About this course:
This module aims to provide an overview of prostate cancer, its risk factors, clinical features, common subtypes, and treatment modalities, along with the most common adverse effects of treatment. This module will also include patient education and methods to improve clinical outcomes when caring for patients who have prostate cancer.
Course preview
Overview of Prostate Cancer
This module aims to provide an overview of prostate cancer, its risk factors, clinical features, common subtypes, and treatment modalities, along with the most common adverse effects of treatment. This module will also include patient education and methods to improve clinical outcomes when caring for patients who have prostate cancer.
By the completion of this learning activity, the learner should be able to:
- discuss the epidemiology of prostate cancer in the US and the pathophysiology of the disease
- identify the risk factors, signs and symptoms, primary classifications, and subtypes of prostate cancer
- summarize prostate cancer screening guidelines and the meaning of active surveillance and “watchful waiting” as core components of patient education
- explore treatment modalities for prostate cancer, including surgery, radiation, hormonal treatment, antiandrogen therapy, chemotherapy, management of skeletal-related events, and immunotherapy
- describe the most common adverse effects, monitoring parameters of systemic treatments, and highlight the pertinent aspects of patient education
Epidemiology
Aside from skin cancer, prostate cancer is the most common cancer among men in the US. According to the American Cancer Society (ACS, 2023a), one in eight men will be diagnosed with prostate cancer during their lifetime. In 2023, there will be an estimated 288,300 new diagnoses and 34,700 deaths from prostate cancer. Prostate cancer is primarily an aging disease, most commonly diagnosed in men 65 years and older. According to the Centers for Disease Control and Prevention (CDC, 2023), 100 out of every 100,00 men will be diagnosed, and about 19 will die from prostate cancer. Prostate cancer is the second leading cause of cancer-related deaths in the US. African American men have the highest incidence of prostate cancer worldwide. Despite these numbers, most prostate cancers are slow-growing, low-grade, and nonlethal. Survival rates vary depending on the disease’s severity and extent. Prostate cancer has a nearly 100% survival rate if in the early stages at diagnosis but declines to approximately 30% for patients with advanced disease. Currently, there are more than 3.1 million prostate cancer survivors in the US. Since early diagnosis improves health outcomes and reduces morbidity and mortality associated with the disease and its treatment, healthcare providers (HCPs) must be aware of the potential warning signs and symptoms to help facilitate a timely diagnosis (ACS, 2023a; Leslie et al., 2023).
Pathophysiology
The prostate gland is part of the male reproductive system and is comparable in size to a walnut. The prostate is located at the base of the penis beneath the bladder and anterior to the rectum. As demonstrated in Figure 1, the prostate surrounds the urethra and has ducts opening into the prostatic portion of the urethra. It comprises muscular and glandular tissue (Leslie et al., 2023). The prostate gland’s primary function is to form and secrete a slightly alkaline prostatic fluid to carry sperm. This fluid also helps sperm survive in the female reproductive tract’s acidic environment. The prostate contracts rhythmically to push the prostate fluid into the urethra, and the fluid leaves the body through the penis’ tip during ejaculation (McCance & Heuther, 2019).
Figure 1
Prostate and Nearby Organs

(National Cancer Institute [NCI], 2007)
The prostate gland includes three major sections that are important when discussing the development of prostate cancer: the peripheral zone (PZ), central zone (CZ), and transitional zone (TZ). As demonstrated in Figure 2, the PZ comprises the prostate’s largest surface area; 60-75% of cancers arise here. Anatomically, the PZ is positioned at the posterior aspect of the gland, closest to the rectal wall. The CZ surrounds the ejaculatory ducts and makes up about 25% of the gland. While less than 5% of prostate cancers originate within this region, these are more aggressive and pose a higher likelihood of invasion into the seminal vesicles. The TZ surrounds the urethra at the connection point with the prostate gland and is relatively small but grows larger with age. It is responsible for up to 20% of prostate cancers but is more commonly associated with benign prostatic hyperplasia (BPH, or prostate gland enlargement). The fibromuscular stroma is a thick, nonglandular smooth muscle layer (Ali et al., 2022; Wang et al., 2018).
Figure 2
Zones of the Prostate Gland

Androgens are sexual hormones required for the formation, growth, and optimal functioning of the prostate gland and regulated by the hypothalamic-pituitary-gonadal (HPG) axis. The HPG refers to the complex interplay between the male endocrine system, particularly the hypothalamus, pituitary gland, and gonadal glands. The two most abundant androgens in males include testosterone and dihydrotestosterone (DHT), with testosterone serving as the primary circulating androgen. These hormones are important in prostate cancer growth and treatment (Clavijo & Hsiao, 2018).
The hypothalamus initiates the production of androgens. The hypothalamus creates and releases gonadotropin-releasing hormone (GnRH), also known as luteinizing hormone-releasing hormone (LHRH). GnRH prompts the pituitary gland to generate and secrete luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH acts on specific cells in the testes to produce testosterone; additional androgens are produced by the adrenal glands (Clavijo & Hsiao, 2018; Fujita & Nonomura, 2019).
Prostate cells take up testosterone, which directly binds to the androgen receptor (AR) or is converted to DHT. DHT is found predominantly in prostate tissue and has a greater affinity for binding to the AR than testosterone. The AR is a steroid receptor transcriptional factor that mediates the actions of both testosterone and DHT. It has a similar structure and functionality to the estrogen receptor (ER) that fuels estrogen-based cancers, such as breast cancer in females. Androgens can fuel the growth of prostate cancer by binding to and activating the AR, which subsequently stimulates the overexpression of specific genes that trigger cell growth. Therefore, the AR is central to prostate cancer, particularly castration-resistant prostate cancer (CRPC; Clavijo & Hsiao, 2018; Fujita & Nonomura, 2019).
Prostate Cancer Subtypes
All prostate cancers arise from glandular epithelial cells, the most abundant cell type within the prostate gland. Adenocarcinomas are the most common types of prostate cancers, which form in the mucus-producing glandular cells. There are three major prostate cancer classifications, summarized in Table 1. While these three disease classifications are mutually exclusive, many patients will transition from hormone-sensitive to castration-resistant over time. Similarly, patients may be diagnosed with early-stage, localized prostate cancer, which can later spread to distant sites, whereas others may have evidence of metastatic disease at the time of diagnosis (ACS, 2023b; Cancer Research UK, 2022; Urology Care Foundation, 2021).
Table 1
Three Major Types of Prostate Cancer
zonaws.com/nce-content/uploads%2F1696870507996-Table+1.png" style="width: 445px;" class="fr-fic fr-dib">
(Urology Care Foundation, 2021)
Risk Factors
All men are at risk for prostate cancer; however, advanced age, African American race, and family history of prostate cancer are the most well-cited risks (US Preventative Services Task Force [USPSTF], 2018). African American men tend to develop prostate cancer at a younger age and endure a more aggressive clinical course than other men. Family history is a significant risk factor. Men with a first-degree relative with prostate cancer (i.e., father or brother) are twice as likely to develop prostate cancer as those with no family history (CDC, 2023; Ignatavicius et al., 2021. Other possible risk factors with weaker associations and less evidence include diets high in saturated fat and obesity; tobacco use is associated with a higher risk of mortality (Ignatavicius et al., 2021; USPSTF, 2018).
Genetic Mutations
Everyone has BRCA1 and BRCA2 genes. These genes function as tumor suppressor genes that prevent cancer by regulating specific cells’ growth and division under physiologic conditions. However, mutations in these genes prevent them from working correctly, risking cancer development. Mutations in the BRCA1/BRCA2 genes are inherited in an autosomal dominant pattern. One copy of the mutated gene in each cell is sufficient to increase the risk of developing cancer. Data from the IMPACT study, which followed 2,900 men with annual PSA screenings for three years, demonstrated that BRCA2 mutation carriers had the most significant risk of developing cancer. Their findings revealed a 5.2% risk in BRCA2 mutation carriers, 3.4% in those with BRCA1 mutations, and 2.7% in men who tested negative for both mutations (Page et al., 2019).
Genetic and Molecular Biomarker Analysis
Due to recent technological advancements and targeted treatment modalities, the National Comprehensive Cancer Network (NCCN, 2023) guidelines recommend that patients with advanced or metastatic prostate cancer undergo specialized genetic and molecular biomarker analysis. These specific analyses evaluate the presence of homologous recombination repair gene mutations (HRRm) and microsatellite instability-high (MSI-H) or mismatch repair deficiency (dMMR). HRR gene mutations can increase the risk of developing more aggressive prostate cancer, and the most common include BRCA1/2, ATM, and CDK12. HRR gene mutations compromise tumor cells’ ability to correct the DNA damage through the loss of HRR. This mechanism can lead to an increased reliance on poly ADP-ribose polymerase (PARP)-mediated DNA repair pathways for survival, thereby opening up the possibility of targeted treatment pathways, such as the use of PARP-inhibitors (i.e., Olaparib [Lynparza]) in patients with advanced BRCA2-mutant prostate cancer. MSI-H and dMMR have clinical implications concerning the potential use and benefit of immunotherapy (specifically, immune checkpoint inhibitors) in several cancers. MSI-H or dMMR are relatively uncommon in prostate cancer, with one study demonstrating a prevalence rate of only 3.2%. These mutations pose the potential for durable therapeutic responses to anti-programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) treatment, such as pembrolizumab (Keytruda), which will be discussed later within the treatment section of this module (NCCN, 2023; Reichert et al., 2019).
Men with a genetic mutation in the HOXB13 gene may also be at increased risk for prostate cancer. HOX proteins are transcription factors that serve various roles in tissue differentiation of developing embryos and organogenesis and contribute to several other cellular processes such as proliferation, differentiation, apoptosis, and migration. The HOXB13 protein also acts as a tumor suppressor, keeping cells from growing and dividing too quickly or uncontrolled. While all individuals have two copies of the HOXB13 gene, mutations in one copy of the HOXB13 gene are associated with an increased risk for early-onset prostate cancer and CRPC. Research suggests that mutations in this gene impair the protein’s tumor suppressor function, resulting in uncontrolled cellular proliferation and cancer development (US National Library of Medicine, 2015).
Signs and Symptoms
Early prostate cancer generally does not cause pain, sexual dysfunction, or any other signs or symptoms. In most cases, affected patients are asymptomatic. As the tumor grows or spreads into surrounding structures, patients may develop uncomfortable urinary symptoms, such as difficulty starting or stopping the flow of urine, a feeling of not being able to empty the bladder or pain with ejaculation. However, many of these symptoms can also occur due to BPH, a common condition affecting many men as they age. An enlarged prostate gland can induce uncomfortable urinary symptoms, including bladder, urinary tract, or kidney problems. With advanced disease, symptoms often result from cancer metastasizing to other areas in the body. Based on cancer spread patterns, the most common symptoms include bone pain, dysuria, hematuria, flank pain, anemia, fatigue, weight loss, and weakness (Ignatavicius et al., 2021; Leslie et al., 2023).
Early Detection and Screening
Given the lack of warning signs and symptoms in early prostate cancer, most patients are diagnosed as a result of health screenings for a serum protein (prostate-specific antigen [PSA]) or from abnormalities detected on a digital rectal exam (DRE; Leslie et al., 2023).
PSA
PSA is a protein generated only by the prostate gland and a tumor marker for prostate cancer. Tumor markers are substances that may be produced by cancer or by the body’s response to cancer’s presence. They are also made in smaller quantities by healthy cells, making them nonspecific. The PSA blood test measures the amount of this protein in the blood. A PSA level of less than 4.0 ng/mL was previously considered normal, and higher than 4.0 ng/mL was deemed abnormal, warranting a biopsy to evaluate for prostate cancer. Over time, these recommendations have changed. The PSA can be elevated in benign conditions such as BPH or prostatitis (infection or inflammation of the prostate gland) or due to specific urologic procedures or medications. Ejaculation, bicycle riding, or strenuous exercise can induce a transient rise in PSA for a short time (Ignatavicius et al., 2021; Leslie et al., 2023; Pagana & Pagana, 2022).
In contrast, commonly prescribed medications for BPH, such as 5-alpha-reductase inhibitors or dihydrotestosterone blockers (i.e., finasteride [Proscar] and dutasteride [Avodart]), are known to decrease PSA by approximately 30 to 50%, leading to false-negative results (Burchum & Rosenthal, 2022). The American Board of Internal Medicine (ABIM) cites the PSA as having “no specific normal or abnormal level” in their 2023 Laboratory Test Reference Ranges (ABIM, 2023). The PSA level varies and rises slowly with age, even without prostate abnormalities. Beyond a screening modality, the PSA test can help to stage, treat, and monitor prostate cancer (Cadet et al., 2019; Ignatavicius et al., 20211).
DRE
As demonstrated in Figure 3, the DRE allows for direct palpation of the PZ region, the most common site of prostate cancer, to assess for lumps, nodularity, or abnormalities that might suggest cancer (ACS, 2021). Despite its continued use in clinical practice, research has demonstrated that the DRE carries a low specificity and sensitivity for detecting early prostate cancer. In 2018, the USPSTF removed the DRE from its prostate cancer screening guidelines.
Figure 3
Digital Rectal Exam (DRE)

(NCI, 2005)
Controversies Over Prostate Cancer Screenings
Screening for prostate cancer is routinely performed throughout the US, but its benefit and value is controversial. It has been the subject of much criticism over the last few decades; it remains unclear if the benefits of prostate cancer screening outweigh the risks for most men. While prostate cancer screenings may lead to earlier diagnosis and treatment, this does not correlate with improved survival (Pagana & Pagana, 2022; Smith et al., 2019). The major shortcoming of prostate cancer screening is the lack of impact and questionable value of the information received, leading to disagreements about its importance amongst many experts. Predicting who will benefit from treatment is impossible for patients whose prostate cancer is detected by screening. Some patients treated may avoid death and disability from prostate cancer, whereas others who are treated would have died of unrelated causes before their cancer became severe enough to spread, affect their health, or reduce the quality and longevity of their lives. PSA specificity and sensitivity are low, and screening is subject to high false-positive rates. A false positive entails an abnormal PSA level but no detectable prostate cancer, whereas a false negative is characterized by a normal PSA level despite the presence of cancer. Approximately 80% of PSA screenings are false-positive. Further, the PSA test has been criticized for flagging too many slow-growing cancers and subjecting patients to invasive procedures, interventions, treatments, and treatment adverse effects when the cancer may never have posed a risk to the patient during their lifetime (Cadet et al., 2019; Leslie et al., 2023; Pagana, 2022).
Current Screening Guidelines
The consensus from the USPSTF, NCCN, and ACS evidence-based screening guidelines is that the decision to perform PSA screening tests should be an individual one. Clinicians are encouraged to discuss the pros and cons of screening with patients and engage in shared decision-making before deciding upon screening. This allows patients to determine if screening is right for them and strives to reduce the overdiagnosis and overtreatment of prostate cancer. Given the controversies over prostate cancer screenings, there remain some discrepancies between the three guidelines. All three guidelines classify high-risk patients as African American or those with a first-degree relative with prostate cancer. A summary of the specific screening guidelines for each organization is outlined in Table 2 (ACS, 2021; NCCN, 2023; USPSTF, 2018).
Table 2
Comparison of Prostate Cancer Screening Guidelines
USPSTF
|
ACS
|
NCCN
|
Based on the recent findings from the IMPACT trial discussed earlier, Page and colleagues (2019) recommend consideration of testing for BRCA mutations under the following circumstances:
- if there is a history of prostate, breast, or ovarian cancer in the immediate family, particularly among younger family members
- if other family members test positive for BRCA1 or BRCA2 mutations
- if the patient is of Ashkenazi Jewish descent (Page et al., 2019)
Diagnosis
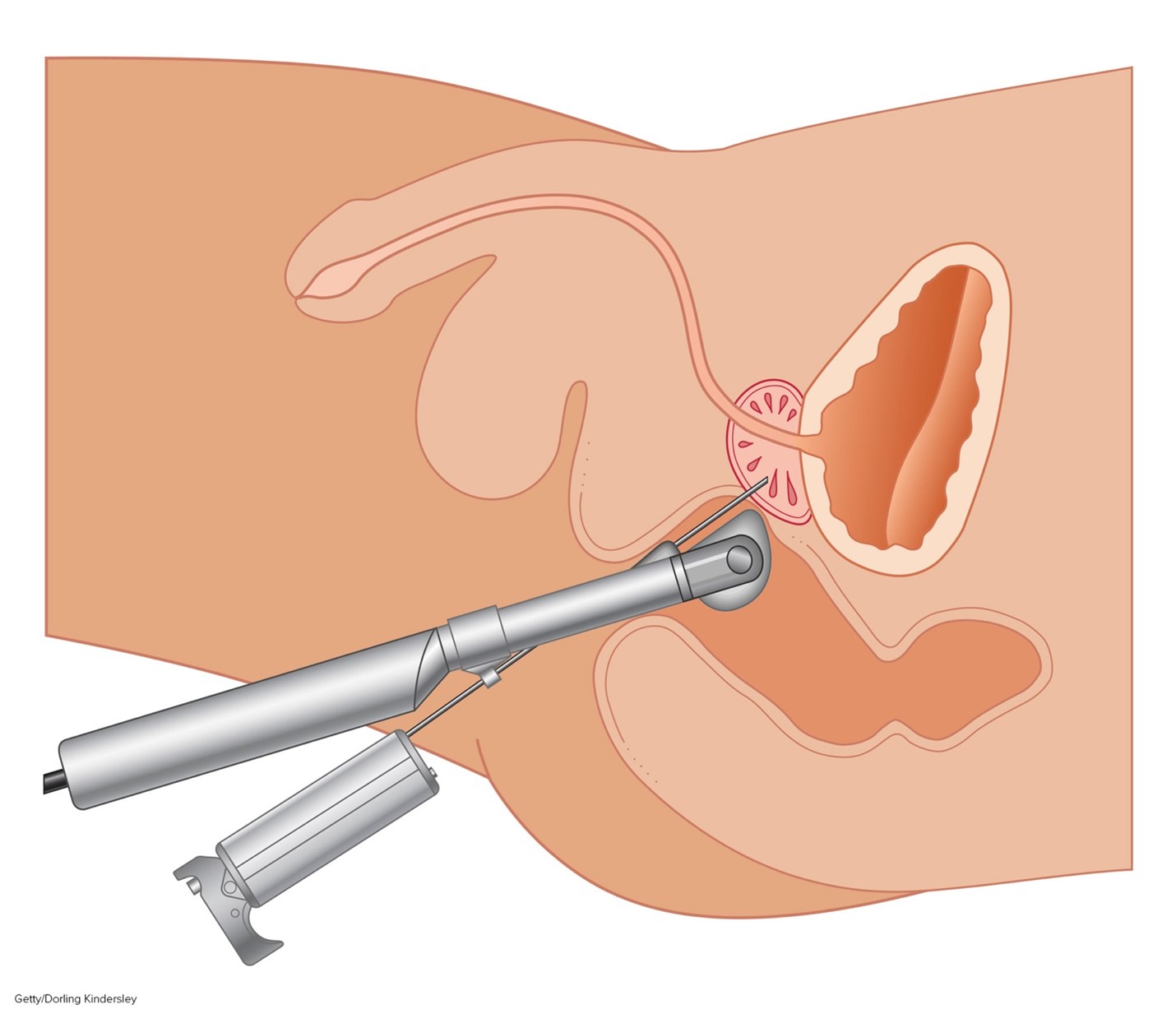
While the PSA and DRE can be useful in detecting prostate gland abnormalities, tissue sampling is necessary to confirm prostate cancer and establish its clinical features. A transrectal ultrasound-guided (TRUS) core biopsy of the prostate is the most common tissue sampling procedure in which a needle is guided through the rectum (transrectal biopsy) or the skin between the scrotum and anus (transperineal biopsy) to remove a small prostate tissue core. This process is repeated several times to obtain up to 12 core tissue samples. The TRUS procedure is demonstrated in Figure 4 (ACS, 2021; Leslie et al., 2023).
Figure 4
Transrectal Ultrasound (TRUS) Prostate Biopsy
Gleason Score
All prostate cancers are subjected to a histologic grading system called the Gleason score, which is vital in predicting prostate cancer’s behavior and determining the best treatment options. Initially developed in the 1960s, the Gleason score is the most widely used and reliable system to define prostate cancer’s aggressiveness (Leslie et al., 2023). The Gleason score is based on the cellular architecture, microscopic arrangement, and pattern of the glands within the prostate tissue. This is distinct from other cancers, which are generally defined by individual cellular characteristics. A histologic grade ranging from one to five is assigned to the tissue to determine the Gleason score. If these cells appear very similar to normal, healthy prostate tissue, they are considered well-differentiated, and a grade one is assigned. If the cells are markedly abnormal and do not have any characteristic features of healthy cells, they are poorly differentiated, and a grade five is assigned. Grades two through four have features between these extremes. Low-grade cancer cells grow and spread more slowly than high-grade cancer cells. Two grades are assigned: one for the most predominant (primary) pattern identified within the tissue sample and one for the second most predominant pattern. Next, the two grades are added together to form the Gleason score, which currently ranges between 6 and 10 (Leslie et al., 2023; Prostate Cancer Foundation [PCF], n.d.).
In 2014, the International Society of Urological Pathology (ISUP) developed a revised prostate cancer grading system called the Grade Groups. The system is more straightforward and was devised to enable patients to understand their risk level better and limit overtreatment (ACS, 2023b). The new system has since been adopted by the World Health Organization (WHO) and incorporated into the NCCN evidence-based guidelines. Compiled through rigorous clinical trial research and annual expert panel reviews, the NCCN provides evidence-based treatment guidelines for each cancer type according to distinct features such as cellular characteristics, genetics, staging, and inheritance patterns. Most NCI-accredited cancer centers utilize the guidelines to guide medical decision-making throughout the patient’s disease trajectory (NCCN, 2023).
The new system compresses the Gleason score into grade groups ranging from one to five, as represented in Table 3. For example, if the Gleason score is presented as 3+4=7, the tumor is primarily graded three, and less of the tumor is a grade four. When combined, they have a Gleason score of 7. If a tumor is all the same grade (i.e., grade 4), then the Gleason score is reported as 4+4=8 (ACS, 2023b).
Table 3
Revised Prostate Cancer Grade Groups
Grade Group | Gleason Score | Gleason Pattern | Description |
1 | ≤6 | ≤ 3+3 | Low grade Well-differentiated |
2 | 7 | 3+4 | Intermediate-grade Moderately-differentiated |
3 | 7 | 4+3 | Intermediate-grade Moderately-differentiated |
4 | 8 | 4+4, 3+5, 5+3 | High-grade Poorly-differentiated |
5 | 9 or 10 | 4+5, 5+4, 5+5 | High-grade Poorly-differentiated |
(ACS, 2023b; NCCN, 2023; PCF, n.d.)
Staging
Prostate cancer is grouped into four stages (I through IV), defined by the extent of the tumor presence and how rapidly the cancerous cells are growing. The four stages are determined by combining the Gleason Score with the Tumor, Nodes, and Metastasis (TNM) system. T stands for the primary tumor and measures the tumor’s size and extent; N stands for lymph node(s) and denotes if cancer has spread into nearby lymph nodes; M stands for metastasis and denotes that cancer has spread to distant sites in the body. Staging is crucial as it helps determine treatment options (Leslie et al., 2023; PCF, n.d.). Radiographic imaging is the only way to identify the presence or absence of distant metastases definitively. However, unlike most cancer diagnoses, systemic imaging studies are not universally recommended for all patients diagnosed with prostate cancer. When imaging studies are indicated, the most commonly performed tests may include one or several of the following: radionuclide bone scan, single-photon emission computerized tomography (SPECT), plain film radiographs (x-rays), ultrasound, computerized tomography (CT scans), positron emission tomography/computed tomography (PET/CT) scan, sodium fluoride PET/CT scan, or advanced magnetic resonance imaging (MRI) techniques such as multiparametric magnetic resonance imaging (mpMRI; NCCN, 2023).
Risk Stratification
No staging system (as described above) has been universally adopted in the national guidelines for prostate cancer. The NCCN risk stratification system assigns a risk category (very low, low, favorable intermediate, unfavorable intermediate, high, and very high) based on several clinical factors. Factors include grade grouping (above), PSA, TNM, and the number of core biopsies found to be positive (NCCN, 2023). Dess and colleagues (2020) developed a clinical prognostic stage group system for prostate cancer patients (STAR-CAP). Factors used in this model include the patient’s TNM (although patients with metastatic disease were not included in this study), Gleason score, age, the percentage/number of positive core biopsies, and pre-treatment PSA. These details are used to assign the patient points that help predict prognosis. An online calculator was set up at www.star-cap.org (Dess et al., 2020)
Management
Active Surveillance versus Observation
Not all patients diagnosed with prostate cancer will require treatment, as it is characteristically slow-growing, and in many cases, patients die from other causes. Active surveillance, or “watchful waiting,” is usually a reasonable option for early-stage prostate cancer patients. The NCI dictionary of cancer terminology defines watchful waiting as:
“closely watching a patient’s condition but not giving treatment unless symptoms appear or change. Watchful waiting is sometimes used in conditions that progress slowly. It is also used when the risks of treatment are greater than the possible benefits. During watchful waiting, patients may be given certain tests and exams. Watchful waiting is sometimes used in prostate cancer. It is a type of expectant management” (NCI, n.d.).
Patients often have anxiety and uncertainties regarding this recommendation due to the natural fears associated with a cancer diagnosis. HCPs should educate patients on the rationale for active surveillance, clarifying how this method is evidence-based and can provide valuable information regarding how quickly the prostate cancer is progressing, if at all. Watchful waiting typically involves long-term monitoring with modalities such as periodic PSA testing, DRE exams, prostate biopsies, symptom assessment, and imaging studies until clinicians and patients collectively decide that treatment should be implemented (NCCN, 2023; Urology Care Foundation, 2021).
The NCCN (2023) guidelines suggest active surveillance for patients with:
- very-low-risk disease and a life expectancy of 10 years or more
- some with low-risk disease and a life expectancy of 10 years or more
They also suggest considering active surveillance in some patients with favorable intermediate-risk disease and a life expectancy of more than 10 years (e.g., those with a low percentage of Gleason 4, low tumor volume, low PSA density, or low genomic risk). This decision should be based on confirmatory testing with a tissue biopsy, advanced imaging studies with PSA density calculation, or molecular tumor analysis within 6-12 months of initial diagnostic biopsy. Active surveillance should be tailored to each patient but typically includes a PSA every 6+ months, DRE every 12+ months, tissue biopsy every 12+ months, and/or a mpMRI every 12+ months. This monitoring allows the patient’s tumor grade, risk category, and management plan to be reassessed regularly (NCCN, 2023).
Observation, by contrast, typically includes a history and physical every 12+ months. Observation is preferred/recommended for those with:
- asymptomatic very low or low-risk disease and a life expectancy of less than 10 years
- asymptomatic intermediate-risk disease and a life expectancy of 5 years or less
Observation can also be considered for anyone with asymptomatic disease (regardless of assessed risk) and a life expectancy of 5 years or less (NCCN, 2023).
Treatment Modalities
The decision to initiate treatment is multifactorial and based on the patient’s age, life expectancy, coexisting medical conditions, specific genetic mutations, symptom burden, and cancer stage. Treatment for prostate cancer is divided into two major categories: localized and systemic therapies, as outlined in Figure 5. Patients with early-stage and localized disease are treated with curative intent. These patients are most commonly offered surgical intervention, followed by adjuvant (post-surgery) radiation therapy. Patients with advanced or metastatic prostate cancer are more likely to be treated with a combination of systemic therapies and are subject to relapse and remission (NCCN, 2023).
Figure 5
Prostate Cancer Treatment Modalities

(Selchick, 2020)
Surgical Management
Radical prostatectomy is the most common surgical procedure if the cancer is confined to the prostate. The entire prostate gland, and usually the seminal vesicles, surrounding tissue, lymph nodes, nerves, and veins, are removed. A portion of the urethra is also removed, and the remaining urethra is anastomosed at the bladder neck. A urinary catheter is inserted for 1-2 weeks while the patient heals. Open procedures may be completed through a retropubic (anterior) or perineal (inferior) approach, although a laparoscopic approach is more common now in the US. This approach may be done with or without robotic assistance, tends to limit blood loss, and hastens recovery time. There may be an increased risk of intestinal injury with laparoscopic and robotic techniques. If lymph nodes are removed, lymphocele formation is also a risk (ACS, 2019). Many patients will require adjuvant treatment to reduce the risk of recurrence, especially if adverse pathological or laboratory features are found during surgery, which includes positive surgical margin(s), seminal vesicle invasion, extracapsular extension, or a detectable PSA level following surgery (NCCN, 2023).
Erectile dysfunction (ED) or impotence is one of the most common risks associated with radical prostatectomy. Since the nerves that control the patient’s ability to have an erection are adjacent to the prostate gland, they may be damaged, severed, or removed during the surgery. Approximately 50% of patients with intact nerves will regain some ability to have an erection, but it can take 6 to 12 months. Patients with other health conditions that impair their ability to maintain an erection, such as diabetes or vascular issues, will have a more difficult time regaining their normal function. Urinary incontinence and damage to the urethra is another common risk associated with radical prostatectomy. Following surgery, up to 50% of patients will develop urinary incontinence, and the degree of incontinence can range from intermittent dribbling and stress incontinence to continuous leakage, requiring the use of pads or incontinence briefs. Historically, the rates of these complications are about the same regardless of surgical approach or technique (ACS, 2019). However, a recent study in Taiwan may indicate that the long-term complications of robot-assisted procedures are less compared to laparoscopic or open procedures. While this was a large study (over 1,400 patients), it was retrospective, meaning confounding factors may have influenced the findings. Still, at a mean follow-up of 36 months (3 years), patients undergoing robot-assisted procedures reported a shorter hospital stay, less chance of severe pain, less chance of requiring a blood transfusion, and reduced rates of ED, incontinence, and hernia development (Wu et al., 2021).
The adverse effects of prostate cancer treatment on sexual function and quality of life are well-cited. Urinary and sexual dysfunction are the most frequently reported adverse effects of prostate cancer treatment and among the most distressing. If left untreated, ED can lead to significant physical, psychological, and interpersonal consequences and impair quality of life and overall well-being. HCPs are tasked with addressing these issues with sensitivity, empathy, and compassion, fostering a safe and nonjudgmental environment for patients to express their concerns openly. HCPs should be prepared to educate patients and their partners, offer psychosocial support, and connect patients with resources, support groups, and medical specialists to manage these long-term effects (Yarbro et al., 2018).
While not used to cure prostate cancer, a transurethral resection of the prostate (TURP) may be used to treat symptoms of advanced prostate cancer. The central portion of the prostate surrounding the urethra is removed through a urethral approach with a resectoscope. A urinary catheter is typically inserted for a day or so to allow for swelling to decrease. Like all surgical procedures, potential adverse effects include bleeding, infection, damage to adjacent organs, and anesthesia effects. Prolonged inactivity also increases the risk of blood clots (ACS, 2019).
Radiation Therapy
Radiation therapy is a localized cancer treatment that uses high-energy rays of electron, proton, or neutron beams to destroy cancer cells. The primary objective is to deliver a precisely measured radiation dose to a defined tumor with as little injury as possible to surrounding healthy tissue. Radiation induces damage to cancer cells, leading to biological changes in the DNA, rendering them incapable of reproducing or spreading. All healthy and cancerous cells are vulnerable to the effects of radiation and may be injured or destroyed; however, most normal cells can repair themselves and remain functional. The total radiation dose is hyper-fractionated, which means it is administered in smaller divided doses, or fractions, rather than all at once. Hyper-fractionation allows healthy cells a chance to recover between dosing. Each dose is called a fraction, and the total number of fractions depends on the tumor size, location, reason for treatment, patient’s overall health, performance status, goals of therapy, and consideration of any other treatments the patient is receiving. Radiation can be delivered externally or internally; some patients may receive both types (Yarbro et al., 2018). For prostate cancer, radiation therapy may be administered for early-stage and low-grade prostate cancer confined to the prostate gland or as part of the treatment regimen alongside hormonal therapy (ACS, 2023c). The two most common types of radiotherapy used for prostate cancer include internal brachytherapy and external beam radiation therapy (EBRT), which will be described in this subsection (NCCN, 2023).
Brachytherapy. Prostate brachytherapy is a minimally invasive radiation therapy that implants a small wire or radioactive seeds directly into the prostatic tissue. Brachytherapy delivers high and concentrated doses of radiation to the prostate gland from inside the body. Hormone therapy is sometimes given for three to six months before brachytherapy to shrink the prostate gland and optimize its effectiveness. The two brachytherapy types include a temporary high-dose rate (HDR) or a permanent low-dose rate (LDR). HDR brachytherapy is usually performed on an outpatient basis under general anesthesia. Several catheters, which are attached to a machine that contains radioactive pellets, are placed into the prostate gland. The catheters transmit concentrated bursts of high-dose radiation pellets into the tumor bed. This is generally performed once or twice and takes about 15 minutes (Memorial Sloan Kettering Cancer Center [MSKCC], n.d.).
LDR brachytherapy is an outpatient procedure that requires general anesthesia. Permanent radioactive seeds are implanted into the prostate gland, slowly releasing radiation over several months. Up to 100 seeds may be inserted depending on the size of the prostate. The radiation emitted from the seeds only travels a short distance, limiting the amount of damage to nearby healthy tissues. The radioactivity lessens after several weeks or a few months. Patients are usually advised to stay at least three feet away from pregnant women and small children for a specified period. This precaution is recommended to avoid any unnecessary risk. While there is little clinical data available to validate this recommendation, and the duration of time is not yet defined, the statement is endorsed by the International Atomic Energy Agency (IAEA) within their Radiation Protection of Patients (RPOP) recommendations. Patients should be counseled that detection systems can sometimes pick up low radiation levels at airports, so they should carry a physician’s note or radiation card regarding their treatment to avoid security problems. Overall, patients can safely go about their regular routines and lifestyles without potentially exposing others (ACS, 2023c; IAEA, n.d.; MSKCC, n.d.). Brachytherapy is a recommended option within the 2023 NCCN guidelines for the treatment of localized disease:
- initial treatment in very low-risk patients with more than 20 years of life expectancy
- initial treatment in low-risk patients with at least 10 years of life expectancy
- initial treatment in favorable intermediate-risk patients with at least 5 years of life expectancy
- initial treatment in unfavorable intermediate-risk patients with at least 5 years of life expectancy in combination with EBRT and possibly androgen deprivation therapy (ADT)
- initial treatment in high or very high-risk patients with more than 5 years of life expectancy (or symptomatic) in combination with EBRT and ADT
- in the context of radiation therapy recurrence, if the patient’s life expectancy is more than 5 years, the tissue biopsy is positive for malignancy, but imaging studies indicate no metastatic disease (NCCN, 2023)
EBRT. EBRT is radiation delivered from a source outside the body directly to the cancer site. EBRT uses a linear accelerator to generate and deliver high-energy x-rays in small daily doses. Intensity-modulated radiation therapy (IMRT) is the most common type of EBRT for prostate cancer. It uses a computerized machine that travels around the patient as it delivers the radiotherapy. This technology allows for the more precise shaping and aiming of the radiation beams at the prostate, covering several angles and increasing the beams' intensity (strength). This limits the radiation dose to the surrounding healthy tissues and enables the patient to tolerate a higher radiation dose to cancer, improving clinical outcomes and survival (ACS, 2023c; Ignatavicius et al., 2021). EBRT is currently recommended within the 2023 NCCN guidelines for:
- initial treatment in very low-risk patients with more than 20 years of life expectancy
- initial treatment in low-risk patients with at least 10 years of life expectancy
- initial treatment in favorable intermediate-risk patients with at least 5 years of life expectancy
- initial treatment in unfavorable intermediate-risk patients with at least 5 years of life expectancy in combination with ADT and possibly brachytherapy
- initial treatment in high or very high-risk patients with more than 5 years of life expectancy (or symptomatic) in combination with ADT and/or brachytherapy or abiraterone (Zytiga, a form of hormone therapy)
- initial treatment in high or very high-risk patients with 5 years or less of life expectancy (and asymptomatic)
- initial treatment in regional-risk patients with more than 5 years of life expectancy (or symptomatic) in combination with ADT and/or abiraterone (Zytiga)
- adjuvant therapy in very low-risk patients with more than 20 years of life expectancy following prostatectomy if adverse features are found
- adjuvant therapy in low-risk patients with at least 10 years of life expectancy following prostatectomy if adverse features are found
- adjuvant therapy in favorable intermediate-risk patients with more than 10 years of life expectancy following prostatectomy and pelvic lymph node dissection if adverse features or lymph node metastases are found
- adjuvant therapy in unfavorable intermediate-risk patients with more than 10 years of life expectancy following prostatectomy and pelvic lymph node dissection if adverse features or lymph node metastases are found
- adjuvant therapy in high or very high-risk patients with more than 5 years of life expectancy (or symptomatic) following prostatectomy and pelvic lymph node dissection if adverse features or lymph node metastases are found
- adjuvant therapy in regional-risk patients with more than 5 years of life expectancy (or symptomatic) following prostatectomy and pelvic lymph node dissection if adverse features or lymph node metastases are found (NCCN, 2023)
Radiation Adverse Effects. LDR brachytherapy carries a risk for the migration of the implanted seeds. Additional adverse effects of radiation include fatigue, lymphedema in the legs or genital region, urinary incontinence, radiation cystitis (inflammation of the bladder), urethral stricture, and radiation proctitis (inflammation of the rectum). There is also a risk of delayed ED, which may develop months or years after radiation therapy (ACS, 2023c; Ignatavicius et al., 2021).
For a more in-depth review of radiation therapy and nursing implications, refer to the Oncology Nursing Part 1: Surgical and Radiation Oncology NursingCE course.
Androgen Deprivation Treatment (ADT)
ADT is also called testosterone-depleting therapy and is the most common systemic treatment modality for prostate cancer. Testosterone cannot discriminate between healthy tissue receptors and those of cancerous tissue and is the primary fuel for cancer growth. ADT deprives the body of androgens by lowering (or depleting) the testosterone level. Prostate cancer usually stops growing in response, at least for a while. CRPC occurs when the tumor cells become resistant to ADT therapy and resume growing again despite the hormone-blocking treatments. The most common approaches to androgen deprivation include castration, antiandrogens, and combined androgen blockade. The term castration refers to removing 90 to 95% of the testosterone expected in a healthy male, and this may be achieved surgically or chemically. Orchiectomy is the surgical removal of the testicles, a permanent and irreversible procedure that can reduce the testosterone level in the blood by 90 to 95%. Chemical castration is much more common and involves the use of LHRH agonists or LHRH antagonists (NCI, 2021).
LHRH Agonists. LHRH agonists (sometimes called LHRH analogs) mimic LHRH’s action by occupying the receptors on the pituitary glands. Administration of these drugs causes the pituitary to secrete LH and the testicles to increase testosterone production temporarily, igniting a “testosterone flare”. LHRH agonists have a longer half-life than physiologic LHRH, so they occupy the receptor with greater affinity. Due to the continued presence of high levels of the LHRH agonist, the pituitary gland eventually stops producing LH, halting testosterone production. LHRH agonists are administered by injection or may be implanted under the skin. Currently, the three primary LHRH agonists used to treat prostate cancer in the US include leuprolide acetate (Lupron Depot), goserelin acetate (Zoladex), and triptorelin pamoate (Trelstar; NCI, 2019). These agents are outlined below in Table 4.
All medications in this class are associated with an initial testosterone flare, which may cause an acute worsening of symptoms. This initial setback is particularly concerning for patients with advanced prostate cancer as it can lead to significant bone pain and ureter or bladder outlet obstruction. The temporary testosterone flare is usually counteracted with oral antiandrogen therapy (described below) for several weeks (NCI, 2019). In severe cases, it can induce spinal cord compression (SCC) in patients with metastatic disease to the spinal column. SCC is the compression of the spinal cord by malignant tumor invasion into the epidural space, and symptoms may include the following:
- sudden onset of acute back pain that worsens in the supine recumbent position and may be relieved by sitting
- neurologic dysfunction, such as numbness
- bowel or bladder dysfunction such as incontinence, passing very little urine, or inability to urinate or defecate
- altered gait or difficulty ambulating
Initiating treatment as early as possible is critical and involves administering corticosteroids to reduce vasogenic edema within the spinal cord, thereby improving neurologic dysfunction and relieving pain. Definitive treatment typically involves radiation therapy or neurosurgery (Klemencic & Perkins, 2019; Olsen et al., 2019).
Table 4
LHRH Agonists
Drug & Dosing | Adverse Effects, Warnings, Precautions |
Leuprolide acetate (Lupron Depot) |
|
Goserelin acetate (Zoladex) |
|
Triptorelin pamoate (Trelstar) |
|
(US Food and Drug Administration [FDA], 2020b, 2020c, 2022b)
LHRH Antagonists. LHRH antagonists, also known as GnRH antagonists, prevent LHRH from binding to its receptors in the pituitary gland, thereby inhibiting LH and testosterone production. Unlike LHRH agonists, LHRH antagonists do not cause a testosterone flare. Degarelix (Firmagon) is the only LHRH antagonist currently approved for prostate cancer treatment in the US (NCI, 2021). Degarelix (Firmagon) is administered as a subcutaneous injection. Patients should be counseled on the most common side effects, including mild injection site reactions (localized pain, erythema, swelling, or induration), hot flashes, and weight gain. Patients must undergo periodic cardiac monitoring (with an EKG) and liver function tests. Although rare, hypersensitivity reactions have been reported (FDA, 2015a).
Antiandrogen Therapy. Most antiandrogens are AR inhibitors or antagonists. These medications differ from LHRH antagonists by directly competing with androgens at the AR within the prostate gland. Testosterone circulates through the body but cannot interact with the prostate gland or promote cancer growth. These oral medications are rarely used as monotherapy since they do not block testosterone production but may be used when other forms of ADT stop working effectively. Instead, they are prescribed concurrently with LHRH agonists or in patients who have undergone a bilateral orchiectomy. This combined regimen is referred to as a combined androgen blockade. There are two major classes of antiandrogens: first-generation and second-generation. First-generation antiandrogens are credited with establishing the AR blockade as an effective treatment strategy in prostate cancer; however, they do not completely block all AR activity. These agents primarily block androgen activity in the testes. First-generation antiandrogens currently approved for use in the US include bicalutamide (Casodex), flutamide (Eulexin), and nilutamide (Nilandron). Second-generation antiandrogens were developed to enhance the first-generation drugs’ mechanisms and bypass resistance to therapy. Second-generation agents have increased specificity and higher affinity for ARs. There are four second-generation antiandrogens currently approved by the FDA: enzalutamide (Xtandi), abiraterone acetate (Zytiga), apalutamide (Erleada), and darolutamide (Nubeqa). These agents are described in Table 5. Abiraterone acetate (Zytiga) was the first second-generation antiandrogen approved by the FDA in 2011. It is slightly different from others in the class as it prevents androgen biosynthesis and testosterone production in the testes, the adrenal glands, and the tumor by inhibiting the activity of a necessary enzyme, CYP17. Before the introduction of abiraterone (Zytiga), ketoconazole (Nizoral, an antifungal) and aminoglutethimide (Cytadren) were used off-label as CYP17 inhibitors (NCI, 2021; Rice et al., 2019).
Table 5
Antiandrogen Medications
Drug & Dosing | Adverse Effects, Warnings, Precautions |
First-Generation Antiandrogens | |
Bicalutamide (Casodex)
|
|
Flutamide (Eulexin)
|
|
Nilutamide (Nilandron) |
|
Second-Generation Antiandrogens | |
Enzalutamide (Xtandi) |
|
Abiraterone acetate (Zytiga) |
|
Apalutamide (Erleada) |
|
Darolutamide (Nubeqa) |
|
(FDA, 2015b, 2017, 2018, 2019, 2021, 2022a, 2023; NCI, 2021; Rice et al., 2019)
Chemotherapy
Chemotherapy works by destroying quickly dividing cells and is not commonly used as an upfront treatment option for prostate cancer. Due to the slow-growing nature of the disease and the high toxicity associated with chemotherapy, it is generally reserved for more advanced, widespread disease stages that have become refractory to the effects of ADT. When used as salvage therapy for progressive disease, chemotherapy can extend longevity and improve the quality of life in many patients. As a class, chemotherapy agents are high-risk, hazardous drugs administered to destroy as many cancer cells with as minimal effect on healthy cells as possible. Chemotherapy generally interferes with the normal cell cycle, impairing DNA synthesis and cell replication, thereby preventing cancer cells from dividing and multiplying. Since cancer cells tend to divide rapidly, chemotherapy targets cells that divide quickly. As a result, it also impacts healthy cells that divide quickly, such as those within the gastrointestinal tract, skin cells, and bone marrow. The most common chemotherapy agents used for prostate cancer include docetaxel (Taxotere), paclitaxel (Taxol), cabazitaxel (Jevtana), carboplatin (Paraplatin), and vinblastine (Velban; NCCN, 2023; Olsen et al., 2019).
Adverse effects of chemotherapy are inevitable due to the nonspecific nature of cytotoxic therapy; they simultaneously impact healthy cells along with cancerous cells. However, adverse effects vary based on the drug type, dosage, duration of treatment, and specific patient factors. Not all patients respond similarly, and not all chemotherapy agents pose the same risks. Assessment and education are the most critical components to ensuring timely recognition, intervention, and management of adverse effects experienced by each patient. Many adverse effects, such as nausea, can be primarily thwarted by implementing appropriate prevention strategies and medications. As a group, the most common adverse effects include decreased blood counts (anemia, thrombocytopenia, neutropenia), fatigue, nausea, anorexia, alopecia (hair loss), mucositis (mouth sores), diarrhea, skin changes, and peripheral neuropathy (damage to the sensory nerves; Burchum & Rosenthal, 2022; Olsen et al., 2019;).
For a more in-depth review of chemotherapy and nursing implications, refer to the Oncology Nursing Part 2: Chemotherapy and Oncologic Emergencies and Oncology Administration NursingCE courses and earn up to 12 ANCC contact hours.
PARP Inhibitors
PARP inhibitors are targeted agents, small molecule inhibitors that block the PARP. The PARP protein is critical in cell growth, regulation, and repair, helping cancer cells repair themselves and survive. This inhibition leads to cancer cell death. PARP inhibitors have revolutionized how BRCA–mutant cancers, such as ovarian and breast cancer, are treated. Their value has recently been discovered in BRCA-mutant prostate cancers (Yarbro et al., 2018). On May 15, 2020, the FDA granted accelerated approval of rucaparib (Rubraca) for patients with BRCA-mutant, metastatic CRPC who have been previously treated with AR-directed therapy and taxane-based (i.e., docetaxel [Taxotere] or paclitaxel [Taxol]) chemotherapy. Rucaparib (Rubraca) is given orally twice daily with or without food. Patients receiving rucaparib should receive an LHRH agonist concurrently or should have previously undergone bilateral orchiectomy. The most common adverse effects include fatigue, anemia, anorexia, rash, nausea, vomiting, diarrhea, constipation, thrombocytopenia, and increased transaminase levels (Oncology Nursing Society [ONS] Voice, 2020). Olaparib (Lynparza) is another oral PARP inhibitor that has proven efficacy in treating BRCA-mutant breast and ovarian cancers. The FDA approved Olaparib (Lynparza) for metastatic CRPC treatment in combination with an LHRH agonist or following bilateral orchiectomy. It is only approved for use in those with HRR gene mutations, including BRCA. It is dosed orally twice daily. Adverse reactions include nausea/vomiting/diarrhea, fatigue, anemia, anorexia, headache, neutropenia, dysgeusia, cough, dyspnea, dizziness, dyspepsia, leukopenia, thrombocytopenia, and abdominal pain (FDA, 2020a).
Immunotherapy
Sipuleucel-T (Provenge) is a cellular immunotherapy customized using each patient’s immune cells to create a vaccine. The patient’s immune cells are collected through leukapheresis and then developed into a vaccine to boost the immune system’s ability to attack prostate cancer. The cells are subsequently returned to the patient through an IV infusion about 72 hours later. The patient is given three doses, about two weeks apart. Sipuleucel-T (Provenge) is only currently approved for use in patients with metastatic CRPC who are asymptomatic or minimally symptomatic. Further, eligible patients must not have liver metastases and have a life expectancy greater than six months to receive this medication. In phase 3 clinical trial, Sipuleucel-T has been shown to extend average survival by only about four months; however, this translates to a 22% reduction in mortality risk for patients in this category. The infusion is generally well-tolerated, and the most common adverse effects include fever, chills, infusion-related reactions, and headache (Burchum & Rosenthal, 2022; Lexicomp, 2022; NCCN, 2023).
Pembrolizumab (Keytruda) is an immunotherapy that blocks the PD-1 pathway and triggers the immune system to recognize the cancer cells as foreign and attack them. Pembrolizumab (Keytruda) has demonstrated clinically significant results, including improved survival and quality of life across various cancer types. With regard to prostate cancer, pembrolizumab (Keytruda) is approved for metastatic CRPC patients with MSI-H or dMMR mutations who have progressed through at least one line of systemic treatment. As cited earlier, MSI-H and dMMR mutations are relatively uncommon in prostate cancers; however, pembrolizumab (Keytruda) has demonstrated remarkable and durable responses in those who express these mutations. Abida and colleagues (2018) found that in 11 patients with MSI-H/dMMR CRPC, 6 patients (54.5%) had more than a 50% decline in PSA levels, while 4 patients had radiographic responses with reduced tumor burden on imaging. Further, 5 of the 6 responders remained on treatment, with the most prolonged ongoing response extending to 89 weeks. While the number of patients with MSI-H/dMMR CRPC is small and clinical research continues, these findings support the clinical significance of this mutation in treatment planning (Reichert et al., 2019).
Cryotherapy
Cryotherapy, also referred to as cryosurgery or cryoablation, is a minimally invasive surgery that uses thin needles and controlled freezing gas to destroy the cancer cells. It still lacks rigorous, long-term survival data. It is offered to specific patients with locally advanced prostate cancer alongside other treatments. Ultrasound guidance facilitates the placement of the cryoprobes into the prostate since cryotherapy destroys both healthy and cancerous tissue that it comes in contact with. Argon gas is subsequently injected into the prostate, promoting the formation of ice crystals inside and around the cells. The freezing and thawing process destroys the cancer cells by dehydrating them, causing extreme changes in the pH levels and preventing blood flow, blocking necessary nutrients. While the procedure is generally well-tolerated, it is normal to experience soreness and hematuria for one or two days following the procedure. Potential complications include urinary incontinence, injury to the rectum, and ED. The NCCN guidelines do not recommend this as an initial treatment option for most patients. Still, it may be considered in the context of radiation therapy recurrence (disease that recurs following radiation therapy) if the patient’s life expectancy is at least 5 years, the tissue biopsy is positive for malignancy, but imaging studies indicate no metastatic disease (NCCN, 2023).
Adverse Effects
Skeletal-Related Events (SREs)
Since bone is one of the most common sites of metastases for prostate cancer, bone health and preventing SREs is an important part of prostate cancer treatment. Denosumab (XGeva) and zoledronic acid (Zometa) are injectable bisphosphonates that reduce the risk for disease-related skeletal complications, such as fracture, SCC, or the need for palliative surgery or radiation therapy to the bones. Under physiologic conditions, the body is continuously breaking down and rebuilding bone to maintain the strength and health of the bones. Osteoclasts are responsible for bone resorption. In patients with bone metastases from cancer, the breakdown and rebuilding of bones can become overactive, weakening the bones and leading to serious complications such as fractures. RANK is a protein essential for osteoclasts' formation, function, and survival. Denosumab (XGeva) binds to the RANK ligand (RANKL). By binding to the RANKL, denosumab (XGeva) inhibits osteoclastic activity, decreasing bone resorption and increasing bone mass and strength. The most common adverse effects include hypocalcemia, weakness, fatigue, acute kidney injury, nausea, diarrhea, infusion reactions, and flu-like symptoms. Although rare, atypical subtrochanteric and diaphyseal femoral fractures have been reported in patients receiving bisphosphonate therapy; these fractures occur after minimal or no trauma. Patients should be counseled on the importance of adequate calcium and vitamin D intake through dietary sources or supplementation to reduce the risk for hypocalcemia and atypical fractures. Additionally, all bisphosphonates carry a risk for osteonecrosis of the jaw (ONJ), a severe medication complication resulting in progressive bone destruction in the maxillofacial region. The pathophysiology of ONJ is poorly understood, but it can lead to significant morbidity, infection, and reduced quality of life. The risk of ONJ is heightened with dental extractions or implants. Patients should be educated on risk reduction strategies such as maintaining good oral hygiene practices, routine follow-up with their dentist, and avoiding dental implants or extractions. All patients should undergo a baseline dental evaluation before starting bisphosphonate therapy (NCCN, 2023).
Sexual Dysfunction
The adverse effects of prostate cancer treatment on sexual function and quality of life are well-cited. ED is the most frequently reported adverse effects of prostate cancer treatment and among the most distressing. Left untreated, ED can lead to significant physical, psychological, and interpersonal consequences and impair quality of life and overall well-being. The most commonly prescribed oral agents include phosphodiesterase 5 (PDE5) inhibitors, such as tadalafil (Cialis), sildenafil (Viagra), vardenafil (Levitra), and avanafil (Stendra). The most common adverse effects of PDE5 inhibitors include flushing, headache, dizziness, dyspepsia, myalgias, and rhinitis. On average, these medications take approximately one hour to take effect, and the erection-boosting effects can last up to 36 hours. Contraindications to PDE5 inhibitor use include any concurrent use of nitrates (i.e., nitroglycerin [Nitrostat] or isosorbide mononitrate [Imdur]). According to Murphy and colleagues (2018), nitroglycerin (Nitrostat) must be withheld for at least 12 hours after avanafil (Stendra), 24 hours following the last dose of sildenafil (Viagra) or vardenafil (Levitra), and 48 hours following tadalafil (Cialis). Further, all PDE5 inhibitors have cardiovascular precautions and must be used with caution in patients with any of the following:
- MI, stroke, or life-threatening cardiac arrhythmia within the last six months
- hypotension (BP <90/50 mmHg) or hypertension (BP >170/100 mmHg)
- unstable angina, angina during sexual intercourse, or congestive heart failure
Alpha-blockers are commonly used to treat hypertension (i.e., prazosin [Minipress]) and prostate issues (i.e., tamsulosin [Flomax]). These medications are of significant concern with the concomitant use of PDE5 inhibitors. While dosing recommendations differ based on the specific type of alpha-blocker used, all PDE5 inhibitors administered concomitantly with antihypertensives or alcohol may lower blood pressure and should be used cautiously (Murphy et al., 2018).
References
Ali, A., Du Feu, A., Oliveira, P., Choudhury, A., Bristow, R. G., & Baena, E. (2022). Prostate zones and cancer: Lost in transition. Nature reviews. Urology, 19(2), 101–115. https://doi.org/10.1038/s41585-021-00524-7
American Board of Internal Medicine. (2023). ABIM laboratory test reference ranges - July 2023. https://www.abim.org/~/media/ABIM%20Public/Files/pdf/exam/laboratory-reference-ranges.pdf
American Cancer Society. (2019). Surgery for prostate cancer. https://www.cancer.org/cancer/prostate-cancer/treating/surgery.html
American Cancer Society. (2021). Screening tests for prostate cancer. https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/tests.html
American Cancer Society. (2023a). Key statistics for prostate cancer. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
American Cancer Society. (2023b). Prostate pathology. https://www.cancer.org/treatment/understanding-your-diagnosis/tests/understanding-your-pathology-report/prostate-pathology.html
American Cancer Society. (2023c). Radiation therapy for prostate cancer. https://www.cancer.org/cancer/prostate-cancer/treating/radiation-therapy.html
American Society of Clinical Oncology. (2020). TAPUR study shows encouraging results for olaparib in BRCA-mutated advanced prostate and pancreatic cancers. https://www.asco.org/about-asco/press-center/news-releases/tapur-study-shows-encouraging-results-olaparib-brca-mutated
Burchum, J. R., & Rosenthal, L. D. (2022). Lehne’s pharmacology for nursing care (11th ed.). Elsevier. (785-787, 1264-1265
Cancer Research UK. (2022). Types of prostate cancer. https://www.cancerresearchuk.org/about-cancer/prostate-cancer/stages/types
The Centers for Disease Control and Prevention. (2023). Prostate cancer. https://www.cdc.gov/cancer/prostate/index.htm
Clavijo, R. I., & Hsiao, W. (2018). Update on male reproductive endocrinology. Translational Andrology and Urology, 7(Suppl 3), S367-S372. https://doi.org/10.21037/tau.2018.03.25
Fujita, K., & Nonomura, N. (2019). Role of androgen receptor in prostate cancer: A review. World Journal of Men’s Health, 37(3); 288-295. https://doi.org/10.5534/wjmh.180040
Ignatavicius, D. D., Workman, M. L., & Rebar, C. R., & Heimgartner, N. M. (2021). Medical-surgical nursing (10th ed.). Elsevier.
International Atomic Energy Agency. (n.d.). Brachytherapy – What patients need to know. Retrieved August 2023 from https://www.iaea.org/resources/rpop/patients-and-public/brachytherapy
Klemencic, S., & Perkins, J. (2019). Diagnosis and management of oncologic emergencies. West J Emerg Medicine, 20(2), 316-322. https://doi.org/10.5811/westjem.2018.12.37335
Leslie, S. W., Soon-Sutton, T. L., Sajjad, H., & Siref, L. E. (2023). Prostate cancer. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK470550/
Lexicomp. (2022). Adult drug information handbook (31st ed.). Wolters Kluwer Clinical Drug Information, Inc., 1755-1756 2064.
McCance, K. L., & Heuther, S. E. (2019). Pathophysiology: The biologic basis for disease in adults and children. (8th ed.). Elsevier.
Memorial Sloan Kettering Cancer Center. (n.d.). Brachytherapy for prostate cancer. Retrieved August 2023 from https://www.mskcc.org/cancer-care/types/prostate/treatment/brachytherapy
National Cancer Institute. (n.d.). NCI dictionaries: Watchful waiting. Retrieved August 2023 from https://www.cancer.gov/publications/dictionaries/cancer-terms/def/watchful-waiting
National Cancer Institute. (2005). Exam, digital rectal [image]. https://visualsonline.cancer.gov/details.cfm?imageid=7136
National Cancer Institute. (2007). Prostate and nearby organs [image]. https://visualsonline.cancer.gov/details.cfm?imageid=4280
National Cancer Institute. (2021). Hormone therapy for prostate cancer. https://www.cancer.gov/types/prostate/prostate-hormone-therapy-fact-sheet#r5
National Comprehensive Cancer Network. (2023). NCCN guidelines version 3.2023: Prostate cancer. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
Olsen, M., LeFebvre, K., & Brassil, K. (2019). Chemotherapy and immunotherapy guidelines and recommendations for practice. (1st Ed.). Oncology Nursing Society
Oncology Nursing Society Voice. (2020). FDA grants accelerated approval to rucaparib for BRCA-mutated, metastatic, castration-resistant prostate cancer.
https://voice.ons.org/news-and-views/fda-grants-accelerated-approval-to-rucaparib-for-brca-mutated-metastatic-castration
Pagana, K. D. & Pagana, T. J. (2022). Mosby’s manual of diagnostic and laboratory tests (7th ed.). Elsevier. 400-401.
Page, E. C., Bancroft, E. K., Brook, M. N., Assel, M., Al Battat, M. H., Thomas, S., Taylor, N., Chamberlain, A., Pope, J., Raghallaigh, H. N., Evans, D. G., Rothwell, J., Maehle, L., Grindedal, E. M., James, P., Mascarenhas, L., McKinley, J., Side, L., Thomas, T., … Eeles, R. A. (2019). Interim results from the IMPACT study: Evidence for prostate-specific antigen screening in BRCA2 mutation carriers. European Urology, 76(6),831-842. https://doi.org/10.1016/j.eururo.2019.08.019
Prostate Cancer Foundation. (n.d.). Gleason score and grade group. Retrieved August 2023 from https://www.pcf.org/about-prostate-cancer/diagnosis-staging-prostate-cancer/gleason-score-isup-grade/
Reichert, Z. R., Urrutia, J., & Alumkal, J. J. (2019). Microsatellite instability as an emerging biomarker for checkpoint inhibitor response in advanced prostate cancer. JAMA Oncology, 5(4), 478-479. https://doi.org/10.1001/jamaoncol.2018.5789
Rice, M. A., Malhotra, S. V., & Stoyanova, T. (2019). Second-generation antiandrogens: From discovery to standard of care in castration-resistant prostate cancer. Frontiers in Oncology, 9(801), 1-12. https://doi.org/10.3389/fonc.2019.00801
Selchick, F. (2020). Prostate cancer treatment modalities [image].
Smith, R. A., Andrews, K. S., Brooks, D., Fedewa, S. A., Manassaram-Baptiste, D., Saslow, D., & Wender, R. C. (2019). Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA: A Cancer Journal for Clinicians, 69(3), 184-210. https://doi.org/10.3322/caac.21557
Urology Care Foundation. (2021). What is advanced prostate cancer? https://www.urologyhealth.org/urologic-conditions/advanced-prostate-cancer
US Food and Drug Administration. (2015a). Highlights of prescribing information: FIRMAGON (degarelix for injection). https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022201s009lbl.pdf
US Food and Drug Administration. (2015b). Highlights of prescribing information: Nilutamide tablets. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/207631Orig1s000lbl.pdf
US Food and Drug Administration. (2017). Highlights of prescribing information: CASODEX® (bicalutamide). https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020498s028lbl.pdf
US Food and Drug Administration. (2018). Highlights of prescribing information. ZYTIGA® (abiraterone acetate) tablets. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/202379s024lbl.pdf
US Food and Drug Administration. (2019). Highlights of prescribing information: XTANDI® (enzalutamide). https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/203415s015lbl.pdf
US Food and Drug Administration. (2020a). Highlights of prescribing information: Lynparza (Olaparib). https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208558s014lbl.pdf
US Food and Drug Administration. (2020b). Highlights of prescribing information: TRELSTAR® (triptorelin pamoate for injectable suspension). http://www.trelstar.com/pdf/TrelstarPrescribingInformation_May2020.pdf
US Food and Drug Administration. (2020c). Highlights of prescribing information: ZOLADEX® (goserelin acetate implant). http://documents.tersera.com/zoladex-us/10.8mg_MagnumPI.pdf
US Food and Drug Administration. (2021). Flutamide (Eulexin) prescribing information. https://www.accessdata.fda.gov/spl/data/6c1fd5b4-b5c7-4e48-8bb1-36f0cacf6f42/6c1fd5b4-b5c7-4e48-8bb1-36f0cacf6f42.xml
US Food and Drug Administration. (2022a). Highlights of prescribing information: Darolutamide (Nubeqa). https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/212099s002lbl.pdf
US Food and Drug Administration. (2022b). Highlights of prescribing information: LUPRON DEPOT® (leuprolide acetate for depot suspension). https://www.rxabbvie.com/pdf/lupronuro_pi.pdf
US Food and Drug Administration. (2023). Highlights of prescribing information: ERLEADA® (apalutamide) tablets. http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/ERLEADA-pi.pdf
US National Library of Medicine. (2015). HOXB13 gene. https://ghr.nlm.nih.gov/gene/HOXB13
US Preventive Services Task Force. (2018). Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA, 319(18), 1901-1913. https://doi.org/10.1001/jama.2018.3710
Wang, G., Zhao, D., Spring, D. J., & DePinho, R. A. (2018). Genetics and biology of prostate cancer. Genes & Development, 32, 1105-1140. https://doi.org/10.1101/gad.315739.118
Winer, A., Bodor, J. N., & Borghaei, H. (2018). Identifying and managing the adverse effects of immune checkpoint blockade. Journal of Thoracic Disease, 10(Supple 3); S480-S489. https://doi.org/10.21037/jtd.2018.01.111
Wu, S. Y., Chang, C. L., Chen, C. I., & Huang, C. C. (2021). Comparison of acute and chronic surgical complications following robot-assisted, laparoscopic, and traditional open radical prostatectomy among men in Taiwan. JAMA Network Open, 4(8), e2120156. https://doi.org/10.1001/jamanetworkopen.2021.20156
Yarbro, C. H., Wujcik, D., & Gobel, B. H. (Eds.). (2018). Cancer nursing: Principles and practice. (8th ed.). Jones & Bartlett Learning.