maintenance care and do not impede daily activities, including bathing or swimming, like PICC lines or tunneled catheters. Ports are also associated with a lower risk of infection than other CVCs (CDC, 2019; Flick & Winters, 2023).
CPN Feeding and CVC Care
CPN can be initiated after a patient has been hemodynamically resuscitated; has had their glucose, electrolyte, and acid-base homeostasis established; and can tolerate the fluid volumes involved. CPN is administered using filtration that suits the type of solution/emulsion and infuses via an electronic device equipped with appropriate alarms. Strict adherence to aseptic techniques is advised during all aspects of CPN and CVC care to reduce the risk of central line-associated bloodstream infection (CLABSI). A dedicated line must be allotted for all CPN infusions and labeled for CPN use. No other medications, infusions, or therapies should be infused through the dedicated CPN lumen. According to INS guidelines, the CVC should not be used for routine blood sampling since it can significantly increase the risk of CLABSI. The administration sets for CPN must be replaced at least every 24 hours, and current recommendations advise changing the administration set with each new CPN container or bag. Nurses are responsible for monitoring and reporting any potential signs of infection, such as erythema, edema, pain or tenderness, drainage, subcutaneous pocket or tunnel, and induration at the exit site or over the pocket. Signs of systemic infection include pyrexia, chills, rigors, lethargy, disorientation, and confusion in previously alert patients (CDC, 2019; Gorski et al., 2021; Hamden & Puckett, 2023).
While CPN formula composition is beyond the scope of this learning activity, the formula typically consists of a high-osmolarity solution with a high proportion of protein in the form of essential and nonessential amino acids. Carbohydrates are included in the form of dextrose monohydrate. In general, the goal dose of dextrose is 4 to 5 mg/kg/min in stable patients and less than 4 mg/kg/min in those who are critically ill. The administration of carbohydrates is essential, but too much can lead to refeeding syndrome (see next section) and hyperglycemia, which can increase the risk of infection and organ dysfunction. Fatty acids are provided in an isotonic solution. They are especially beneficial in patients who require restricted fluid intake or when dextrose administration must be limited due to hyperglycemia. When lipids are added to a dextrose and amino acid mixture, it is referred to as a total nutrient admixture or a 3-in-1 mixture (Dudek, 2022; Grodner et al., 2023).
CPN should be discontinued as soon as EN can adequately nourish the patient. Evidence-based guidelines recommend that the dose of CPN should be tapered down as food intake increases. Once a patient tolerates one-half to three-quarters of their food requirement by the enteral route and there are no other barriers to further improvements in intake, PN should be discontinued. PN should never be suspended to entice increased oral intake. Stopping PN too quickly or abruptly may lead to hypoglycemia. It may take 1 to 2 weeks for an individual to feel hunger after the cessation of PN. PN does not induce anorexia, and stopping PN does not boost a patient's appetite (Dudek, 2022; Grodner et al., 2023).
Complications and Management
Complications associated with CPN occur in two major categories: CVC-related issues (CLABSI, thrombosis, catheter malfunction) and CPN-related issues (refeeding syndrome, metabolic abnormalities, organ dysfunction such as hepatic injury; Dudek, 2022; Grodner et al., 2023; Hamdan & Puckett, 2023).
CLABSI
CLABSI is the most common complication associated with CPN, as several studies have demonstrated an increased risk of infectious complications in patients receiving CPN compared to other patients with CVCs. Proper aseptic insertion technique, meticulous dressing care, and dedicating a single port to CPN reduce this risk. Strict infection control protocols are strongly advised regardless of the type of catheter placed to reduce the risk of CLABSI (Gorski et al., 2021). This includes the following measures:
- handwashing
- aseptic site and hub care (wearing gloves and prepping the site with topical antiseptics)
- port sterilization before access
- close monitoring of catheter site appearance for redness or inflammation (Gorski et al., 2021)
Many institutional policies enforce the use of chlorhexidine-impregnated dressings over CVCs to reduce the risk of CLABSI rates. If an individual is sensitive to chlorhexidine, it is recommended that povidone-iodine or 70% alcohol be used when providing site care (Gorski et al., 2021).
Catheter Malfunction
Catheter malfunction (clogging [occlusion] or breakage) is another common complication of CVCs. Catheter occlusion may arise from blood, fat solutions in many PN formulas, or a precipitate (abnormal crystal formation in a solution). An occluded catheter is typically managed by instilling a de-clotting agent such as alteplase into the catheter to dissolve the clot. When a catheter is cracked, leaking, or broken, the catheter must be repaired or replaced as soon as possible. Symptoms of a fractured catheter can include pain or burning when flushing, swelling, redness, paresthesia, or leakage at the insertion site (Gorski et al., 2021).
Catheter-Associated Thrombosis
Thrombosis of a blood vessel around a CVC is a serious potential complication of CPN that can be life-threatening if not managed. A thrombus is a stationary clot attached to the vessel wall composed of fibrin and other blood cells. A thrombus can reduce or obstruct blood flow within the vessel, depriving tissues and organs of vital nutrients and inducing tissue ischemia and hypoxia. Since thrombosis can block blood flow in veins and arteries, complications depend on the clot's location. Although not commonly associated with CVCs, the most severe and dangerous complication of venous thrombosis is a pulmonary embolism, a blood clot within the lung. A thrombus detaches from the vessel wall and circulates within the bloodstream, causing a sudden vessel blockage (an embolism). A pulmonary embolism is most commonly caused by deep vein thrombosis (DVT), as a piece of the blood clot within the extremity breaks off, travels through the veins, and lodges in the lung's blood vessels. A pulmonary embolism can completely obstruct blood flow and induce sudden death. It can also cause hypoxia, leading to permanent damage to the lungs or other organs in the body due to insufficient oxygen (Gorski et al., 2021; McCance & Huether, 2019; Zaccone et al., 2023).
Thrombotic complications associated with CVCs vary based on the device and insertion site. Overall, upper extremity DVT accounts for 3% to 4% of all DVTs. However, 85% of upper extremity DVTs are related to CVCs. Risk factors for catheter-related thrombosis include larger, multi-lumen catheters. PICCs are associated with higher rates of thrombosis than other types of CVCs. Placement via the subclavian is associated with lower rates of thrombosis than those inserted into the jugular or femoral sites. Thrombosis is associated with distressing symptoms (limb swelling, pain, or loss of function), heightened morbidity, catheter dysfunction, increased risk of infection, and higher costs of care. If there is a DVT present but the catheter is properly placed and functional, it is not recommended to remove the CVC, as insertion of a new CVC is associated with a higher risk of DVT at the new site (Gorski et al., 2021; Leib et al., 2023; Zaccone et al., 2023).
For more information on CVCs, including the identification and management of catheter-associated thrombosis, refer to the following NursingCE courses:
- Venous Thromboembolism
- Blood Clotting and Bleeding Disorders
- Vascular Access Devices
CPN Feeding Complications
CPN is associated with a higher risk of feeding complications than EN. The most common metabolic complication of CPN is hyperglycemia, particularly in patients with insulin resistance due to diabetes mellitus, high-dose glucocorticoid therapy, or severe systemic inflammation. Hyperglycemia is worsened by the high glucose levels (such as dextrose) in most CPN formulas. Since critically ill patients have underlying alterations in glucose metabolism, they are more prone to the hyperglycemic complications of CPN. Blood glucose should be monitored at least every 6 hours, and short-acting insulin may be added to the CPN or administered subcutaneously as indicated to maintain normal average glucose concentrations. Reactive hypoglycemia can occur when high-dextrose, non-insulin-containing CPN is abruptly discontinued. Therefore, to avoid reactive hypoglycemia, CPN should be tapered down slowly before stopping, as noted earlier. Volume overload and fluid retention comprise another common complication of CPN. Hypertonic dextrose CPN formulas stimulate a more intense insulin response than oral glucose. Insulin is a potent antinatriuretic and antidiuretic hormone, potentiating sodium and water retention (Dudek, 2022; Mueller et al., 2017; Thomas, 2022).
In patients receiving CPN, fluid retention is likely when the total fluid administration exceeds 2 L/day, particularly when patients have high nutritional requirements requiring larger fluid volumes or are not experiencing significant GI losses. Volume overload can be prevented by reducing infusion rates to minimize the need for exogenous insulin therapy, limiting sodium intake, and avoiding overfeeding. Hypertriglyceridemia is a relatively common complication of CPN when the rate of lipid infusion exceeds plasma triglyceride clearance capacity. This is especially concerning for ICU patients since sepsis, renal failure, metabolic alterations in glucose metabolism, and multiple organ failure reduce triglyceride clearance, as outlined earlier in this module. Furthermore, severe pancreatitis is a rare but possible complication of unmanaged severe hypertriglyceridemia. Prolonged CPN, especially when it delivers excess calories, can lead to hepatic dysfunction, typically manifesting with elevated serum liver enzyme tests (LFTs). Elevated LFTs usually happen within 2 to 4 weeks of initiating CPN but typically normalize, even when CPN is continued. Hepatic complications such as fatty liver and intrahepatic cholestasis occur more frequently with continuous CPN. These conditions can usually be managed by transitioning to cyclic CPN (CPN is infused for only 8 hours per day; Hamdan & Puckett, 2023; Mueller et al., 2017; Thomas, 2022).
Refeeding Syndrome
Refeeding syndrome is a potentially fatal condition characterized by severe electrolyte and fluid shifts and metabolic abnormalities in malnourished patients receiving nutritional support. There is no standardized definition or diagnostic criteria for refeeding syndrome, and it can be associated with significant morbidity and mortality. While refeeding syndrome can result from oral, enteral, or parenteral nutrition, it most frequently appears with CPN. The primary clinical features of refeeding syndrome include alterations in fluid balance, abnormal glucose metabolism, electrolyte disturbances (hypophosphatemia, hypomagnesemia, hypokalemia, and hypocalcemia), vitamin B1 (thiamine) deficiency, and hyperglycemia. Metabolic and electrolyte disturbances are primarily caused by the increased use of these nutrients for carbohydrate metabolism. The most sensitive electrolyte is phosphorus; therefore, hypophosphatemia is a classic sign and is present in nearly all cases. Signs appearing on a physical examination may include:
- Cardiovascular: cardiac arrhythmias (torsades de pointes, ventricular tachycardia, QT prolongation), hypotension, fluid retention (edema), heart failure, cardiac arrest
- Respiratory: shortness of breath, pulmonary edema, respiratory failure
- Neurologic: weakness, paresthesia (numbness/tingling), myalgias, vertigo, confusion (Dudek, 2022; Persaud-Sharma et al., 2022; Siobal et al., 2021)
Symptoms typically present within 2 to 5 days of starting nutritional support, but some patients can present with asymptomatic phosphate depletion (Berlana, 2022; Dudek, 2022; Siobal et al., 2021).
The primary goal of nutritional support is the prevention of refeeding syndrome. Prevention strategies involve identifying and managing underlying risk factors (severe malnutrition, prolonged nothing by mouth status, diuretic use). Such strategies include assessments of baseline electrolytes and correcting any underlying deficits before initiating EN or CPN. SCCM/ASPEN guidelines recommend adding thiamine supplementation to nutritional support formulas in patients at risk of thiamine deficiency (such as patients with underlying alcohol abuse disorders or dependency). The typical thiamine replacement dose is 100 mg daily for 5 to 7 days. Early identification of the warning signs of refeeding syndrome is vital to minimize complications (Dudek, 2022; McClave et al., 2016; Siobal et al., 2021).
Ethical Considerations in Nutritional Support
Before initiating EN or CPN, the ethical issues surrounding nutritional support should be considered. Artificial nutrition and hydration is a medical intervention and requires the same discussion and decision-making as other medication treatments. Ethics is a discipline studied in nearly all professions that encompasses standards of conduct. Ethics provide the fundamental structure for putting morals or rules of conduct into practice. Despite several misconceptions, ethics do not change with emotions or feelings and are not legally binding. Medical ethics refers to a set of moral standards that govern the behaviors and practices of health care professionals. Issues in medical ethics often involve life and death. Ethical arguments related to the role of artificial nutrition and hydration (ANH) are well-cited in the literature on critically ill and dying patients. ANH ethical issues include patient and family wishes, including the review of advanced care planning documents (if available), quality of life, goals of care, and the risks and benefits of nutritional therapy in the context of the patient's diagnosis, prognosis, and long-term care goals (Mueller et al., 2017; Schwartz et al., 2021).
Providing optimal care to critically ill patients requires keen awareness and an understanding of the principles of medical ethics, including:
- Autonomy: patient's right to self-determination
- Beneficence: doing what is good or in the best interest of the patient
- Nonmaleficence: avoidance of the infliction of intentional harm to the patient
- Justice: fairness in the delivery of health care (Schwartz et al., 2021)
With growing attention on medical futility, the withdrawal or withholding of medical interventions, and aid-in-dying, nurses should not only appreciate the ethical and legal ramifications surrounding these topics but also examine their beliefs and personal biases to ensure care is delivered without prejudice (American Nurses Association [ANA], 2017).
According to the American Medical Association (AMA, n.d.), a competent patient with decision-making capacity has the right to decline any medical intervention (including ANH) or request that an intervention be stopped, even when that decision is expected to lead to death. Declining nutrition is a prime example of a patient exercising their right to autonomy. The ANA (2017) position statement on nutrition and hydration for end-of-life care identifies comparable views regarding the acceptance or refusal of clinically appropriate food and fluids, whether delivered by oral or artificial means. Patient (or surrogate) decisions must be respected, provided the decision is based on accurate information and represents patient preferences. If a patient lacks capacity, the patient's surrogate may decline or ask to stop an intervention on the patient's behalf. When an intervention is no longer helpful in achieving the patient's goals for care or desired quality of life, it may be ethically withdrawn. Nutritional support is not indicated when a patient's prognosis does not warrant continued aggressive therapies or the patient or caregiver refuses nutritional support. The AMA (n.d.) and the ANA (2017) mutually agree that there is no ethical, moral, or legal difference between withholding care (never starting it) or withdrawing care (stopping it). Furthermore, decisions about accepting or forgoing nutrition and hydration should be honored, including choices about artificially delivered nutrition (as a means of hastening death). The AMA (n.d.) urges nurses to work collaboratively with the health care team to identify, understand, and advocate for interventions that are congruent with the patient's goals and preferences early in the course of illness; these steps can help avoid complex ethical dilemmas at the end of life (ANA, 2017).
References
Academy of Nutrition and Dietetics. (2021). Malnutrition measures specification manual: Version 2.0 – March 2021. https://malnutritionquality.org/wp-content/uploads/Malnutrition-Measures-Specification-Manual_v2_03252021.pdf
Adeyinka, A., Rouster, A. S., & Valentine, M. (2022). Enteric feedings. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK532876
American Board of Internal Medicine. (2024). ABIM laboratory test reference ranges – January 2024. https://www.abim.org/Media/bfijryql/laboratory-reference-ranges.pdf
American Medical Association. (n.d.). Code of medical ethics: Caring for patients at the end of life. Retrieved February 6, 2024, from https://code-medical-ethics.ama-assn.org/chapters/caring-patients-end-life
American Nurses Association. (2017). Nutrition and hydration at the end of life. https://www.nursingworld.org/~4af0ed/globalassets/docs/ana/ethics/ps_nutrition-and-hydration-at-the-end-of-life_2017june7.pdf
Applied Medical Technology. (n.d.-a). AMT original low profile button G-tube. Retrieved February 15, 2024, from https://www.appliedmedical.net/enteral/amt-button
Applied Medical Technology. (n.d.-b). MiniOne balloon button: Low profile balloon button G-tube. Retrieved February 15, 2024, from https://www.appliedmedical.net/enteral/minione/balloon
Berlana, D. (2022). Parenteral nutrition overview. Nutrients, 14, 1–24. https://doi.org/10.3390/nu14214480
Boeykens, K., Holvoet, T., & Duysburgh, I. (2023). Nasogastric tube insertion length measurement and tip verification in adults: A narrative review. Critical Care, 27, 317. https://doi.org/10.1186/s13054-023-04611-6
Canadian Malnutrition Task Force. (2017). Subjective global assessment form. Canadian Nutrition Society. https://www.nutritioncareincanada.ca/sites/default/uploads/files/SGA%20Tool%20EN%20colour_2017(1).pdf
Canadian Malnutrition Task Force. (2022). Subjective global assessment (SGA)- Diagnosing malnutrition. Canadian Nutrition Society. https://nutritioncareincanada.ca/resources-and-tools/hospital-care-inpac/assessment-sga
Centers for Disease Control and Prevention. (2019). Frequently asked questions about catheters. https://www.cdc.gov/hai/bsi/catheter_faqs.html
Centers for Disease Control and Prevention. (2022). Defining adult overweight & obesity. https://www.cdc.gov/obesity/basics/adult-defining.html
Compat. (n.d.). A guide for selecting the appropriate nasogastric tube. Retrieved February 6, 2024, from https://www.compat.com/wp-content/uploads/2022/02/compat-web-educational-page-types-nasogastric-tubes-31jan2022-en.pdf
Compher, C., Bingham, A. L., McCall, M., Patel, J., Rice, T. W., Braunschweig, C., & McKeever, L. (2022). Guidelines for the provision of nutritional support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. Journal of Parenteral and Enteral Nutrition, 46(1), 12–41. https://doi.org/10.1002/jpen.2267
D'Cruz, J. R. & Cascella, M. (2023). Feeding jejunostomy tube. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK562278
DeLegge, M. H. (2024). Gastrostomy tubes: Placement and routine care. UpToDate. Retrieved February 7, 2024, from https://www.uptodate.com/contents/gastrostomy-tubes-placement-and-routine-care
Doley, J. (2022). Enteral nutrition overview. Nutrients, 14(11), 2180. https://doi.org/10.3390/nu14112180
Dudek, S. G. (2022). Nutrition essentials for nursing practice (9th ed.). Wolters Kluwer.
Ferguson, M., Capra, S., Bauer, S., & Banks, M. (1999). Development of a valid and reliable malnutrition screening tools for adult acute hospital patients. Nutrition, 15(6), 458–464. https://doi.org/10.1016/S0899-9007(99)00084-2
First Medical Company. (n.d.). PICC lines. Retrieved February 7, 2024, from https://www.firstmedical.co.za/products/picc-lines
Flick, A. I. & Winters, R. (2023). Vascular tunneled central catheter access. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK557614
Gonzalez, R. & Cassaro, S. (2023). Percutaneous central catheter. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK459338
Gorski, L. A., Hadaway, L., Hagle, M. E., Broadhurst, D., Clare, S., Kleidon, T., Meyer, B. M., Nickel, B., Rowley, S., Sharpe, E., & Alexander, M. (2021). 2021 infusion therapy standards of practice updates (8th ed.). Journal of Infusion Nursing, 44(4), 1–232. https://doi.org/10.1097/NAN.0000000000000436
Grodner, M., Escott-Stump, S., & Dorner, S. (2023). Nutritional foundations and clinical applications: A nursing approach (8th ed.). Elsevier.
Hamdan, M. & Puckett, Y. (2023). Total parenteral nutrition. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK559036
Hodin, R. A. & Bordeianou, L. (2023). Inpatient placement and management of nasogastric and nasoenteric tubes in adults. UpToDate. Retrieved February 7, 2024, from https://www.uptodate.com/contents/inpatient-placement-and-management-of-nasogastric-and-nasoenteric-tubes-in-adults
Hu, L., Peng, K., Huang, X., Wang, Z., Wu, Q., Xiao, Y., Hou, Y., He, Y., Zhou, X., & Chen, C. (2022). Ventilator-associated pneumonia prevention in the intensive care unit using postpyloric tube feeding in China (VIP study): Study protocol for a randomized controlled trial. Trials, 23, 478. https://doi.org/10.1186/s13063-022-06407-5
Ignatavicius, D. D., Workman, M. L., Rebar, C. R., & Heimgartner, N. M. (2021). Medical-surgical nursing: Concepts for interprofessional collaborative care (10th ed.). Elsevier.
Inayat-Hussain, A., Falck, H., Oorschot, S., Picardo, S., & So, K. (2023). Peripheral parenteral nutrition: An evaluation of its use, safety and cost implications in a tertiary hospital setting. Clinical Nutrition ESPEN, 56, 215–221. https://doi.org/10.1016/j.clnesp.2023.05.020
Jamieson, N. C. & Tadi, P. (2023). Feeding tube. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK559044
Jensen, G. L., Cederholm, T., Correia, M. I., Gonzalez, M. C., Fukushima, R., Higashiguchi, T., de Baptista, G. A., Barazzoni, R., Blaauw, R., Coats, A. J., Crivelli, A., Evans, D. C., Gramlich, L., Fuchs-Tarlovsky, V., Keller, H., Llido, L., Malone, A., Mogensen, K. M., Morley, J. E., … Van Gossum, A. (2019). GLIM criteria for the diagnosis of malnutrition: A consensus report from the global clinical nutrition community. Journal of Parenteral and Enteral Nutrition, 43(1), 32–40. https://doi.org/10.1002/jpen.1440
The Joint Commission. (2022). Nutritional and functional screening - Requirement. https://www.jointcommission.org/standards/standard-faqs/critical-access-hospital/provision-of-care-treatment-and-services-pc/000001652
Keller, U. (2019). Nutritional laboratory markers in malnutrition. Journal of Clinical Medicine, 8(6), 775. https://doi.org/10.3390/jcm8060775
Kesari, A. & Noel, J. Y. (2023). Nutritional assessment. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK580496
Kolikof, J., Peterson, K., & Baker, A. M. (2023). Central venous catheter. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK557798
Kroc, L., Fife, E., Piechocka-Wochniak, E., Soltysik, B., & Kostka, T. (2021). Comparison of Nutrition Risk Screening 2002 and Subjective Global Assessment Form as short nutrition assessment tools in older hospitalized adults. Nutrients, 13(1), 255. https://doi.org/10.3390/nu13010225
Lambell, K. J., Tatucu-Babet, O., Chapple, L., Gantner, D., & Ridley, E. J. (2020). Nutrition therapy in critical illness: A review of the literature for clinicians. Critical Care, 24(35), 1–11. https://doi.org/10.1186/s13054-020-2739-4
Leib, A. D., England, B. S., & Kiel, J. (2023). Central line. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK519511/#_article-19125_s10_
Malik, Z. (2023). How to insert a nasogastric tube. Merck Manual Professional Version. https://www.merckmanuals.com/professional/gastrointestinal-disorders/how-to-do-gastrointestinal-procedures/how-to-insert-a-nasogastric-tube
McCance, K. L. & Huether, S. E. (2019). Pathophysiology: The biologic basis for disease in adults and children (8th ed.). Elsevier.
McClave, S. A., Taylor, B. E., Martindale, R. G., Warren, M. M., Johnson, D. R., Braunschweig, C., McCarthy, M. S., Davanos, E., Rice, T. W., Cresci, G. A., Gervasio, J. M., Sacks, G. S., Roberts, P. R., Compher, C., the Society of Critical Care Medicine, & the American Society for Parenteral and Enteral Nutrition. (2016). Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient. Journal of Parenteral and Enteral Nutrition, 40(2), 159–211. https://doi.org/10.1177/0148607115621863
Mueller, C. M., Lord, L. M., Marian, M., McClave, S. A., & Miller, S. J. (Eds.). (2017). The ASPEN adult nutrition core curriculum (3rd ed.). American Society for Parenteral and Enteral Nutrition.
Ogobuiro, I., Gonzales, J., Shumway, K. R., & Tuma, F. (2023). Physiology, gastrointestinal. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK537103
Pagana, K. D., Pagana, T. J., & Pagana, T. N. (2022). Mosby's manual of diagnostic and laboratory tests (7th ed.). Elsevier.
Parrish, C. R., & McCray, S. (2019a). Part I enteral feeding barriers: Pesky bowel sounds & gastric residual volumes. Practical Gastroenterology, 43(1), 35–50. https://practicalgastro.com/wp-content/uploads/2019/07/Part-I-Enteral-Feeding-Barriers-Pesky-Bowel-Sounds-Gastric-Residual-Volumes.pdf
Parrish, C. R. & McCray, S. (2019b). Part II enteral feeding: Eradicate barriers with root cause analysis and focused intervention. Practical Gastroenterology, 43(2), 14–33. https://med.virginia.edu/ginutrition/wp-content/uploads/sites/199/2019/02/Parrish-Barriers-in-EN-February-2019.pdf
Persaud-Sharma, D., Saha, S., & Trippensee, A. W. (2022). Refeeding syndrome. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK564513
Potter, P. A., Perry, A. G., Stockert, P. A., & Hall, A. M. (2023). Fundamentals of nursing (11th ed.). Elsevier.
Reber, E., Gomes, F., Vasiloglou, M. F., Schuetz, P., & Stanga, Z. (2019). Nutritional risk screening and assessment. Journal of Clinical Medicine, 8(7), 1065. https://doi.org/10.3390/jcm8071065
Schwartz, D. B., Barrocas, A., Annetta, M. G., Stratton, K., McGinnis, C., Hardy, G., Wong, T., Arenas, D., Turon-Findley, M. P., Kliger, R. G., Corkins, K. G., Mirtallo, J., Amagai, T., & Guenter, P. (2021). Ethical aspects of artificially administered nutrition and hydration: An ASPEN position paper. Nutrition in Clinical Practice, 36(2), 254–267. https://doi.org/10.1002/ncp.10633
Selchick, F. (2021). French (Fr) sizes of feeding tubes [image].
Seres, D. (2024). Nutrition support in intubated critically ill adult patients: Initial evaluation and prescription. UpToDate. Retrieved February 7, 2024, from https://www.uptodate.com/contents/nutrition-support-in-intubated-critically-ill-adult-patients-initial-evaluation-and-prescription
Shashidhar, H. R. (2022). Malnutrition clinical presentation. https://emedicine.medscape.com/article/985140-clinical#b2
Siobal, M. S., Baltz, J. E., & Wright, J. (2021). A guide to the nutritional assessment and treatment of the critically ill patient (2nd ed.). American Association for Respiratory Care. https://www.aarc.org/wp-content/uploads/2014/11/nutrition_guide.pdf
Skipper, A., Coltman, A., Tomesko, J., Charney, P., Porcari, J., Piemonte, T. A., Handu, D., & Cheng, F. W. (2020). Position of the Academy of Nutrition and Dietetics: Malnutrition (undernutrition) screening tools for all adults. Journal of the Academy of Nutrition and Dietetics, 120(4), 709–713. https://doi.org/10.1016/j.jand.2019.09.011
Thomas, D. R. (2022). Total parenteral nutrition. Merck Manual Professional Version. https://www.merckmanuals.com/professional/nutritional-disorders/nutritional-support/total-parenteral-nutrition-tpn
Tse, A., & Schick, M. A. (2022). Central line placement. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK470286
van Braak, H., Gorter, R. R., van Wijk, M. P., & de Jong, J. R. (2022). Laparoscopic Roux-en-Y feeding jejunostomy as a long-term solution for severe feeding problems in children. European Journal of Pediatrics, 182, 601–607. https://doi.org/10.1007/s00431-022-04705-3
van Zanten, A. R., De Waele, E., & Wischmeyer, P. E. (2019). Nutrition therapy and critical illness: Practical guidance for the ICU, post-ICU, and long-term convalescence phases. Critical Care, 23(368), 1–10. https://doi.org/10.1186/s13054-019-2657-5
White, J. V., Guenter, P. Jensen, G., Malone, A., Schofield, M., Academy Malnutrition Work Group, ASPEN Malnutrition Task Force, & ASPEN Board of Directors. (2012). Consensus statement of the Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). Journal of Parenteral and Enteral Nutrition, 36(3), 275–283. https://doi.org/10.1177/0148607112440285
Yahia, A., Szlavecz, A., Knopp, J. L., Razak, N. N. A., Samah, A. A., Shaw, G., Chase, J. G., & Benyo, B. (2022). Estimating enhanced endogenous glucose production in intensive care unit patients with severe insulin resistance. Journal of Diabetes Science and Technology, 16(5), 1208–1219. https://doi.org/10.1177/19322968211018260
Zaccone, V., Santoro, L., Guerrieri, E., Diblasi, I., Roncarati, I., Viticchi, G., Vecchiarelli, P., Santoliquido, A., Fiore, F., Molfino, A., Landi, F., Moroncini, G., Gasbarrini, A., Muscaritoli, M., & Falsetti, L. (2023). Prevention and treatment of catheter-related venous thrombosis in long-term parenteral nutrition: A SINuC position statement. Frontiers in Nursing, 10, 1–11. https://doi.org/10.3389/fnut.2023.1106327
Zierle-Ghosh, A. & Jan, A. (2023). Physiology, body mass index. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK535456